Abstract
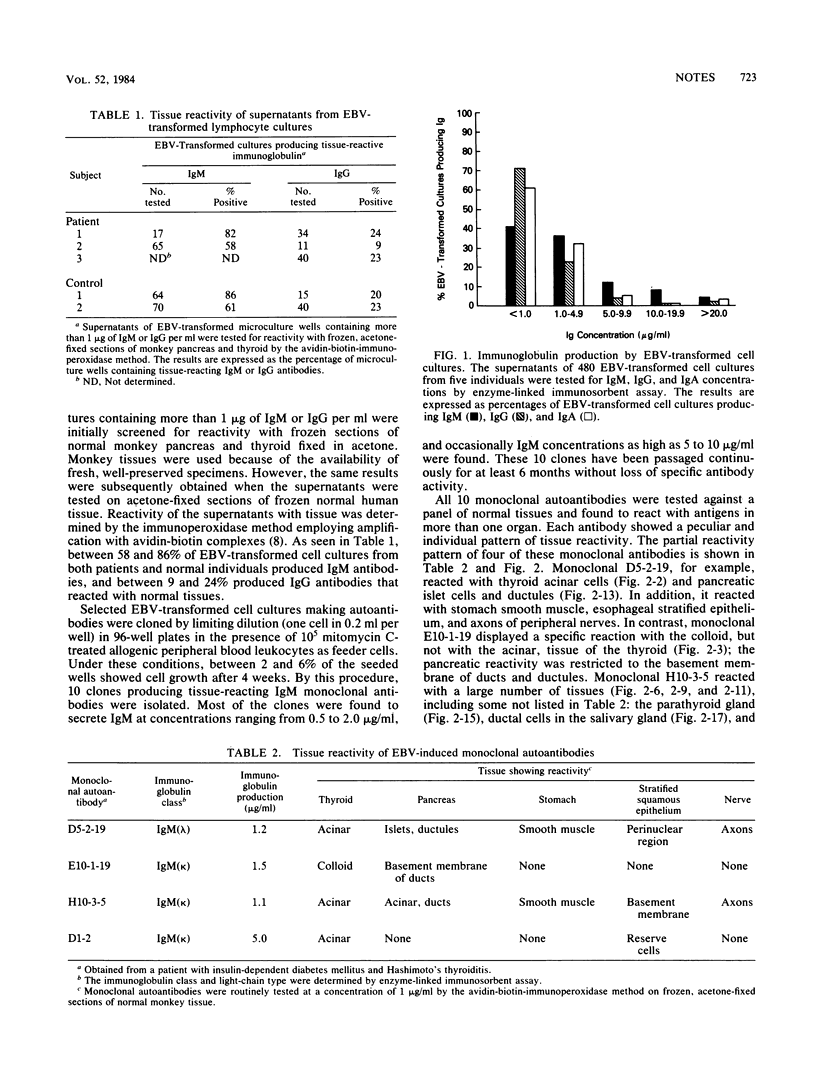
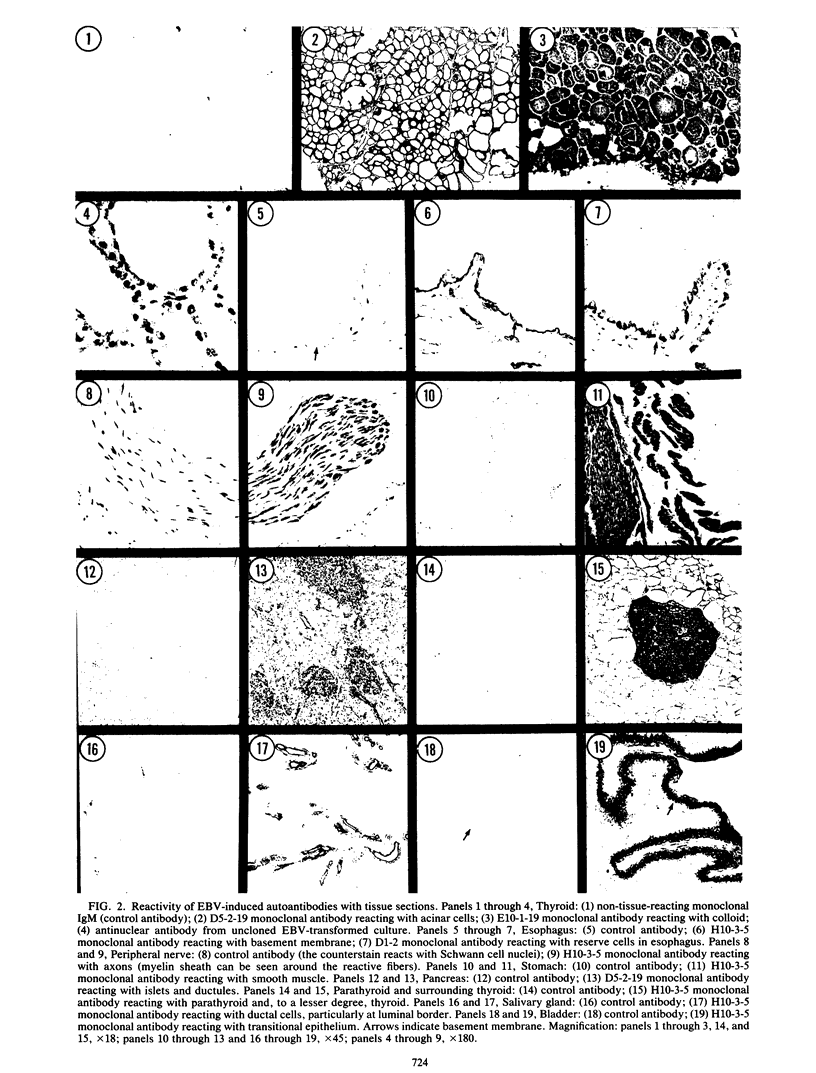
Peripheral blood lymphocytes from normal individuals and patients with autoimmune abnormalities such as insulin-dependent diabetes mellitus and thyroiditis were infected with Epstein-Barr virus, and the culture supernatants were tested for autoantibodies that reacted with normal tissues. Between 58 and 86% of Epstein-Barr virus-transformed cultures produced immunoglobulin M antibodies, and between 9 and 24% of the transformed cultures produced immunoglobulin G antibodies that reacted with normal tissues. Ten Epstein-Barr virus-transformed clones secreting human immunoglobulin M monoclonal autoantibodies were isolated. Four of these monoclonal autoantibodies were studied in depth and found to react with antigens in multiple organs, including thyroid, pancreas, stomach, smooth muscle, and nerves. It is concluded that Epstein-Barr virus can trigger the production of autoantibodies without infecting the target cells to which the autoantibodies are directed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Faber V. Antibodies to smooth muscle and other tissue components in infectious mononucleosis. Scand J Infect Dis. 1978;10(1):1–5. doi: 10.3109/inf.1978.10.issue-1.01. [DOI] [PubMed] [Google Scholar]
- Bird A. G., Britton S. A live human B-cell activator operating in isolation of other cellular influences. Scand J Immunol. 1979;9(6):507–510. doi: 10.1111/j.1365-3083.1979.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Bird A. G., Britton S. A new approach to the study of human B lymphocyte function using an indirect plaque assay and a direct B cell activator. Immunol Rev. 1979;45:41–67. doi: 10.1111/j.1600-065x.1979.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Bird A. G., Britton S., Ernberg I., Nilsson K. Characteristics of Epstein-Barr virus activation of human B lymphocytes. J Exp Med. 1981 Sep 1;154(3):832–839. doi: 10.1084/jem.154.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., Horita M., Suzuki H., Notkins A. L. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science. 1983 Apr 15;220(4594):304–306. doi: 10.1126/science.6301002. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., McClintock P. R., Essani K., Ray U. R., Yagihashi S., Notkins A. L. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983 Jul 7;304(5921):73–76. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- Holborow E. J., Hemsted E. H., Mead S. V. Smooth muscle autoantibodies in infectious mononucleosis. Br Med J. 1973 Aug 11;3(5875):323–325. doi: 10.1136/bmj.3.5875.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Kamo I., Furukawa S., Tada A., Mano Y., Iwasaki Y., Furuse T., Ito N., Hayashi K., Satoyoshi E. Monoclonal antibody to acetylcholine receptor: cell line established from thymus of patient with Myasthenia gravis. Science. 1982 Feb 19;215(4535):995–997. doi: 10.1126/science.6297000. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Roder J. C. Requirements for the establishment of high-titered human monoclonal antibodies against tetanus toxoid using the Epstein-Barr virus technique. J Immunol. 1981 Oct;127(4):1275–1280. [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Linder E., Kurki P., Andersson L. C. Autoantibody to "intermediate filament" in infectious mononucleosis. Clin Immunol Immunopathol. 1979 Dec;14(4):411–417. doi: 10.1016/0090-1229(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Satoh J., Prabhakar B. S., Haspel M. V., Ginsberg-Fellner F., Notkins A. L. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med. 1983 Jul 28;309(4):217–220. doi: 10.1056/NEJM198307283090405. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Izak G., Cohen S., Ehrenfeld M., Flechner I. Continuous production of monoclonal rheumatoid factor by EBV-transformed lymphocytes. Nature. 1980 Oct 2;287(5781):443–445. doi: 10.1038/287443a0. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Klein G., Koskimies S., Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977 Sep 29;269(5627):420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- Sutton R. N., Emond R. T., Thomas D. B., Doniach D. The occurrence of autoantibodies in infectious mononucleosis. Clin Exp Immunol. 1974 Jul;17(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Zurawski V. R., Jr, Haber E., Black P. H. Production of antibody to tetanus toxoid by continuous human lymphoblastoid cell lines. Science. 1978 Mar 31;199(4336):1439–1441. doi: 10.1126/science.204013. [DOI] [PubMed] [Google Scholar]