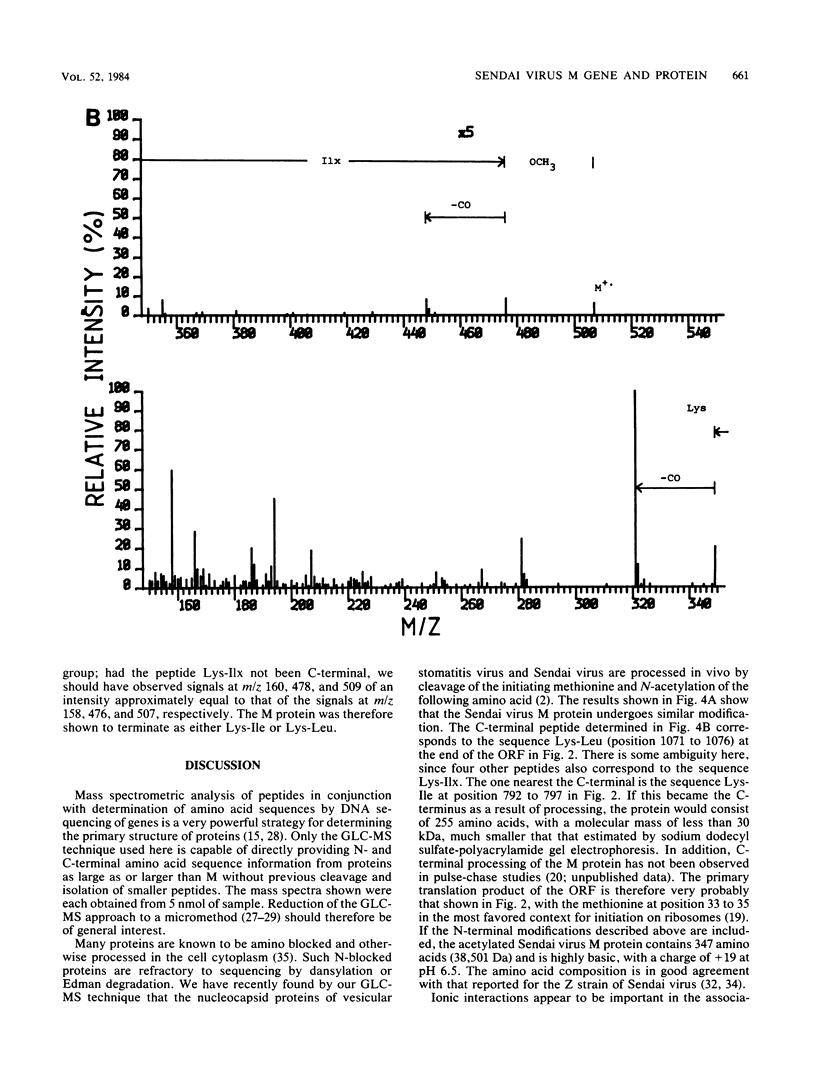
Abstract
The nucleotide sequence of the Sendai virus M (matrix or membrane) gene region was determined from cloned genomic DNA, and the limits of the M mRNA were determined by S1 nuclease mapping. The M mRNA is 1,173 nucleotides long and contains a single long open reading frame coding for a protein of 348 amino acids. The amino acid sequences of the N- and C-terminal peptides of the M protein were obtained by mass spectrometric analysis and correspond to those predicted from the open reading frame, with the N terminus modified in vivo by cleavage of the initiating methionine and acetylation of the following amino acid. The amphiphilic nature of the M protein structure is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Preparation and analysis of the nucleocapsid proteins of vesicular stomatitis virus and sendai virus, and analysis of the sendai virus leader-NP gene region. J Gen Virol. 1984 Apr;65(Pt 4):769–779. doi: 10.1099/0022-1317-65-4-769. [DOI] [PubMed] [Google Scholar]
- Bohn W., Rutter G., Hohenberg H., Mannweiler K. Inhibition of measles virus budding by phenothiazines. Virology. 1983 Oct 15;130(1):44–55. doi: 10.1016/0042-6822(83)90116-2. [DOI] [PubMed] [Google Scholar]
- Bächi T. Intramembrane structural differentiation in Sendai virus maturation. Virology. 1980 Oct 15;106(1):41–49. doi: 10.1016/0042-6822(80)90219-6. [DOI] [PubMed] [Google Scholar]
- Büechi M., Bächi T. Microscopy of internal structures of Sendai virus associated with the cytoplasmic surface of host membranes. Virology. 1982 Jul 30;120(2):349–359. doi: 10.1016/0042-6822(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Dowling P. C., Giorgi C., Roux L., Dethlefsen L. A., Galantowicz M. E., Blumberg B. M., Kolakofsky D. Molecular cloning of the 3'-proximal third of Sendai virus genome. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5213–5216. doi: 10.1073/pnas.80.17.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gething M. J., White J. M., Waterfield M. D. Purification of the fusion protein of Sendai virus: analysis of the NH2-terminal sequence generated during precursor activation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2737–2740. doi: 10.1073/pnas.75.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A. The membrane protein of influenza virus: extraction from virus and infected cell with acidic chloroform-methanol. Virology. 1973 Aug;54(2):369–383. doi: 10.1016/0042-6822(73)90150-5. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Conserved polyadenylation signals in two negative-strand RNA virus families. Virology. 1982 Jul 30;120(2):518–523. doi: 10.1016/0042-6822(82)90055-1. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Smith P. R., Choppin P. W. In vitro assembly of the nonglycosylated membrane protein (M) of Sendai virus. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6232–6236. doi: 10.1073/pnas.79.20.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy W. C., Royal N. J., Biemann K., Putney S. D., Schimmel P. R. Mass spectra of partial protein hydrolysates as a multiple phase check for long polypeptides deduced from DNA sequences: NH2-terminal segment of alanine tRNA synthetase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6531–6535. doi: 10.1073/pnas.77.11.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. A., Nermut M. V. A morphological study of the M-protein of Sendai virus. J Gen Virol. 1977 Jan;34(1):127–136. doi: 10.1099/0022-1317-34-1-127. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E., Swoveland P., Carrigan D. R. Experimental subacute sclerosing panencephalitis: selective disappearance of measles virus matrix protein from the Central nervous system. J Infect Dis. 1981 Aug;144(2):161–169. doi: 10.1093/infdis/144.2.161. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Lackland H., Choppin P. W. Isolation and characterization of the nonglycosylated membrane protein and a nucleocapsid complex from the paramyxovirus SV5. Virology. 1975 Oct;67(2):365–374. doi: 10.1016/0042-6822(75)90438-9. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Lackland H., Choppin P. W. Proteolytic cleavage of subunits of the nucleocapsid of the paramyxovirus simian virus 5. J Virol. 1974 Nov;14(5):1253–1261. doi: 10.1128/jvi.14.5.1253-1261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K., Kocher H. P., Blumberg B. M., Kolakofsky D. An improved procedure, involving mass spectrometry, for N-terminal amino acid sequence determination of proteins which are N alpha-blocked. Biochem J. 1984 Jan 1;217(1):253–257. doi: 10.1042/bj2170253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K., Simona M. G., Offord R. E. Amino acid sequence determination by g.l.c.--mass spectrometry of permethylated peptides. Optimization of the formation of chemical derivatives at the 2-10 nmol level. Biochem J. 1983 Nov 1;215(2):261–272. doi: 10.1042/bj2150261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K., Simona M. G., Offord R. E., Prior C. P., Otto B., Thatcher D. R. A new mass-spectrometric C-terminal sequencing technique finds a similarity between gamma-interferon and alpha 2-interferon and identifies a proteolytically clipped gamma-interferon that retains full antiviral activity. Biochem J. 1983 Nov 1;215(2):273–277. doi: 10.1042/bj2150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- Semba T., Hosaka Y., Sakiyama F. Isolation of the M polypeptide of Sendai virus (HVJ) with column chromatography. Biken J. 1977 Jun;20(2):77–80. [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold F. In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]