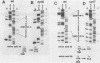Abstract
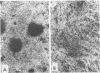
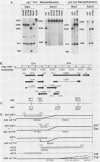
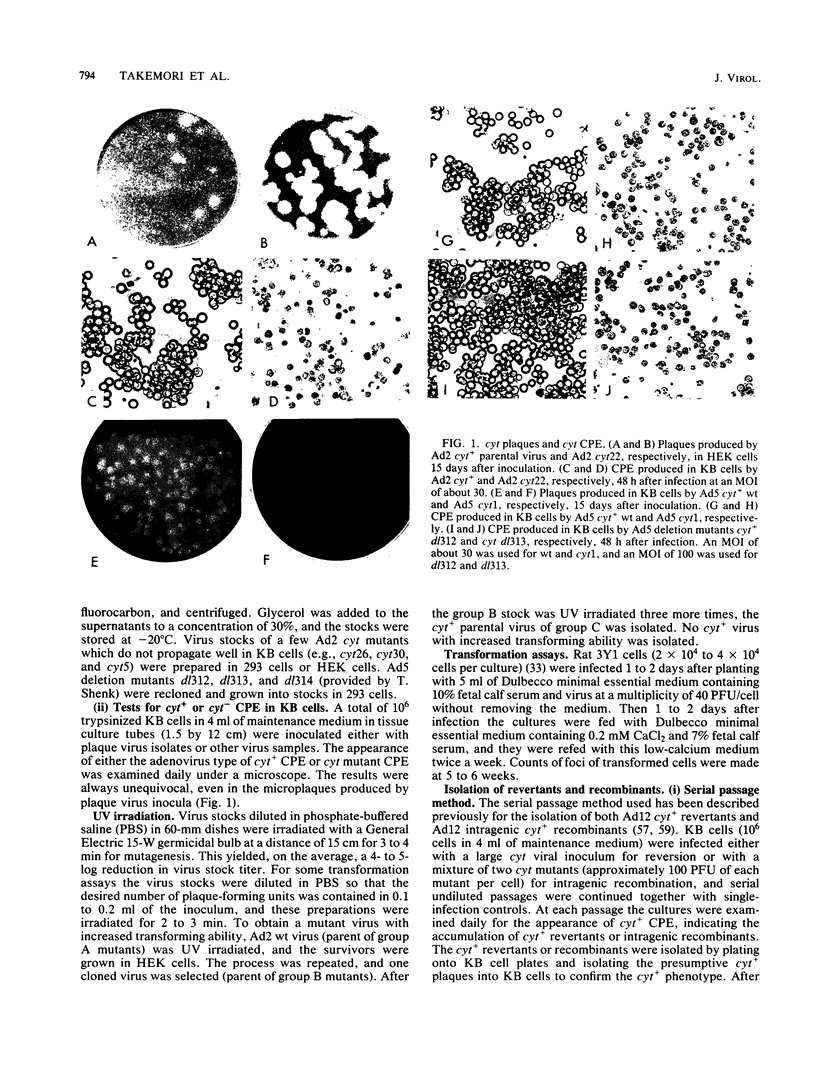
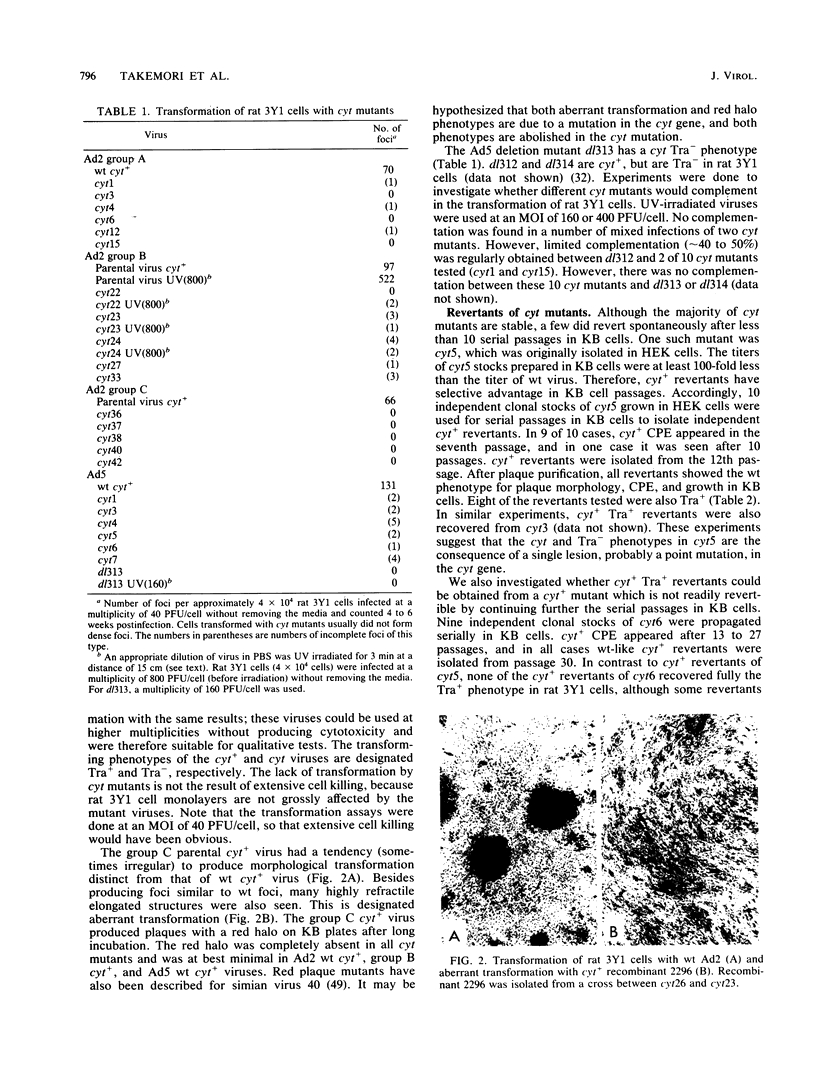
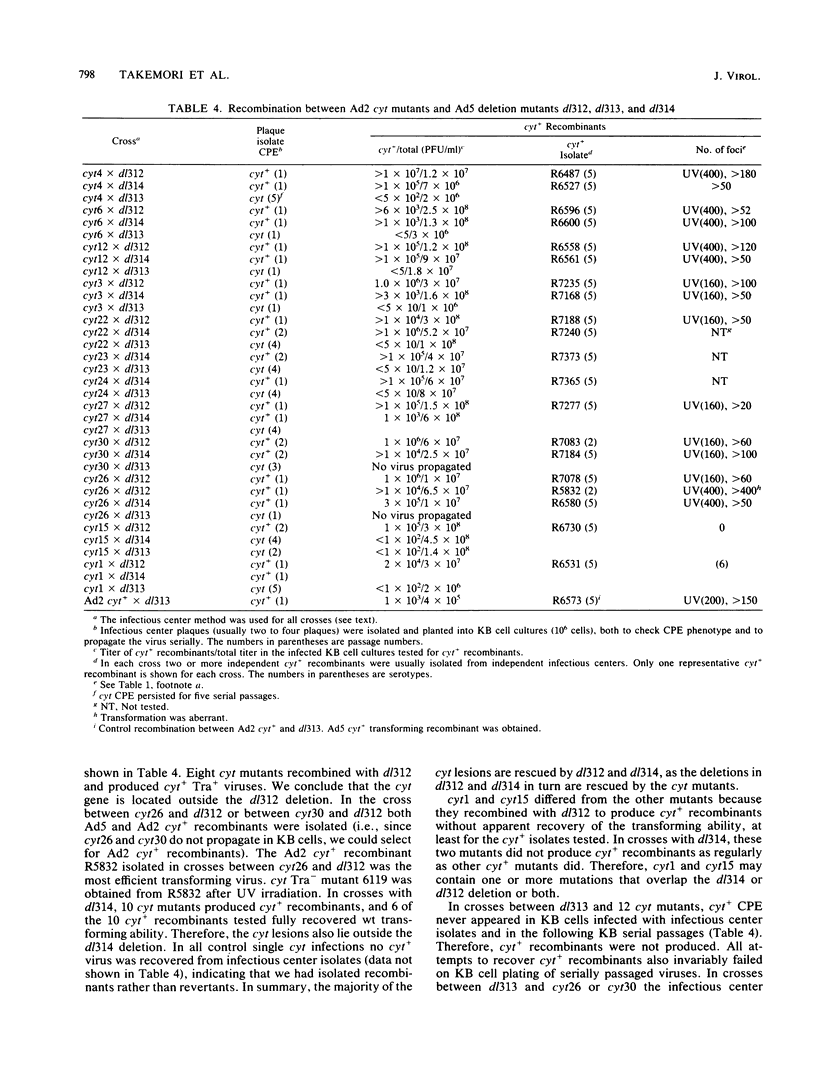
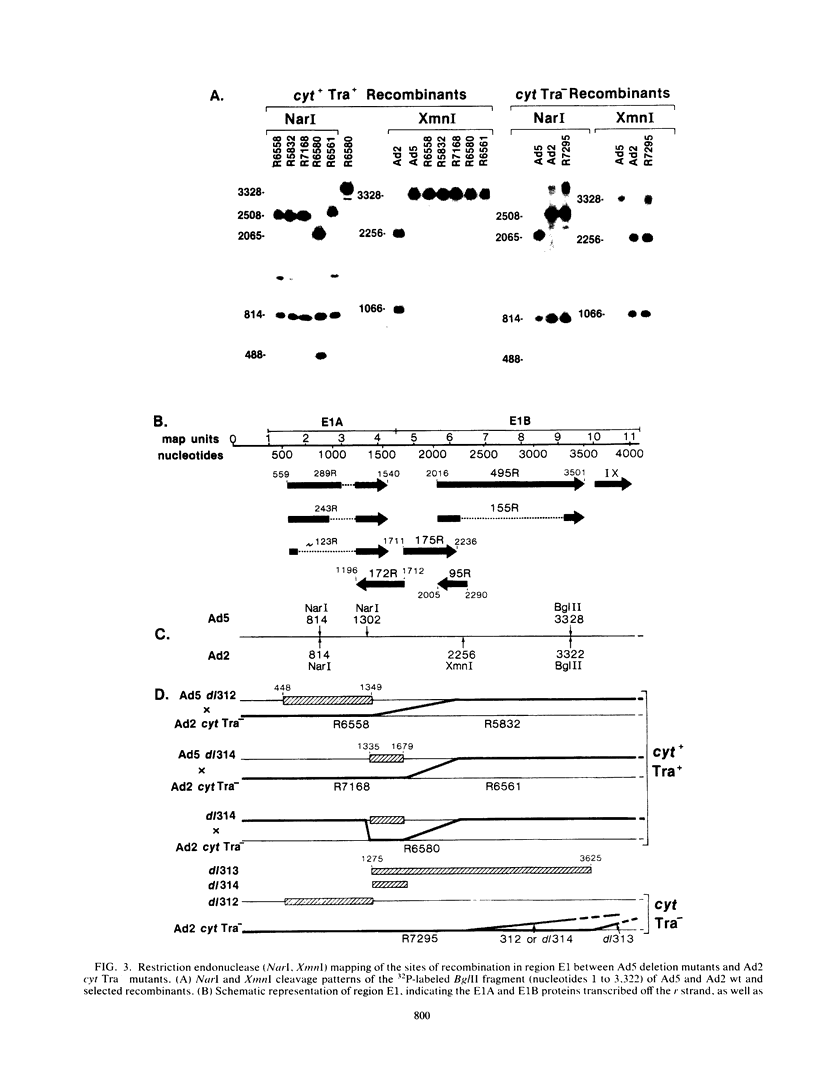
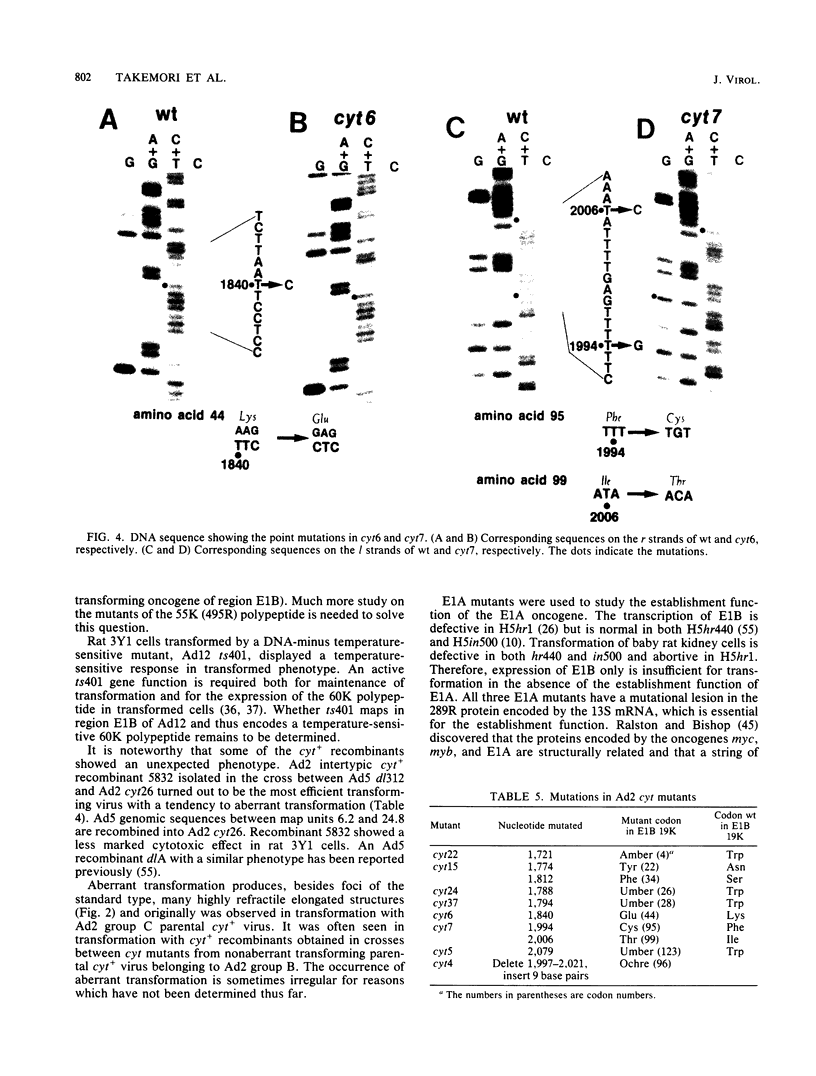
A total of 59 cytocidal (cyt) mutants were isolated from adenovirus 2 (Ad2) and Ad5. In contrast to the small plaques and adenovirus type of cytopathic effects produced by wild-type cyt+ viruses, the cyt mutants produced large plaques, and the cytopathic effect was characterized by marked cellular destruction. cyt mutants were transformation defective in established rat 3Y1 cells. cyt+ revertants and cyt+ intragenic recombinants recovered fully the transforming ability of wild-type viruses. Thus, the cyt gene is an oncogene responsible for the transforming function of Ad2 and Ad5. Genetic mapping in which we used three Ad5 deletion mutants (dl312, dl313, and dl314) as reference deletions located the cyt gene between the 3' ends of the dl314 deletion (nucleotide 1,679) and the dl313 deletion (nucleotide 3,625) in region E1B. Restriction endonuclease mapping of these recombinants suggested that the cyt gene encodes the region E1B 19,000-molecular-weight (175R) polypeptide (nucleotides 1,711 to 2,236). This was confirmed by DNA sequencing of eight different cyt mutants. One of these mutants has a single missense mutant, two mutants have double missense mutations, and five mutants have nonsense mutations. Except for one mutant, these point mutations are not located in any other known region E1B gene. We conclude that the cyt gene codes for the E1B 19,000-molecular-weight (175R) polypeptide, that this polypeptide is required for morphological transformation of rat 3Y1 cells, and that simple amino acid substitutions in the protein can be sufficient to produce the cyt phenotype.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Schmitt R. C., Smart J. E., Lewis J. B. Early region 1B of adenovirus 2 encodes two coterminal proteins of 495 and 155 amino acid residues. J Virol. 1984 May;50(2):387–396. doi: 10.1128/jvi.50.2.387-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Fisher P. B., Ginsberg H. S. Deletion and insertion mutations in early region 1a of type 5 adenovirus that produce cold-sensitive or defective phenotypes for transformation. J Virol. 1984 Mar;49(3):731–740. doi: 10.1128/jvi.49.3.731-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Fisher P. B. Cold-sensitive expression of transformation by a host range mutant of type 5 adenovirus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1352–1356. doi: 10.1073/pnas.80.5.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOLOGY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1607–1620. doi: 10.1073/pnas.45.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Boursnell M. E., Mautner V. Recombination in adenovirus: crossover sites in intertypic recombinants are located in regions of homology. Virology. 1981 Jul 15;112(1):198–209. doi: 10.1016/0042-6822(81)90625-5. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Cheetham B. F., Li P., Parish C. R., Waldron-Stevens L. K., Bellett A. J. Adenovirus-induced alterations of the cell growth cycle: a requirement for expression of E1A but not of E1B. J Virol. 1983 Jan;45(1):192–199. doi: 10.1128/jvi.45.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Carlock L. R., Jones N. C. Transformation-defective mutant of adenovirus type 5 containing a single altered E1a mRNA species. J Virol. 1981 Dec;40(3):657–664. doi: 10.1128/jvi.40.3.657-664.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983 Jul;33(3):759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Brusca J. Physical mapping of a large-plaque mutation of adenovirus type 2. J Virol. 1979 Nov;32(2):623–628. doi: 10.1128/jvi.32.2.623-628.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M., van Ormondt H. Gene organization of the transforming region of adenovirus type 7 DNA. Gene. 1982 May;18(2):143–156. doi: 10.1016/0378-1119(82)90112-3. [DOI] [PubMed] [Google Scholar]
- Ezoe H., Fatt R. B., Mak S. Degradation of intracellular DNA in KB cells infected with cyt mutants of human adenovirus type 12. J Virol. 1981 Oct;40(1):20–27. doi: 10.1128/jvi.40.1.20-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Saito I., Shiroki K., Shimojo H. Isolation of transformation-defective, replication-nondefective early region 1B mutants of adenovirus 12. J Virol. 1984 Jan;49(1):154–161. doi: 10.1128/jvi.49.1.154-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S. Biological and biochemical basis for cell injury by animal viruses. Fed Proc. 1961 Jul;20:656–660. [PubMed] [Google Scholar]
- GINSBERG H. S. Identification and classification of adenoviruses. Virology. 1962 Oct;18:312–319. doi: 10.1016/0042-6822(62)90018-1. [DOI] [PubMed] [Google Scholar]
- Galos R. S., Williams J., Shenk T., Jones N. Physical location of host-range mutations of adenovirus type 5; deletion and marker-rescue mapping. Virology. 1980 Jul 30;104(2):510–513. doi: 10.1016/0042-6822(80)90356-6. [DOI] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Hirst G. K. Mechanism of influenza recombination. I. Factors influencing recombination rates between temperature-sensitive mutants of strain WSN and the classification of mutants into complementation--recombination groups. Virology. 1973 Sep;55(1):81–93. doi: 10.1016/s0042-6822(73)81010-4. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Galos R., Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982 Oct 15;122(1):109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Ito Y. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important for cell transformation. Virology. 1979 Oct 15;98(1):261–266. doi: 10.1016/0042-6822(79)90545-2. [DOI] [PubMed] [Google Scholar]
- Jochemsen H., Daniëls G. S., Hertoghs J. J., Schrier P. I., van den Elsen P. J., van der EB A. J. Identification of adenovirus-type 12 gene products involved in transformation and oncogenesis. Virology. 1982 Oct 15;122(1):15–28. doi: 10.1016/0042-6822(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Lai Fatt R. B., Mak S. Mapping of an adenovirus function involved in the inhibition of DNA degradation. J Virol. 1982 Jun;42(3):969–977. doi: 10.1128/jvi.42.3.969-977.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L. Tumor antigens of human Ad5 in transformed cells and in cells infected with transformation-defective host-range mutants. Cell. 1979 Nov;18(3):781–791. doi: 10.1016/0092-8674(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Ledinko N., Schaeufele J., Soorma O. Adenovirus type 12 gene 401 function and maintenance of transformation. J Virol. 1979 Jan;29(1):250–260. doi: 10.1128/jvi.29.1.250-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledinko N. Transformation-specific antigen induced by oncogenic human adenovirus. Nature. 1978 Aug 24;274(5673):812–813. [PubMed] [Google Scholar]
- Mak I., Mak S. Transformation of rat cells by cyt mutants of adenovirus type 12 and mutants of adenovirus type 5. J Virol. 1983 Mar;45(3):1107–1117. doi: 10.1128/jvi.45.3.1107-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCahon D., Slade W. R. A sensitive method for the detection and isolation of recombinants of foot-and-mouth disease virus. J Gen Virol. 1981 Apr;53(Pt 2):333–342. doi: 10.1099/0022-1317-53-2-333. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Carcinogens induce targeted mutations in Escherichia coli. Cell. 1982 Nov;31(1):5–7. doi: 10.1016/0092-8674(82)90398-1. [DOI] [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Persson H., Katze M. G., Philipson L. Purification of a native membrane-associated adenovirus tumor antigen. J Virol. 1982 Jun;42(3):905–917. doi: 10.1128/jvi.42.3.905-917.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGGS, JL, LENNETTE E. H. SIMIAN VIRUS 40: ISOLATION OF TWO PLAQUE TYPES. Science. 1965 Jan 22;147(3656):408–409. doi: 10.1126/science.147.3656.408. [DOI] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The protein products of the myc and myb oncogenes and adenovirus E1a are structurally related. Nature. 1983 Dec 22;306(5945):803–806. doi: 10.1038/306803a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. R., Levine A. J., Galos R. S., Williams J., Shenk T. Early viral proteins in HeLa cells infected with adenovirus type 5 host range mutants. Virology. 1980 Jun;103(2):475–492. doi: 10.1016/0042-6822(80)90205-6. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Hearing P., Anderson C. W., Halbert D. N., Shenk T., Levine A. J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984 Mar;49(3):692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Solnick D., Anderson M. A. Transformation-deficient adenovirus mutant defective in expression of region 1A but not region 1B. J Virol. 1982 Apr;42(1):106–113. doi: 10.1128/jvi.42.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori N. Genetic studies with tumorigenic adenoviruses. 3. Recombination in adenovirus type 12. Virology. 1972 Jan;47(1):157–167. doi: 10.1016/0042-6822(72)90249-8. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. D. Genetic studies with tumorigenic adenoviruses. II. Heterogeneity of cyt mutants of adenovirus type 12. Virology. 1969 May;38(1):8–15. doi: 10.1016/0042-6822(69)90122-6. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968 Dec;36(4):575–586. doi: 10.1016/0042-6822(68)90189-x. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Van den Elsen P., Houweling A., Van der Eb A. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology. 1983 Jul 30;128(2):377–390. doi: 10.1016/0042-6822(83)90264-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Shimojo H., Hamada C. Less tumorigenic (cyt) mutants of adenovirus 12 defective in induction of cell surface change. Virology. 1972 Dec;50(3):743–752. doi: 10.1016/0042-6822(72)90428-x. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Rowe D. T., Tremblay M. L., McDermott M., Branton P. E. Identification of human adenovirus early region 1 products by using antisera against synthetic peptides corresponding to the predicted carboxy termini. J Virol. 1983 Jun;46(3):1003–1013. doi: 10.1128/jvi.46.3.1003-1013.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]