Abstract
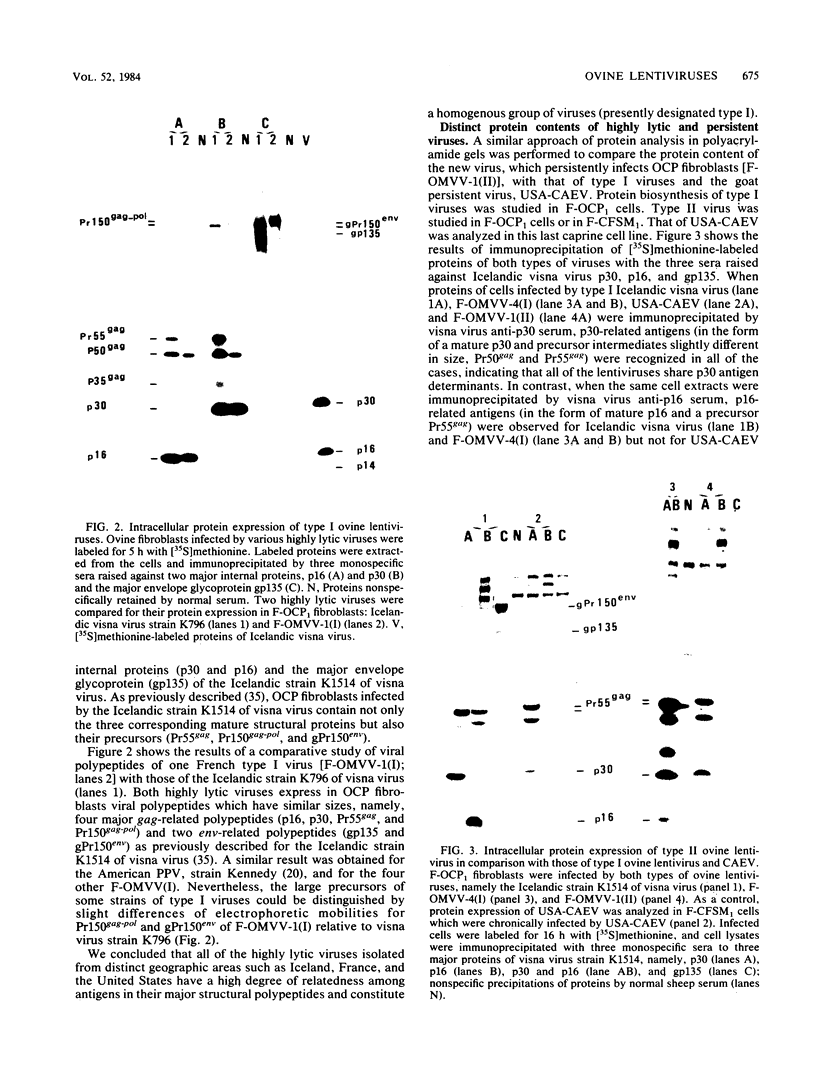
Ovine and caprine lentiviruses share the capacity to induce slowly progressive and inflammatory diseases of the central nervous system (leukoencephalitis or visna), lungs (progressive pneumonia or maedi), and joints (arthritis) in their natural hosts. Studies on their replication indicated that ovine lentiviruses and caprine arthritis-encephalitis virus (CAEV) recently isolated in the United States establish persistent infection in ovine and caprine fibroblasts, whereas older prototype ovine lentiviruses such as Icelandic visna virus or American progressive pneumonia virus irreversibly lyse fibroblast cultures. Since all of the recent isolates were found to be persistent, Narayan et al. (J. Gen. Virol. 59:345-356, 1982) concluded that the highly lytic viruses were only tissue-culture-adapted strains. In the present report, we isolated new ovine lentiviruses from French sheep with naturally occurring progressive pneumonia which are either highly lytic (five isolates), as are the Icelandic strains of visna virus, or persistent (one isolate), as are CAEV or American persistent ovine lentiviruses. Protein and nucleic acid content analyses of these new highly lytic (type I) and persistent (type II) isolates indicated that type I and type II ovine lentiviruses were genetically distinct, type I and type II viruses being closely related to the Icelandic strains of visna virus and to CAEV, respectively. We conclude that (i) highly lytic ovine lentiviruses, such as the Icelandic prototype strains of visna virus and persistent lentiviruses more related to CAEV, are naturally present in the ovine species, and (ii) irreversible cell lysis induced by highly lytic viruses does not result from a tissue culture adaptation of field isolates that were originally persistent but is instead the consequence of a genetic content distinct from that of persistent viruses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks K. L., Adams D. S., McGuire T. C., Carlson J. Experimental infection of sheep by caprine arthritis-encephalitis virus and goats by progressive pneumonia virus. Am J Vet Res. 1983 Dec;44(12):2307–2311. [PubMed] [Google Scholar]
- Clements J. E., Narayan O. A physical map of the linear unintegrated DNA of Visna virus. Virology. 1981 Aug;113(1):412–415. doi: 10.1016/0042-6822(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Cork L. C. Biochemical characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980 Oct;50(2):423–427. doi: 10.1099/0022-1317-50-2-423. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Hadlow W. J., Crawford T. B., Gorham J. R., Piper R. C. Infectious leukoencephalomyelitis of young goats. J Infect Dis. 1974 Feb;129(2):134–141. doi: 10.1093/infdis/129.2.134. [DOI] [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S., Cheevers W. P., Cork L. C. Chronic arthritis in goats caused by a retrovirus. Science. 1980 Feb 29;207(4434):997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Gaskin J. M., Perk K. Morphological and immunological comparison of caprine arthritis encephalitis and ovine progressive pneumonia viruses. J Virol. 1981 Sep;39(3):914–919. doi: 10.1128/jvi.39.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Filippi P., Brahic M., Vigne R., Tamalet J. Characterization of visna virus mRNA. J Virol. 1979 Jul;31(1):25–30. doi: 10.1128/jvi.31.1.25-30.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Yaniv A., Dvir M., Perk K., Irving S. G., Dahlberg J. E. The caprine arthritis-encephalitis virus is a distinct virus within the Lentivirus group. Virology. 1983 Jan 15;124(1):192–195. doi: 10.1016/0042-6822(83)90305-7. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Baringer J. R. The structural polypeptides of RNA slow viruses. Virology. 1974 Jan;57(1):238–250. doi: 10.1016/0042-6822(74)90124-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Harris J. D., Scott J. V., Traynor B., Brahic M., Stowring L., Ventura P., Haase A. T., Peluso R. Visna virus DNA: discovery of a novel gapped structure. Virology. 1981 Sep;113(2):573–583. doi: 10.1016/0042-6822(81)90185-9. [DOI] [PubMed] [Google Scholar]
- Harter D. H., Choppin P. W. Cell-fusing activity of visna virus particles. Virology. 1967 Feb;31(2):279–288. doi: 10.1016/0042-6822(67)90172-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kennedy R. C., Eklund C. M., Lopez C., Hadlow W. J. Isolation of a virus from the lungs of Montana sheep affected with progressive pneumonia. Virology. 1968 Jul;35(3):483–484. doi: 10.1016/0042-6822(68)90228-6. [DOI] [PubMed] [Google Scholar]
- Klevjer-Anderson P., Cheevers W. P. Characterization of the infection of caprine synovial membrane cells by the retrovirus caprine arthritis-encephalitis virus. Virology. 1981 Apr 15;110(1):113–119. doi: 10.1016/0042-6822(81)90012-x. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Narayan O., Clements J. E., Strandberg J. D., Cork L. C., Griffin D. E. Biological characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980 Sep;50(1):69–79. doi: 10.1099/0022-1317-50-1-69. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Silverstein A. M. Slow virus infection: replication and mechanisms of persistence of visna virus in sheep. J Infect Dis. 1977 May;135(5):800–806. doi: 10.1093/infdis/135.5.800. [DOI] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Oliver R. E., Gorham J. R., Parish S. F., Hadlow W. J., Narayan O. Ovine progressive pneumonia: pathologic and virologic studies on the naturally occurring disease. Am J Vet Res. 1981 Sep;42(9):1554–1559. [PubMed] [Google Scholar]
- Panitch H., Petursson G., Georgsson G., Palsson P. A., Nathanson N. Pathogenesis of visna. III. Immune responses to central nervous system antigens in experimental allergic encephalomyelitis and visna. Lab Invest. 1976 Nov;35(5):452–460. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberson S. M., McGuire T. C., Klevjer-Anderson P., Gorham J. R., Cheevers W. P. Caprine arthritis-encephalitis virus is distinct from visna and progressive pneumonia viruses as measured by genome sequence homology. J Virol. 1982 Nov;44(2):755–758. doi: 10.1128/jvi.44.2.755-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGURDSSON B., GRIMSSON H., PALSSON P. A. Maedi, a chronic, progressive infection of sheep's lungs. J Infect Dis. 1952 May-Jun;90(3):233–241. doi: 10.1093/infdis/90.3.233. [DOI] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Vigne R., Brahic M., Filippi P., Tamalet J. Complexity and polyadenylic acid content of visna virus 60-70S RNA. J Virol. 1977 Jan;21(1):386–395. doi: 10.1128/jvi.21.1.386-395.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne R., Filippi P., Quérat G., Sauze N., Vitu C., Russo P., Delori P. Precursor polypeptides to structural proteins of visna virus. J Virol. 1982 Jun;42(3):1046–1056. doi: 10.1128/jvi.42.3.1046-1056.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitu C., Russo P., Filippi P., Vigne R., Querat G., Giauffret A. Une technique ELISA pour la détection des anticorps anti-virus maedi-visna. Etude comparative avec l'immunodiffusion en gelose et la fixation du complement. Comp Immunol Microbiol Infect Dis. 1982;5(4):469–481. doi: 10.1016/0147-9571(82)90073-x. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Sweet R. W., Gulati S. C., Harter D. H. Nucleic acid sequence relationships among "slow" viruses of sheep. Virology. 1976 Jun;71(2):395–401. doi: 10.1016/0042-6822(76)90367-6. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]