Abstract
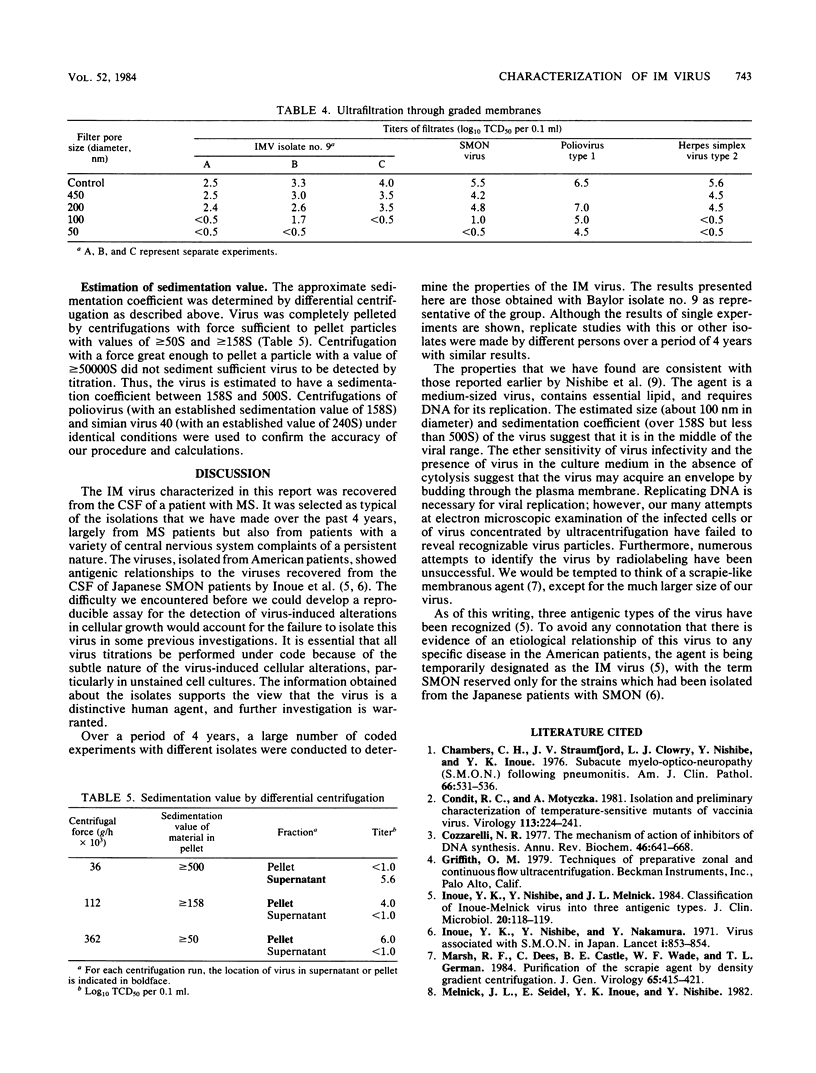
A transmissible agent, the IM virus, antigenically related to the Japanese subacute myelo-optico-neuropathy virus, has been isolated from several human cerebrospinal fluids obtained from American patients with multiple sclerosis and other chronic diseases of the central nervous system. The isolates were propagated in human diploid fibroblast (MRC5) cells, and virus was released into the culture medium in the absence of overt cytolysis. Infection of MRC5 cells resulted in a subtle alteration in the normal growth pattern of the cells. In unstained cultures, the cell changes were so mild that it was necessary to carry out all virus assays under code to eliminate bias. Cells in late passages were more susceptible than vigorously growing cells in early passages. Analysis of the kinetics of replication revealed that newly synthesized progeny virus was first detected about 12 h postinfection, that maximal virus release occurred by 48 h postinfection, and that virus production was persistent throughout an 8-day period. Several inhibitors of DNA synthesis were effective in blocking viral replication, including cytosine arabinoside, iododeoxyuridine, and phosphonoacetic acid. A substantial decrease in infectivity was observed upon treatment of IM virus with ether, suggesting that a lipid-containing structure is essential for infectivity. Ultrafiltration studies approximated the size (diameter) of IM virus to be between 100 and 200 nm.
Full text
PDF

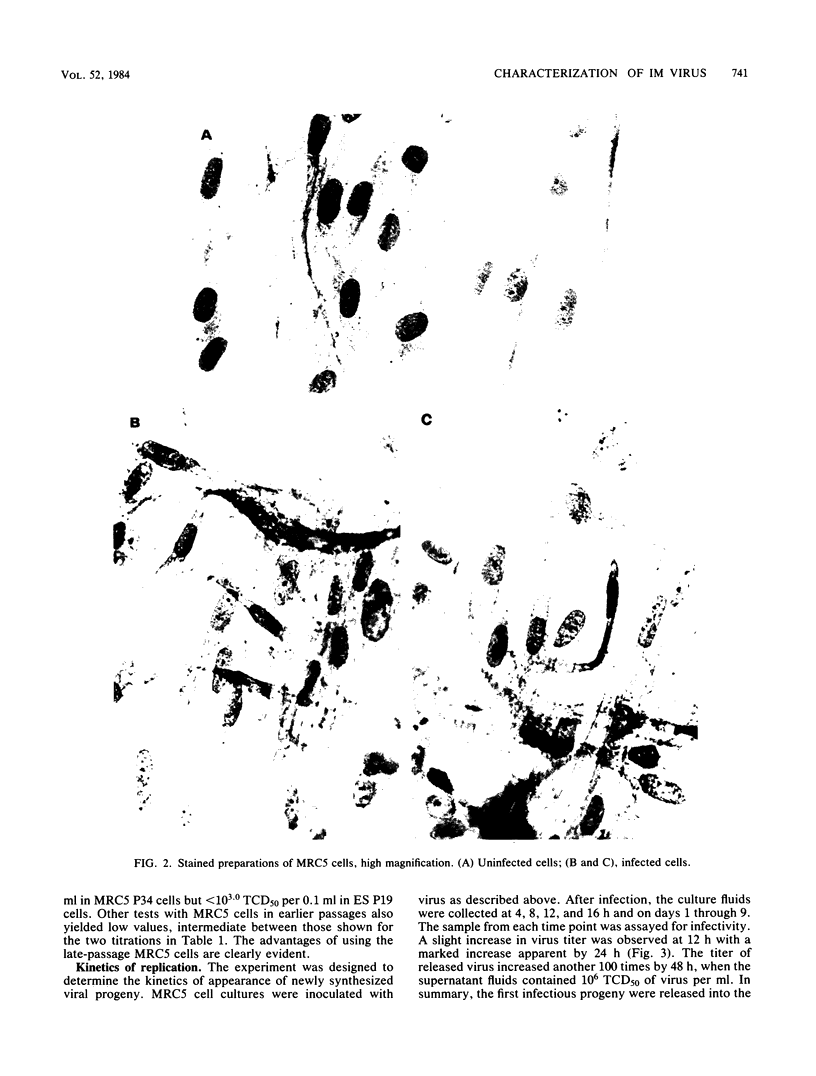

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers C. H., Straumfjord J. V., Clowry L. J., Nishibe Y., Inoue Y. K. Subacute myelo-optico-neuropathy (SMON) following pneumonitis. Serologic confirmation three and a half years after death. Am J Clin Pathol. 1976 Sep;66(3):531–536. doi: 10.1093/ajcp/66.3.531. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Inoue Y. K., Nishibe Y., Melnick J. L. Classification of Inoue-Melnick virus into three antigenic types. J Clin Microbiol. 1984 Jul;20(1):118–119. doi: 10.1128/jcm.20.1.118-119.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. K., Nishibe Y., Nakamura Y. Virus associated with S.M.O.N. in Japan. Lancet. 1971 Apr 24;1(7704):853–854. doi: 10.1016/s0140-6736(71)91513-3. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Dees C., Castle B. E., Wade W. F., German T. L. Purification of the scrapie agent by density gradient centrifugation. J Gen Virol. 1984 Feb;65(Pt 2):415–421. doi: 10.1099/0022-1317-65-2-415. [DOI] [PubMed] [Google Scholar]
- Nishibe Y., Kimura T., Inoue Y. K. Properties of virus associated with subacute myelo-optico-neuropathy. Arch Gesamte Virusforsch. 1973;40(3):189–197. doi: 10.1007/BF01242537. [DOI] [PubMed] [Google Scholar]
- Shipkowitz N. L., Bower R. R., Appell R. N., Nordeen C. W., Overby L. R., Roderick W. R., Schleicher J. B., Von Esch A. M. Suppression of herpes simplex virus infection by phosphonoacetic acid. Appl Microbiol. 1973 Sep;26(3):264–267. doi: 10.1128/am.26.3.264-267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]