Abstract
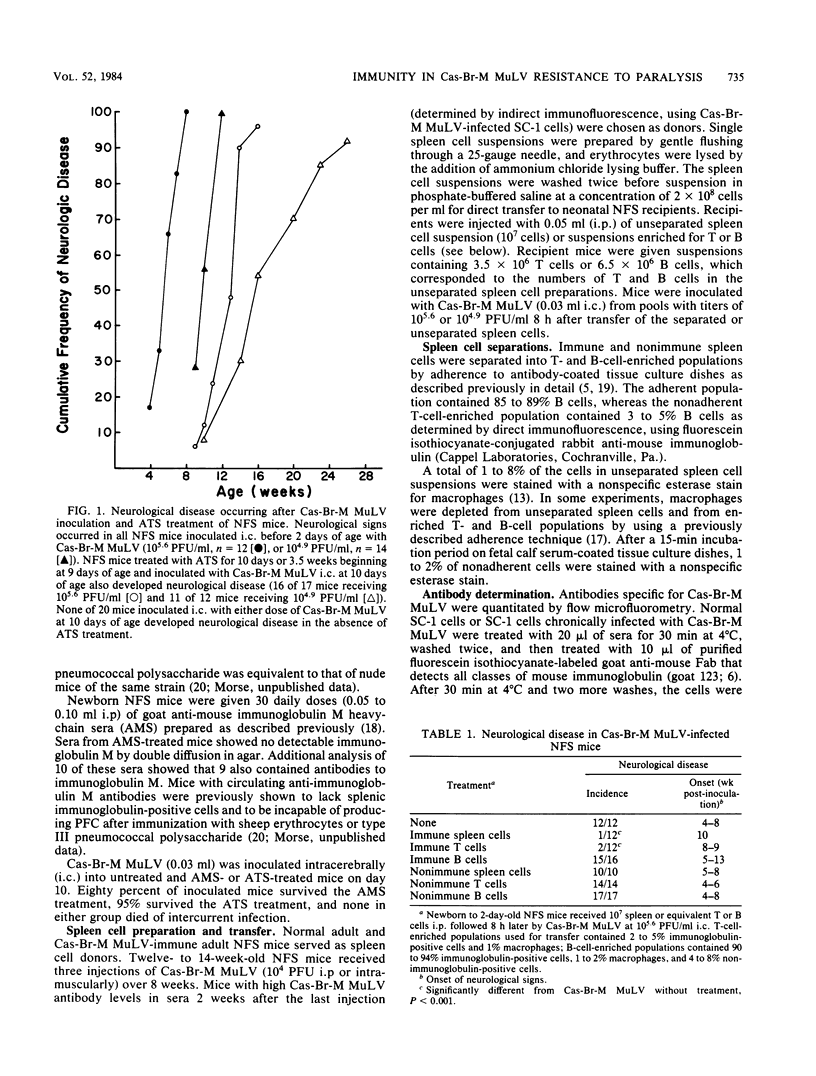
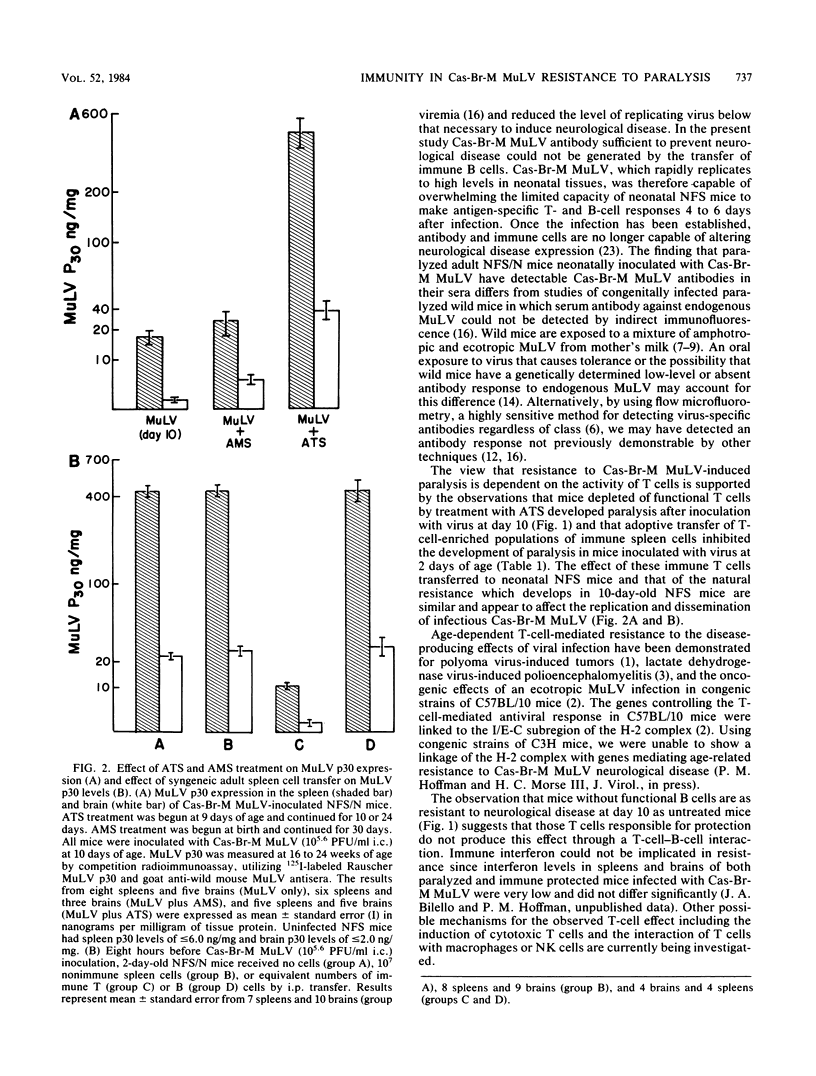
Resistance to the paralytic effects of a wild mouse (Cas-Br-M) murine leukemia virus infection develops with age and is complete by 10 days of age in susceptible NFS mice. The possibility that cell-mediated immunity plays a significant role in this resistance was suggested by the observation that treatment of 10-day-old mice with antithymocyte serum rendered them susceptible to paralysis. By comparison, mice rendered incapable of generating a humoral immune response by treatment from birth to 1 month of age with anti-immunoglobulin M serum did not develop paralysis after challenge with virus at day 10. Transfer of unseparated and T-cell-enriched populations of Cas-Br-M murine leukemia virus-immune spleen cells protected neonatally infected NFS recipients from paralysis; transfer of Cas-Br-M murine leukemia virus-immune populations enriched for B cells delayed the onset but did not ultimately protect neonatally infected NFS mice from paralysis. Transfer of naive adult spleen cells had no protective effect in neonatally infected NFS mice. High-level virus replication occurred in the spleens and brains of all mice that developed paralysis regardless of treatment; low-level virus replication in spleen and barely detectable replication in brain occurred in mice that remained clinically normal. These studies suggest that the age-acquired resistance to the paralytic effect of Cas-Br-M murine leukemia virus infection is immunologically mediated and that T cells may play a major role.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear S. E., Tsichlis P. N., Schwartz R. S. H-2-mediated resistance to an ecotropic type C retrovirus: localization of controlling genes and ontogenic studies of resistance. Immunogenetics. 1980;11(5):451–465. doi: 10.1007/BF01567814. [DOI] [PubMed] [Google Scholar]
- Bentley D. M., Morris R. E. T cell subsets required for protection against age-dependent polioencephalomyelitis of C58 mice. J Immunol. 1982 Feb;128(2):530–534. [PubMed] [Google Scholar]
- Brooks B. R., Swarz J. R., Narayan O., Johnson R. T. Murine neurotropic retrovirus spongiform polioencephalomyelopathy: acceleration of disease by virus inoculum concentration. Infect Immun. 1979 Feb;23(2):540–544. doi: 10.1128/iai.23.2.540-544.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. F., Chused T. M., Morse H. C., 3rd Genetic and functional analyses of the primary in vitro CTL: response of NZB lymphocytes to H-2-compatible cells. Immunogenetics. 1981 Mar 1;12(5-6):445–463. doi: 10.1007/BF01561687. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Chused T. M., Morse H. C., 3rd Genetic control of B- and T-lymphocyte abnormalities of NZB mice in crosses with B10.D2 mice. Immunogenetics. 1981;13(5):421–434. doi: 10.1007/BF00346023. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Estes J. D., Casagrande J., Rasheed S. Prevention of paralysis and suppression of lymphoma in wild mice by passive immunization to congenitally transmitted murine leukemia virus. J Natl Cancer Inst. 1980 Feb;64(2):359–364. doi: 10.1093/jnci/64.2.359. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Estes J. D., Menck H., Parker J. C., Huebner R. J. Unusually high incidence of spontaneous lymphomas in wild house mice. J Natl Cancer Inst. 1973 Jun;50(6):1571–1579. doi: 10.1093/jnci/50.6.1571. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Pitts O. M., Rohwer R. G., Gajdusek D. C., Ruscetti S. K. Retrovirus antigens in brains of mice with scrapie- and murine leukemia virus-induced spongiform encephalopathy. Infect Immun. 1982 Oct;38(1):396–398. doi: 10.1128/iai.38.1.396-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Ruscetti S. K., Morse H. C., 3rd Pathogenesis of paralysis and lymphoma associated with a wild mouse retrovirus infection. Part 1. Age- and dose-related effects in susceptible laboratory mice. J Neuroimmunol. 1981 Sep;1(3):275–285. doi: 10.1016/0165-5728(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Allison A. C., Ward P., Kight N. Identification of human mononuclear leucocyte populations by esterase staining. Clin Exp Immunol. 1977 Nov;30(2):289–298. [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Enjuanes L., Lee J. C., Keller J. The immune response to C-type viruses and its potential role in leukemogenesis. Curr Top Microbiol Immunol. 1982;101:31–49. doi: 10.1007/978-3-642-68654-2_2. [DOI] [PubMed] [Google Scholar]
- Johnson E. D., Monjan A. A., Morse H. C., 3rd Lack of B-cell participation in acute lymphocyte choriomeningitis disease of the central nervous system. Cell Immunol. 1978 Mar 1;36(1):143–150. doi: 10.1016/0008-8749(78)90257-5. [DOI] [PubMed] [Google Scholar]
- Klement V., Gardner M. B., Henderson B. E., Ihle J. N., Stanley A. G., Gilden R. V., Estes J. D. Inefficient humoral immune response of lymphoma-prone wild mice to persistent leukemia virus infection. J Natl Cancer Inst. 1976 Nov;57(5):1169–1173. doi: 10.1093/jnci/57.5.1169. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Miller A., Morse H. C., 3rd, Winkelstein J., Nathanson N. The role of antibody in recovery from experimental rabies. I. Effect of depletion of B and T cells. J Immunol. 1978 Jul;121(1):321–326. [PubMed] [Google Scholar]
- Morse H. C., 3rd, Chused T. M., Boehm-Truitt M., Mathieson B. J., Sharrow S. O., Hartley J. W. XenCSA: cell surface antigens related to the major glycoproteins (gp70) of xenotropic murine leukemia viruses. J Immunol. 1979 Feb;122(2):443–454. [PubMed] [Google Scholar]
- Officer J. E., Tecson N., Estes J. D., Fontanilla E., Rongey R. W., Gardner M. B. Isolation of a neurotropic type C virus. Science. 1973 Sep 7;181(4103):945–947. doi: 10.1126/science.181.4103.945. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Lampert P. W., Lee S., Dixon F. J. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am J Pathol. 1977 Jul;88(1):193–212. [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]



