Abstract
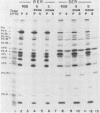
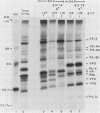
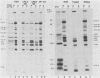
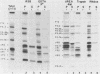
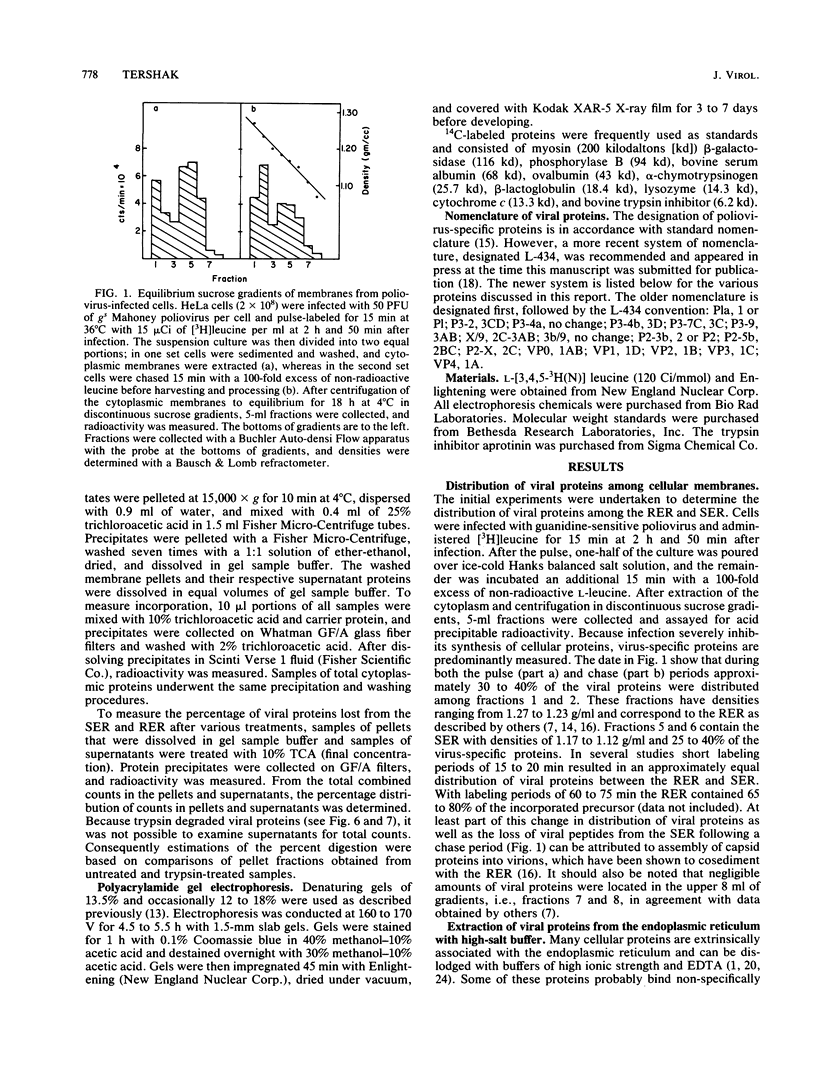
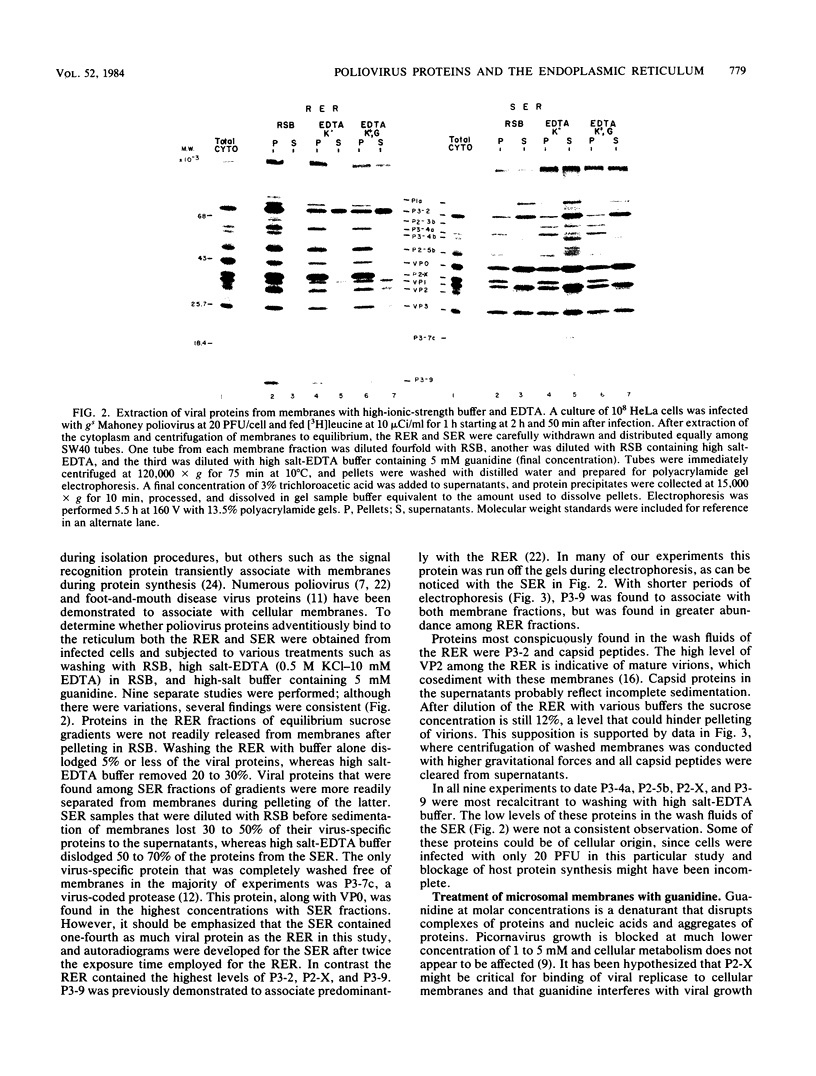
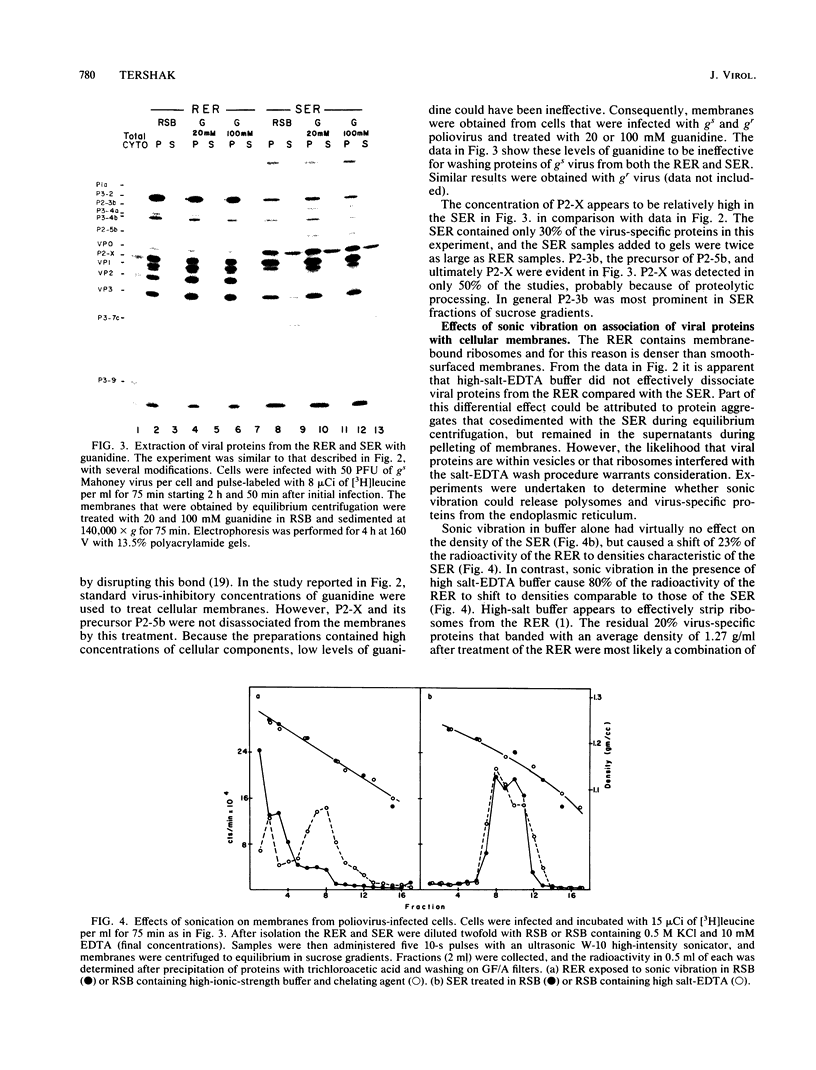
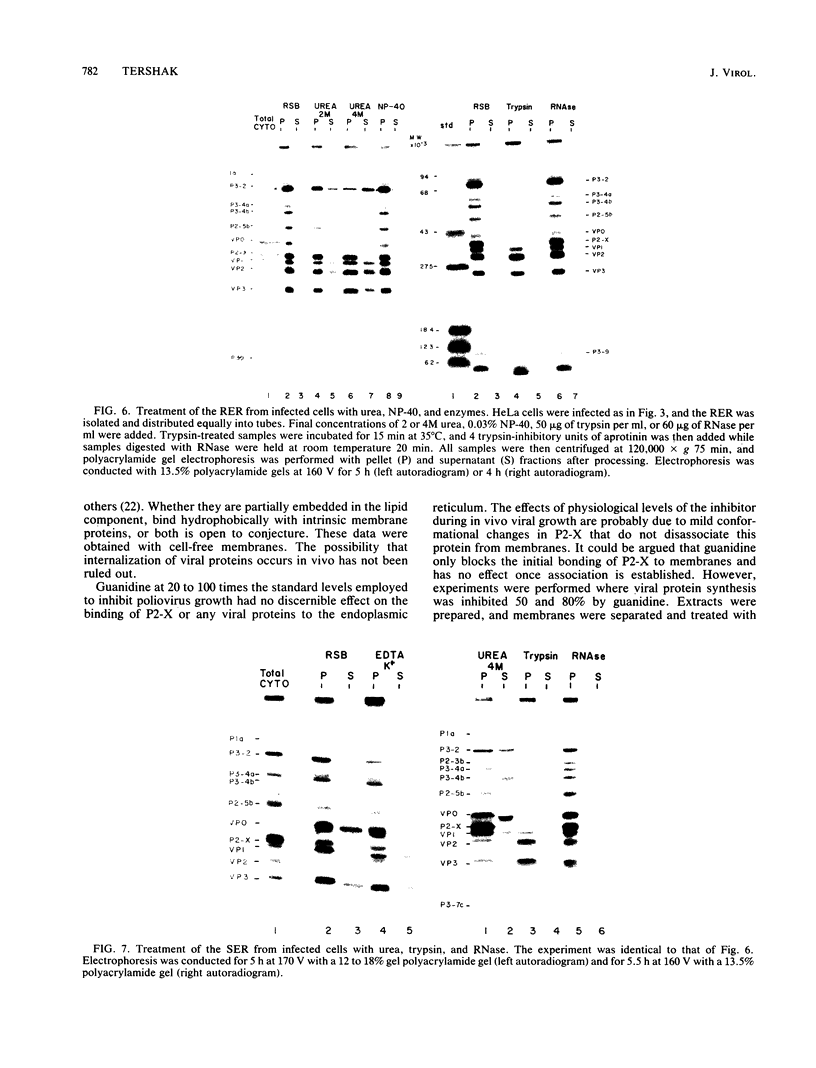
Poliovirus proteins, except P3-7c, are associated with the endoplasmic reticulum after extraction of the cytoplasm and centrifugation of membranes to equilibrium in sucrose gradients. Proteins P3-2, P2-X, and P3-9 are found preferentially among the rough endoplasmic reticulum, whereas P3-7c is located in smooth endoplasmic reticulum fractions. P3-7c is probably not membrane associated, since it can be separated from membranes after centrifugation in buffer. However, P3-4a, P2-5b, P2-X, and P3-9 are avidly bound to membranes and cannot be dislodged with high-ionic-strength buffer containing EDTA or 4 M urea. These proteins are digested by trypsin, indicating peripheral rather than internal localization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amako K., Dales S. Cytopathology of Mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967 Jun;32(2):201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- Anderson-Sillman K., Bartal S., Tershak D. R. Guanidine-resistant poliovirus mutants produce modified 37-kilodalton proteins. J Virol. 1984 Jun;50(3):922–928. doi: 10.1128/jvi.50.3.922-928.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTIMORE D. IN VITRO SYNTHESIS OF VIRAL RNA BY THE POLIOVIRUS RNA POLYMERASE. Proc Natl Acad Sci U S A. 1964 Mar;51:450–456. doi: 10.1073/pnas.51.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Kinetics and location of poliovirus macromolecular synthesis in correlation to virus-induced cytopathology. Virology. 1980 Jan 30;100(2):390–399. doi: 10.1016/0042-6822(80)90530-9. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Mosser A. G. Proteins associated with the poliovirus RNA replication complex. Virology. 1971 Nov;46(2):375–386. doi: 10.1016/0042-6822(71)90039-0. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Etchison D., Ehrenfeld E. Viral polypeptides associated with the RNA replication complex in poliovirus-infected cells. Virology. 1980 Nov;107(1):135–142. doi: 10.1016/0042-6822(80)90279-2. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Robertson B. H., Morgan D. O., Moore D. M., Dowbenko D. Biochemical map of polypeptides specified by foot-and-mouth disease virus. J Virol. 1984 May;50(2):579–586. doi: 10.1128/jvi.50.2.579-586.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Scheid A. S., Tamm I. Chemical and enzymatic characteristics of cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):30–38. doi: 10.1016/0042-6822(72)90235-8. [DOI] [PubMed] [Google Scholar]
- Pallansch M. A., Kew O. M., Semler B. L., Omilianowski D. R., Anderson C. W., Wimmer E., Rueckert R. R. Protein processing map of poliovirus. J Virol. 1984 Mar;49(3):873–880. doi: 10.1128/jvi.49.3.873-880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M., Phillips B. A. In vitro assembly of polioviruses. 3. Assembly of 14 S particles into empty capsids by poliovirus-infected HeLa cell membranes. Virology. 1973 May;53(1):107–114. doi: 10.1016/0042-6822(73)90469-8. [DOI] [PubMed] [Google Scholar]
- Roumiantzeff M., Maizel J. V., Jr, Summers D. F. Comparison of polysomal structures of uninfected and poliovirus infected HeLa cells. Virology. 1971 May;44(2):239–248. doi: 10.1016/0042-6822(71)90256-x. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K., King A. M. Guanidine-resistant mutants of aphthovirus induce the synthesis of an altered nonstructural polypeptide, P34. J Virol. 1982 May;42(2):389–394. doi: 10.1128/jvi.42.2.389-394.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D. Association of protein fractions and lipids from human erythrocyte membranes. II. Studies on a loosely bound protein fraction. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):781–790. doi: 10.1515/bchm2.1973.354.2.781. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Hanecak R., Dorner L. F., Wimmer E. A membrane-associated precursor to poliovirus VPg identified by immunoprecipitation with antibodies directed against a synthetic heptapeptide. Cell. 1982 Feb;28(2):405–412. doi: 10.1016/0092-8674(82)90358-0. [DOI] [PubMed] [Google Scholar]
- Takegami T., Semler B. L., Anderson C. W., Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983 Jul 15;128(1):33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Tershak D. R. Inhibition of poliovirus polymerase by guanidine in vitro. J Virol. 1982 Jan;41(1):313–318. doi: 10.1128/jvi.41.1.313-318.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]