Abstract
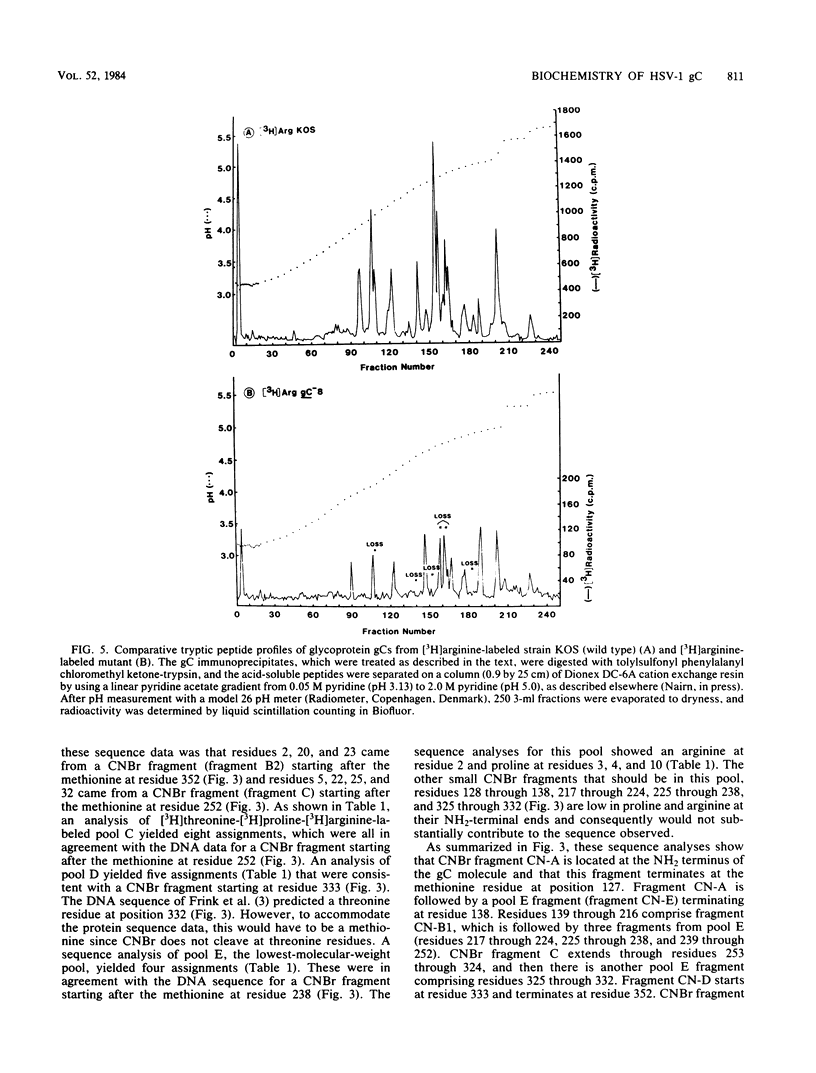
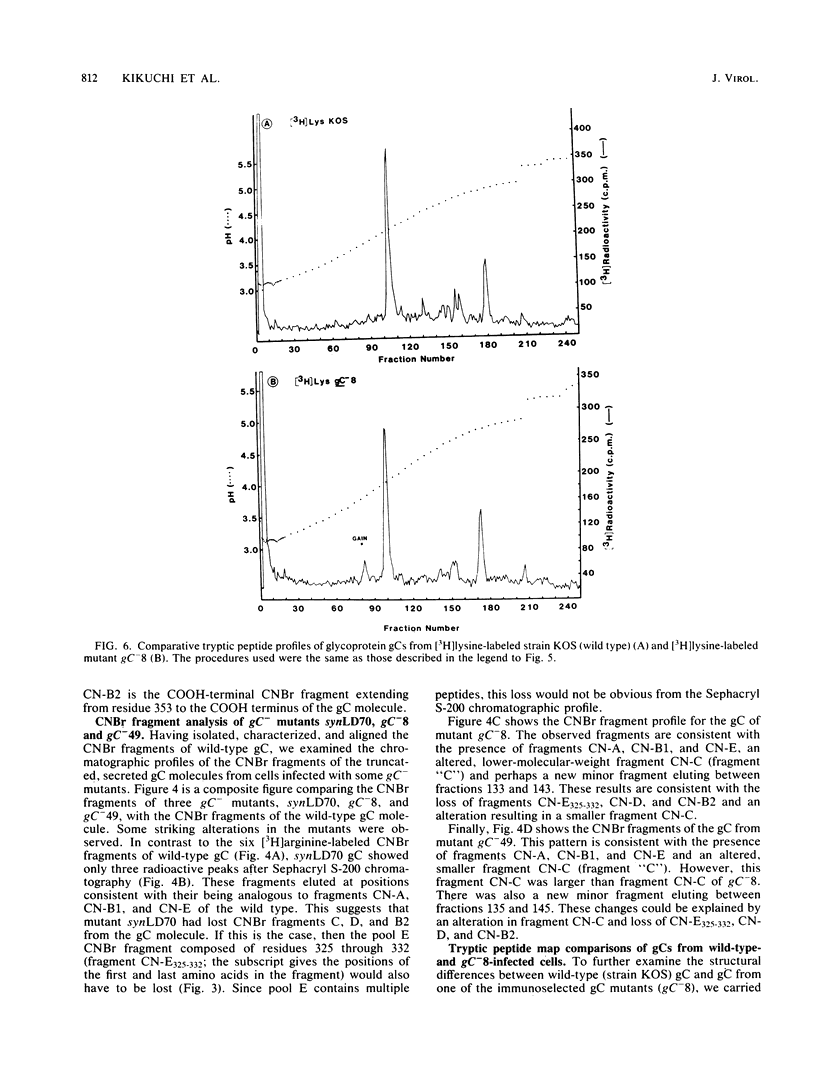
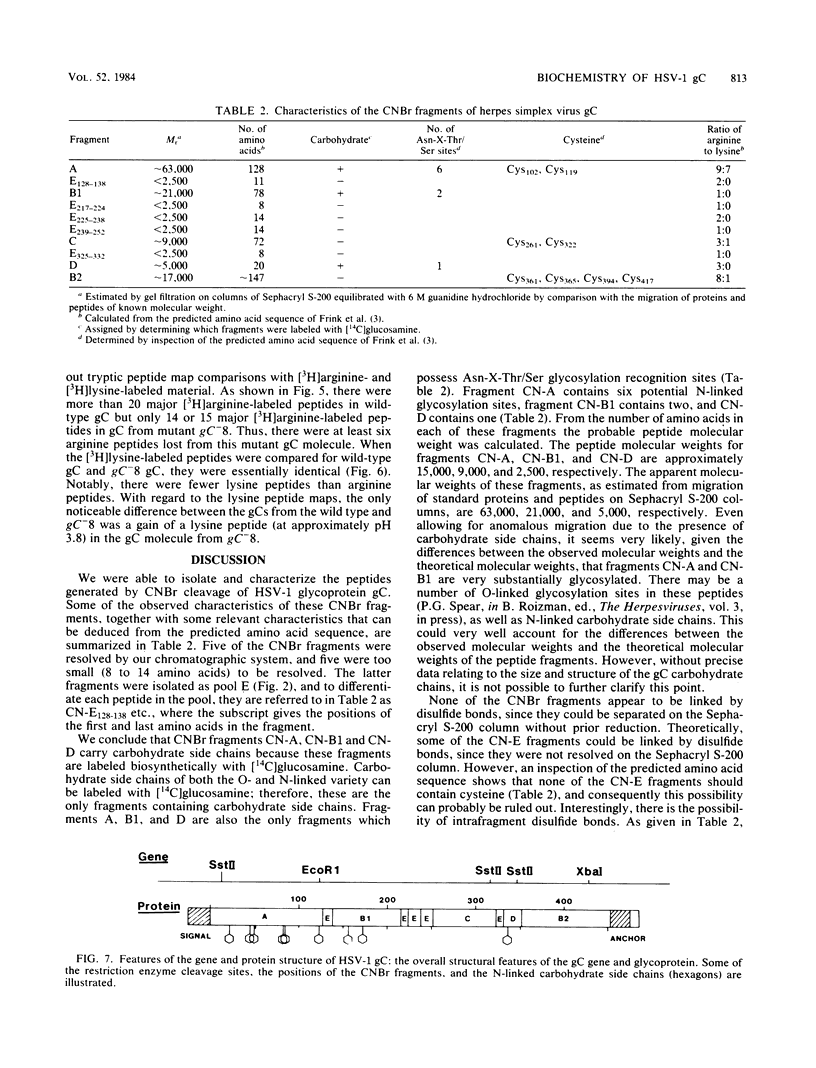
A biochemical characterization of peptides from herpes simplex virus type 1 glycoprotein gC was carried out. We utilized simple micromethods, based on immunological isolation of biosynthetically radiolabeled gC, to obtain gC in pure form for biochemical study. CNBr fragments of gC were prepared, isolated, and characterized. These CNBr fragments were resolved into six peaks by chromatography on Sephacryl S-200 in 6 M guanidine hydrochloride. Only three of the CNBr fragments contained carbohydrate side chains, as judged from the incorporation of [14C]glucosamine. Radiochemical microsequence analyses were carried out on the gC molecule and on each of the CNBr fragments of gC. A comparison of this amino acid sequence data with the amino acid sequence predicted from the DNA sequence of the gC gene showed that the first 25 residues of the predicted sequence are not present in the gC molecule isolated from infected cells and allowed alignment of the CNBr fragments in the gC molecule. Glycoprotein gC was also examined from three gC mutants, synLD70, gC-8, and gC-49. These mutants lack an immunoreactive envelope form of gC but produce a secreted, truncated gC gene product. Glycoprotein gC from cells infected with any of these gC- mutants was shown to have lost more than one CNBr fragment present in the wild-type gC molecule. The missing fragments included the one containing the putative transmembrane anchor sequence. Glycoprotein gC from the gC-8 mutant was also shown, by tryptic peptide map analysis, to have lost more than five major arginine-labeled tryptic peptides arginine-labeled tryptic peptides present in the wild-type gC molecule and to have gained a lysine-labeled tryptic peptide not present in wild-type gC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan J. E., Gates F. T., 3rd, Kimball E. S., Maloy W. L. Radiochemical sequence analysis of biosynthetically labeled proteins. Methods Enzymol. 1983;91:413–434. doi: 10.1016/s0076-6879(83)91039-x. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates F. T., 3rd, Coligan J. E., Kindt T. J. Complete amino acid sequence of rabbit beta 2-microglobulin. Biochemistry. 1979 May 29;18(11):2267–2272. doi: 10.1021/bi00578a021. [DOI] [PubMed] [Google Scholar]
- Glorioso J. C., Levine M., Holland T. C., Szczesiul M. S. Mutant analysis of herpes simplex virus-induced cell surface antigens: resistance to complement-mediated immune cytolysis. J Virol. 1980 Sep;35(3):672–681. doi: 10.1128/jvi.35.3.672-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Marlin S. D., Levine M., Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983 Feb;45(2):672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Levine M., Glorioso J. C. Method for induction of mutations in physically defined regions of the herpes simplex virus genome. J Virol. 1981 Apr;38(1):41–49. doi: 10.1128/jvi.38.1.41-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Vitiello A., Klinman N. R. T-cell and B-cell responses to viral antigens at the clonal level. Annu Rev Immunol. 1983;1:63–86. doi: 10.1146/annurev.iy.01.040183.000431. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard B. F. Herpes simplex virus antigens and antibodies: a survey of studies based on quantitative immunoelectrophoresis. Rev Infect Dis. 1980 Nov-Dec;2(6):899–913. doi: 10.1093/clinids/2.6.899. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]