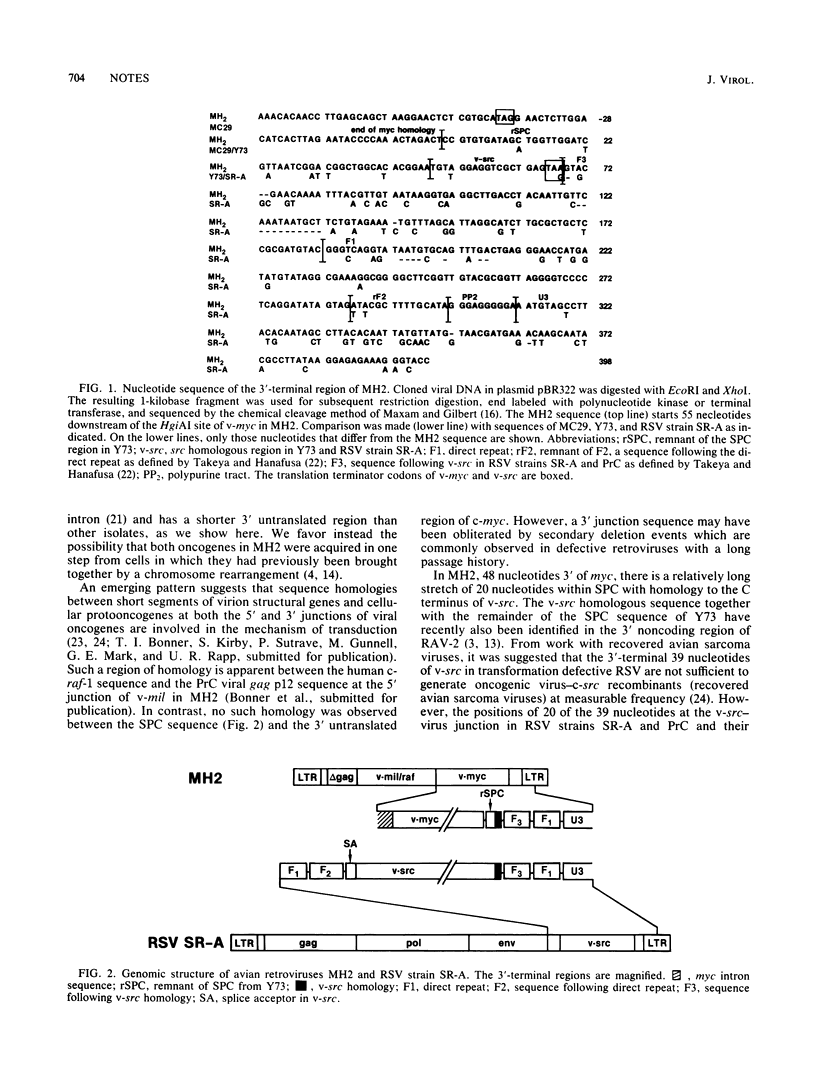
Abstract
We determined the nucleotide sequence of the acute transforming avian retrovirus MH2 from an HgiAI site within the coding region of its oncogene, v-myc, to the KpnI site within the long terminal repeat. Comparison with published sequences from other retroviruses allowed us to identify all sequence elements in this region. We conclude that MH2 contains a unique assembly of 3'-terminal sequences, which includes part of the helper virus-derived SPC region of avian sarcoma virus Y73 and the complete F3 and F1 segments of Rous sarcoma virus strain SR-A.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Bishop J. M., Smith D. H., Chen E. Y., Colby W. W., Levinson A. D. Nucleotide sequence to the v-myc oncogene of avian retrovirus MC29. Proc Natl Acad Sci U S A. 1983 Jan;80(1):100–104. doi: 10.1073/pnas.80.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Katz R. A., Skalka A. M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984 Feb;49(2):557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T., O'Brien S. J., Nash W. G., Rapp U. R., Morton C. C., Leder P. The human homologs of the raf (mil) oncogene are located on human chromosomes 3 and 4. Science. 1984 Jan 6;223(4631):71–74. doi: 10.1126/science.6691137. [DOI] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 1983;2(12):2189–2194. doi: 10.1002/j.1460-2075.1983.tb01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- Hunter E. The mechanism for genetic recombination in the avian retroviruses. Curr Top Microbiol Immunol. 1978;79:295–309. doi: 10.1007/978-3-642-66853-1_7. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Lurz R., Bister K., Bonner T. I., Mark G. E., Rapp U. R. Homologous cell-derived oncogenes in avian carcinoma virus MH2 and murine sarcoma virus 3611. Nature. 1984 Jan 19;307(5948):281–284. doi: 10.1038/307281a0. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Patschinsky T., Bister K. Avian oncovirus MH2: molecular cloning of proviral DNA and structural analysis of viral RNA and protein. J Virol. 1983 Oct;48(1):61–73. doi: 10.1128/jvi.48.1.61-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen H. W., Rückert B., Lurz R., Bister K. Two unrelated cell-derived sequences in the genome of avian leukemia and carcinoma inducing retrovirus MH2. EMBO J. 1983;2(11):1969–1975. doi: 10.1002/j.1460-2075.1983.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Garon C. F., Duesberg P. H., Papas T. S. Avian carcinoma virus MH2 contains a transformation-specific sequence, mht, and shares the myc sequence with MC29, CMII, and OK10 viruses. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6566–6570. doi: 10.1073/pnas.80.21.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Lerner T. L., Hanafusa H. DNA sequence of the Bryan high-titer strain of Rous sarcoma virus: extent of env deletion and possible genealogical relationship with other viral strains. J Virol. 1984 Feb;49(2):549–556. doi: 10.1128/jvi.49.2.549-556.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Primary structure of v-raf: relatedness to the src family of oncogenes. Science. 1984 Apr 20;224(4646):285–289. doi: 10.1126/science.6324342. [DOI] [PubMed] [Google Scholar]
- Mark J., Dahlenfors R., Ekedahl C. Cytogenetics of the human benign mixed salivary gland tumour. Hereditas. 1983;99(1):115–129. doi: 10.1111/j.1601-5223.1983.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Reynolds F. H., Jr, Stephenson J. R. New mammalian transforming retrovirus: demonstration of a polyprotein gene product. J Virol. 1983 Mar;45(3):914–924. doi: 10.1128/jvi.45.3.914-924.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Watson D. K., Schultz R. A., Lautenberger J., Papas T. S. Nucleotide sequence analysis of the proviral genome of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1983 May;80(9):2500–2504. doi: 10.1073/pnas.80.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sutrave P., Bonner T. I., Rapp U. R., Jansen H. W., Patschinsky T., Bister K. Nucleotide sequence of avian retroviral oncogene v-mil: homologue of murine retroviral oncogene v-raf. Nature. 1984 May 3;309(5963):85–88. doi: 10.1038/309085a0. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Beckson M., Anderson S. M., Hanafusa H. Identification of the viral sequence required for the generation of recovered avian sarcoma viruses and characterization of a series of replication-defective recovered avian sarcoma viruses. J Virol. 1984 Mar;49(3):881–891. doi: 10.1128/jvi.49.3.881-891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



