Abstract
Killing of human cells by the parasite Entamoeba histolytica requires adherence via an amebic cell surface lectin. Lectin activity in the parasite is regulated by inside-out signaling. The lectin cytoplasmic domain has sequence identity with a region of the β2 integrin cytoplasmic tail implicated in regulation of integrin-mediated adhesion. Intracellular expression of a fusion protein containing the cytoplasmic domain of the lectin has a dominant negative effect on extracellular lectin-mediated cell adherence. Mutation of the integrin-like sequence abrogates the dominant negative effect. Amebae expressing the dominant negative mutant are less virulent in an animal model of amebiasis. These results suggest that inside-out signaling via the lectin cytoplasmic domain may control the extracellular adhesive activity of the amebic lectin and provide in vivo demonstration of the lectin’s role in virulence.
INTRODUCTION
The intestinal protozoan parasite Entamoeba histolytica is the causative agent of the disease amebiasis. E. histolytica infection results in 50 million cases of invasive amebiasis and 100,000 deaths annually, and is surpassed only by malaria and schistosomiasis as the leading parasitic cause of death. E. histolytica is found worldwide, with the highest morbidity and mortality seen in Central and South America, Africa, and India (WHO, 1997).
Carbohydrate–protein interactions are responsible for the contact-dependent cytotoxicity for which E. histolytica was named. Contact of E. histolytica to host cells is mediated by an amebic lectin specific for galactose (Gal) and N-acetyl-d-galactosamine (GalNAc) (Petri et al., 1987). Amebae are unable to adhere to or kill Chinese hamster ovary (CHO) cell glycosylation mutants which lack Gal/GalNAc-terminal oligosaccharides. Adherence and cytolysis of human colonic epithelial cells, neutrophils, macrophages, and T lymphocytes is blocked in vitro by 50 mM Gal or GalNAc (Ravdin and Guerrant, 1981; Ravdin et al., 1985; Burchard and Bilke, 1992).
The Gal/GalNAc lectin is a heterodimeric molecule composed of a transmembrane heavy (170-kDa) subunit and a glycosylphosphatidylinositol-anchored light (31/35-kDa) subunit which are linked by disulfide bonds (Petri, 1996). The light subunit is encoded by a family of at least seven genes (lgl) which share 79–85% amino acid sequence identity. There are five heavy subunit (hgl) gene family members in E. histolytica strain HM1:IMSS which exhibit >89% nucleotide sequence identity (Ramakrishnan et al., 1996). The amino-terminal 1209 residues are extracellular, with the carbohydrate-binding domain located between amino acids 898–998 of the heavy subunit (Mann et al., 1991; Dodson, Mann, and Petri, unpublished data). The carboxyl-terminal 41 amino acids of the heavy subunit comprise the only cytoplasmic portion of the lectin.
The carbohydrate-binding function of the lectin is regulated, apparently due to changes in the structure of the lectin as opposed to changes in lectin number. For example, mAbs against epitopes 1 and 2 of the heavy subunit enhance trophozoite adherence by activating the Gal/GalNAc lectin (Petri et al., 1990). Cytochalasins B and D inhibit lectin-mediated adherence, presumably by disrupting lectin interactions with the cytoskeleton (Ravdin and Guerrant, 1981). The ability to control lectin activity may be especially important because lectin binding to Gal/GalNAc oligosaccharides is of extremely high affinity (Kd = 8.2 × 10−11 M−1) (Chadee et al., 1988). Without a mechanism to modulate lectin activity, amebae might be unable to detach from mucins and epithelial cells as they invade the host.
The integrin family of adherence proteins is a well-studied example of dynamic regulation of adhesive function. Several integrins are activated to a high-affinity state by extracellular signals or by anti-integrin antibodies. Activation is independent of changes in integrin number or microenvironment. The cytoplasmic tails of the integrin subunits are crucial regulators of integrin activity, both via interaction with the cytoskeleton and by transduction of signals to the extracellular integrin domains. The lectin-heavy subunit cytoplasmic domain has sequence identity with the β2 and β7 integrin cytoplasmic tails, including amino acids implicated in control of integrin adhesiveness (Figures 1 and 2A).
Figure 1.
Sequence identity of the lectin-heavy subunit (hgl1) cytoplasmic tail with the β2 and β7 integrin cytoplasmic tails. Identical amino acids are in bold, amino acids implicated in integrin inside-out signaling are highlighted (∗), and gaps placed to maximize sequence alignment are shown as (:).
Figure 2.
Lectin–GFP fusion protein constructs. (A) Schematic representation of the heavy subunit of the Gal/GalNAc lectin. SP, signal peptide; C-W, cysteine and tryptophan-rich region (3% cysteine, 2% tryptophan); C-free, cysteine-free region; C-rich, cysteine-rich region (11% cysteine); TM, transmembrane; CT, cytoplasmic tail. (B) 224, lectin–GFP fusion protein. (C). 324, lectin–GFP fusion protein lacking the cytoplasmic tail.
For this reason we focused on the potential role of the lectin cytoplasmic domain in control of adhesiveness of the lectin. We hypothesized that an intracellular factor interacts with the lectin cytoplasmic domain to regulate amebic adherence. Inducible expression of a fusion protein containing the cytoplasmic tail of the lectin resulted in a dominant negative effect on the extracellular adhesive activity of the wild-type lectin. The dominant negative effect appeared to be at the level of regulation of lectin activity, since the structure and cell surface concentration of the wild-type lectin were unaltered. The dominant negative effect was lost upon mutation of the lectin cytoplasmic domain amino acids with identity to the β integrins. Amebae induced to express the dominant negative lectin mutant were defective in the ability to form liver abscesses, providing the first in vivo evidence of the importance of the lectin, and its adhesive regulation, in virulence.
MATERIALS AND METHODS
Entamoeba histolytica and CHO Cells—Culture and Other Methods
E. histolytica strain HM1:IMSS trophozoites were grown in TYI-S-33 medium containing penicillin (100 U/ml−1) and streptomycin sulfate (100 μg/ml−1) in either 12-ml screw cap tubes, 75-cm2 flasks, or 25-cm2 flasks at 37°C (Diamond et al., 1978). Amebae to be used for stable transfection were grown in 75-cm2 flasks to a density of 5.3–6.6 × 104 trophozoites/ml−1 (logarithmic phase growth). CHO cells were grown in 25-cm2 flasks in 5 ml of MEM-α (Life Technologies, Grand Island, NY) supplemented with 10% FBS, penicillin (100 U/ml−1), and streptomycin (100 μg/ml−1) at 37°C and 5% CO2. For use in in vitro assays, CHO cells were released from the monolayer by a 3-min incubation in 0.25% trypsin.
E. histolytica adherence and cytolysis were assayed using the method of Ravdin and Guerrant (1981). Electroporation of E. histolytica followed the protocol described previously by Ramakrishnan et al. (1997). Erythrophagocytosis was measured according to the method of Trissl et al. (1978).
Construction of Inducible Expression Vector
As described previously, the tetO operator sequence was introduced downstream of the TATA box of the hgl5 promoter by ligating in annealed oligonucleotides that generate a BglII site at one end (Ramakrishnan et al., 1997). Plasmid pGIR208 described previously has the BglII site proximal to the TATA box; companion plasmid pGIR209 had the BglII site distal to the TATA box. Target genes for inducible expression were cloned at the BglII site of pGIR209, thus placing their translational start very close to the transcription start within the tetO sequence.
Construction of Lectin–Green Fluorescent Protein (GFP) Fusion Expression Vectors
A lectin-heavy subunit–GFP fusion protein was constructed in the following manner: First, an NcoI digest was performed on a full-length hgl clone (kindly provided by J. M. Dodson, University of Virginia). This was followed by blunt-ending with Klenow enzyme (Life Technologies) and ligation of an XbaI linker onto the blunt end. Next, an EcoRI digest was performed which liberated bp 179-3460 of hgl. The entire ORF of GFP was PCR amplified (Pfu DNA polymerase) with the forward primer 5′-ctactgtctagaCATATGAGTAAA GGAGAAGA-3′ and the reverse primer 5′-ctactggaattcTTTGTA TAGTTCATCCATGC-3′. After digestion with XbaI and EcoRI, GFP was ligated into these sites of the hgl construct. The construct was then digested with XbaI and filled in with Klenow to yield an in-frame product. The lectin–GFP fusion was then PCR amplified with the forward primer 5′-ctactgggatccAAATGAAATTATTAT TATTAA-3′ and the reverse primer 5′-ctactggtcgacTTATCCATT GAATGTTGCTG-3′. After digestion with BamHI and SalI, the fragment was ligated into the BglII and SalI sites of pGIR209 creating plasmid pGIR224.
As a control, a vector to inducibly express the lectin–GFP fusion protein, which lacked the lectin cytoplasmic domain, was constructed. Using pGIR224 as template with the forward primer 5′-cta ctgggatccAAATGAAATTATTATTATTAA-3′ and the reverse primer 5′-cagtaggtcgacttaAAATAATCCAATAGAAAC-3′, the desired lectin–GFP DNA was PCR amplified using Pfu DNA polymerase. Following digestion with BamHI and SalI, the fragment was ligated into the BglII and SalI sites of pGIR209 creating plasmid pGIR324. Constructs were sequenced in their entirety to rule out cloning or PCR artifacts.
Mutations in the cytoplasmic tail of the 224 construct were created using a two-step PCR method. For each mutation, one new primer was synthesized to replace the original bases. The primer used for the Y → A transition was 5′-ACTAATGAAAATGCAGAAGCTGT TGGAGCAGATAATGAA-3′. (The bases in bold represent the desired mutations.) This primer was used with a second primer, 5′-AAATGATGTTCACTTTATTT-3′, which hybridized to the 3′ end of hgl 3′ region approximately 100 bp downstream of the stop codon. This first round product was then used as a primer for a second round of PCR with a new primer, 5′-CTACTGGGATCC AAATGAAATTATTATTATTAAA-3′ (BamHI site used for cloning underlined, bold bases represent start codon), which hybridized in the hgl 5′ region, allowing the synthesis of the entire hgl–GFP fusion protein with the appropriate mutation. The TIT… Y → AAA… A mutation was synthesized utilizing the same strategy except with primer 5′-ATGAAGAATGCCATTGCAGCAGCTAATGAAAAT GCAGAAGCTGTTGGAGCAGATAAT-3′ (bold bases indicate mismatch). The second round PCR products were then digested with BamHI and SalI, and ligated into vector pGIR209 which had been digested with BglII and SalI. Colonies were screened by restriction analysis and then sequenced to ensure that there were no PCR-induced errors and that our base substitutions had been incorporated.
FACS Analysis
Trophozoites (1–2 × 106) were first washed with cold PBS and resuspended in 180 μl of PBS and incubated on ice for 45 min with 20 μl of lectin mAb ascites or 40 μg of purified lectin mAb. Amebae were washed twice in cold PBS, resuspended in 180 μl of PBS, and 20 μl of antimouse IgG conjugated to FITC (Sigma, St. Louis, MO) were added and incubated for 45 min on ice. After two washes in PBS, amebae were resuspended in 1 ml of TYI-S-33 medium. Samples were analyzed using a Becton Dickinson FACScan Flow Cytometer equipped with an air-cooled argon laser at an excitation of 488 nm. An acquisition gate was set on forward scatter (relative size) × side scatter (granularity or complexity) to exclude debris. The instrument was adjusted based on negative and background controls. Fifteen thousand gated events were collected and analyzed. Fluorescence data was collected in a 4-decade log mode, forward scatter and side scatter were collected in a linear mode.
Immunofluorescence and Confocal Microscopy
For immunofluorescent staining, approximately 2 × 105 amebae per sample were chilled and washed in PBS. Amebae were then fixed in 3% paraformaldehyde for 30 min at room temperature, permeabilized in 0.25% Triton X-100 for 30 s, washed twice in PBS, and resuspended in a rabbit-derived primary antibody at a dilution of 1:33 for 1 h. Amebae were then washed twice in PBS and resuspended in goat anti-rabbit-FITC (Sigma) at a dilution of 1:64 for 1 h. Amebae were washed twice in PBS and mounted on glass slides in Vecta Shield (Vector Laboratories, Burlingame, CA). Amebae were visualized using a Zeiss LSM 410 laser scanning confocal microscope equipped with an argon/krypton laser. To generate final images, four averages at 8 s each were compiled via a Zeiss 63×, plan-apochromat (numerical aperture, 1.40) objective, with laser excitation at 488 nm appropriate for FITC.
Amebic Liver Abscess Model
One day prior to challenge, amebae strains 224 and 324 were induced with 5 μg/ml doxycycline in TYI-S33 medium. The gerbils in the induced group received drinking water containing 2.5 mg/ml doxycycline and 5% sucrose. The control animals received water containing 5% sucrose alone. The animals were challenged by direct hepatic inoculation with 5 × 105 amebic trophozoites using the method of Chadee and Meerovitch (1985). Gerbils were killed 5–8 d after challenge and liver abscess weights were determined.
RESULTS
Optimization of the tetO Inducible Promoter System for Protein Expression
In the tetracycline-inducible system previously developed for use in E. histolytica, the tetO sequence was incorporated into the 5′ untranslated region of the induced luciferase RNA in plasmid construct pGIR204 (Ramakrishnan et al., 1997). Although the induced transcription was efficient, the induced RNA was not very well translated, presumably due to the presence of a dyad structure conferred by the inverted repeats of the tetO sequence. The expression vector was therefore redesigned so as to prevent the formation of the dyad structure. It has been shown that the site of transcription initiation in the hgl5 promoter is primarily determined by the presence of the TATA box at about −30 from the transcription start (Singh et al., 1997). The endogenous sequences downstream of the TATA box in the expression vector were replaced with the tetO sequence so that transcription would initiate within the inverted repeats of the operator sequence, thereby generating a transcript that cannot form a hairpin loop at the 5′ end. Mapping of the RNA by primer extension showed that transcription initiation occurred within the tetO sequence, as predicted (our unpublished results). The luciferase expression of this construct showed good repression under normal conditions (0.3 U/cell) and was induced 100-fold on addition of 5 mg/ml tetracycline (40 U/cell) at 15 μg/ml hygromycin and 6 μg/ml G418 for maintenance of episomes. The fold induction and induced levels with this construct were approximately fivefold higher than with previous constructs.
Inducible Expression of a Fusion Protein Containing the Lectin Cytoplasmic Domain
To investigate the role of the lectin-heavy subunit cytoplasmic tail in adherence and cell killing, a portion of the coding region of the hgl gene was fused in-frame with the GFP (Cormack et al., 1996), generating the lectin–GFP fusion protein 224 (Figure 2B). This protein maintained the amino-terminal signal peptide, the putative transmembrane domain, and the cytoplasmic tail of the lectin (Mann et al., 1991). Lectin–GFP fusion protein 324 was created by deleting the cytoplasmic tail sequences from the 224 construct via PCR (Figure 2C). These lectin–GFP fusion proteins were designed to be expressed from the pGIR209 plasmid to allow for inducible expression when introduced into E. histolytica (Ramakrishnan et al., 1997).
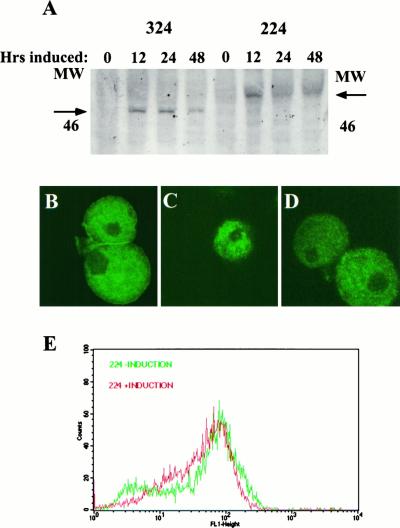
After establishment of stable transfected amebae lines, a Western blot was performed to verify expression of the lectin–GFP proteins. (Detection of the lectin–GFP fusion proteins was always through the use of antibody to GFP because the fusion proteins were not autofluorescent and were not recognized by antibodies to the lectin-heavy subunit.) Figure 3A shows the expression of the 324 and 224 proteins during a time course of induction with 5 μg/ml−1 tetracycline. Expression of the two proteins was approximately equivalent, as judged by Western blot. The 324 protein migrated at the expected size of approximately 47 kDa. With the addition of the 4-kDa cytoplasmic tail, 224 migrated as predicted at approximately 51 kDa. The expression of both proteins was repressed in the absence of tetracycline.
Figure 3.
Inducible expression of the 224 and 324 fusion proteins in E. histolytica. (A) Time course of expression of lectin–GFP fusion proteins after induction with tetracycline. Stably transfected pGIR324/308 and pGIR224/308 amebae were induced to express the 324 and 224 fusion proteins, respectively, with tetracycline for the indicated times. Amebic proteins were analyzed by SDS-PAGE followed by Western blotting with anti-GFP antibodies. The positions of the 324 and 224 fusion proteins and the 46-kDa size marker are shown. (B–D) Intracellular location of lectin–GFP fusion proteins. Control amebae (B) and amebae induced to express the 224 (C) or 324 (D) fusion proteins were fixed in paraformaldehyde, permeabilized, and immunostained with polyclonal antibody to the lectin (B) or to GFP (C and D). Amebae were then incubated with FITC-conjugated sheep anti-rabbit IgG, mounted, and visualized by confocal microscopy. (E) Surface expression of the endogenous lectin is not altered by expression of the 224 lectin–GFP fusion protein. Amebae were incubated with antilectin antibody. After incubation with an FITC-conjugated secondary antibody, amebae (uninduced or induced to express 224 for 24 h) were analyzed by single parameter FACS for surface fluorescence.
To ascertain the percentage of stable, transfected amebae that were actually expressing the lectin–GFP proteins, 224 amebae were induced for 24 h and immunostained using antibody to GFP. Stained amebae were visualized by both bright field and fluorescent microscopy to assess the expression of the 224 protein by individual amebae. All amebae examined were expressing the lectin–GFP protein (our unpublished results). In addition, the intensity of fluorescence suggested that the amebae were expressing the protein at similar levels. Background staining was minimal as staining for lectin–GFP proteins was only evident upon induction, and omission of the GFP antibody yielded no significant staining of cells.
Intracellular Location of the Lectin–GFP Fusion Proteins 224 and 324
As these lectin–GFP proteins maintained the membrane signal sequence, it was theoretically possible that the proteins would localize to the cell surface. The cellular locations of the lectin–GFP proteins were ascertained by immunofluorescent staining and confocal microscopy. Cell surface staining of amebae with lectin antibody has been previously demonstrated (Petri et al., 1987). The staining of the lectin was evident at the cell surface as a border which surrounded the cells; lectin was also visualized within the cytoplasm (Figure 3B). In contrast, the pattern of staining for 324 (Figure 3C) and 224 (Figure 3D) showed a decided lack of surface staining and a punctate pattern of staining within the cytoplasm, suggesting a vesicular location. Consistent with these results, FACS analyses of amebae expressing 324 and 224 were also negative for surface expression (our unpublished results).
Wild-Type Lectin Cell Surface Concentration and Structure Is Unchanged by 224 Expression
To verify that lectin–GFP fusion protein 224 expression did not alter the expression of the endogenous lectin at the surface, cell surface lectin was quantitated by FACS analysis using lectin mAb H85 (which did not recognize the lectin–GFP fusion proteins). Uninduced and 24-h induced 224 amebae were stained with mAb H85 (Mann et al., 1993) followed by incubation with a FITC-conjugated antimouse secondary antibody. Analysis of these amebae by single parameter FACS showed that the cell surface expression of the lectin as assessed by antilectin mAb H85 was not significantly altered on induction of 224 (Figure 3E). Cell surface lectin expression as measured by the lectin activating antilectin mAb 3F4 was also unaltered by expression of the 224 fusion protein (our unpublished results). The lack of a difference in 3F4 mAb staining on induction of the 224 fusion protein is consistent with the 3F4 mAb having the same affinity for active and inactive lectin (as 224 induction inactivated the lectin, see below). A similar observation has been made for the integrin activating mAb KIM-127 (Cai and Wright, 1995). These data indicated that expression of 224 did not perturb endogenous lectin expression on the surface of amebae.
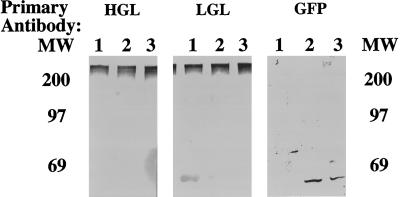
To determine whether 224 associated with the heavy or light subunits of the endogenous lectin, amebae expressing 224 were lysed under nonreducing conditions. Under nonreducing conditions the lectin migrated on SDS-PAGE as a 260-kDa heterodimer composed of the heavy and light subunits (Petri et al., 1989; McCoy et al., 1994). As shown in Figure 4, both the heavy and light subunits comigrated as a single band of 260 kDa, with no new bands being evident which would correlate to a heavy or light subunit/224 complex. The GFP blot showed that 224 was only present at its predicted monomeric size of 51 kDa. These data demonstrated that by Western blot, 224 was not covalently associated with the endogenous heavy or light subunits of the lectin.
Figure 4.
Lack of association of lectin–GFP fusion protein 224 with endogenous lectin subunits. Amebic proteins were separated under nonreducing conditions by SDS-PAGE and transferred to PVDF membranes. The blot was cut into three equivalent blots and each blot was incubated with antibody to either a lectin-heavy subunit, lectin-light subunit, or GFP. The blots were then incubated in alkaline phosphatase-conjugated secondary antibody and developed in substrate for alkaline phosphatase. The position of the molecular mass markers (in kDa) is shown. 1, amebae expressing luciferase as a control; 2 and 3, independent amebae transfectants expressing the 224 fusion protein.
Inducible Expression of Lectin Cytoplasmic Domain Decreases Amebic Adherence
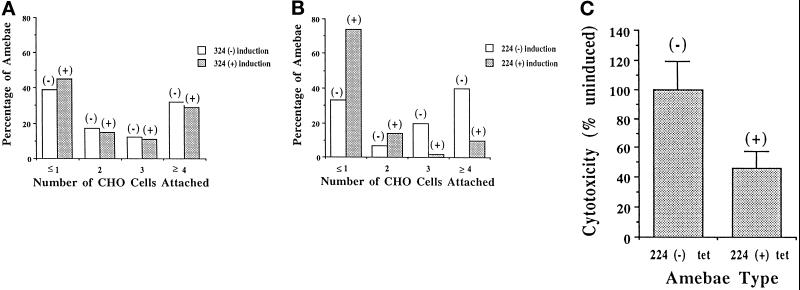
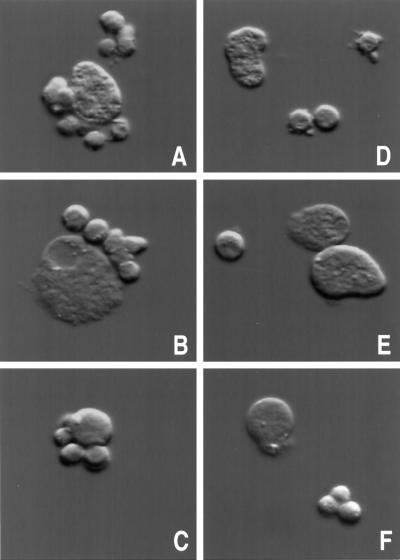
We hypothesized that the extracellular adhesive activity of the lectin is controlled via interactions of cytoplasmic regulatory factors with the cytoplasmic tail of the lectin. Expression of the 224 fusion protein containing the cytoplasmic tail would be predicted to alter the regulation of the endogenous lectin if it competes with the endogenous lectin for the regulatory protein(s). We therefore measured the ability of amebae to adhere (at 4°C) to target cells on induction of the 224 and 324 fusion proteins. As shown in Figure 5A, following induction of the 324 construct (which lacks the lectin cytoplasmic domain), the ability of amebae to adhere to CHO cells was unchanged. However, when amebae were induced to express the 224 fusion protein containing the lectin cytoplasmic domain, there was a marked decrease in the ability of the amebae to adhere to CHO cells (58% decrease, p < 0.0001) (Figure 5B). The decrease in adherence was evidenced as an increase in the number of amebae that had ≤1 CHO cells attached. Correspondingly, these was a decrease in the number of amebae that had 3 or ≥4 CHO cells attached. Photomicrographs showing examples of adherent uninduced 224 amebae and nonadherent induced 224 amebae are shown in Figure 6. Because the endogenous lectin’s cell surface concentration and subunit structure were unaltered and because recruitment to the cell surface of cytoplasmic stores of the endogenous lectin does not occur at 4°C, we concluded that the decreased adherence was a dominant negative effect of the 224 protein on regulation of lectin activity.
Figure 5.
Effect of induction of lectin–GFP fusion proteins on amebic adherence to and killing of CHO cells. Amebae [uninduced or induced to express either 324 (A) or 224 (B) for 24 h] were incubated with CHO cells. An adherence assay was performed and the number of CHO cells adhered to each ameba was determined. (C) Inhibition of cytolysis of CHO cells on inducible expression of lectin–GFP fusion protein 224. Amebae (uninduced or induced to express 224 for 24 h) were incubated with 51Cr-labeled CHO cells and treated as in the adherence assay. Dextran was added to the amebae/CHO pellet to prevent further adherence events from occurring. The pellet was incubated at 37°C to allow for cytolysis of the CHO cells and the supernatant was removed and assayed for 51Cr release.
Figure 6.
Photomicrographs of amebae-expressing lectin–GFP fusion protein 224 adhering to CHO cells. An adherence assay was performed with amebae (uninduced or induced to express 224 for 24 h). Representative amebae were photographed using a microscope equipped with differential interference contrast (DIC) optics. (A–C) Uninduced 224 amebae and adhered CHO cells. (D–F) Induced 224 amebae and nonadhered CHO cells.
Effect of Expression of Lectin Cytoplasmic Domain on Amebic Cytolysis
Because killing of target cells by E. histolytica is contact dependent, a decrease in the ability to adhere to target cells should result in decreased killing (Ravdin and Guerrant, 1981). The effect of 224 expression on the ability of amebae to kill 51Cr-labeled CHO cells was tested. Following adherence at 4°C, the amebae–CHO cell rosettes were resuspended in dextran (to prevent additional amebae–CHO cell interactions) and lysis of the attached CHO cells was measured at 37°C. The amount of 51Cr released into the supernatant was quantitated and used as a measure of cell lysis. Amebae that were induced to express 224 showed a 54% decrease in comparison to uninduced amebae (p = 0.031) in their ability to lyse labeled CHO cells (Figure 5C). Therefore, adherence and cytolysis were proportionately decreased in amebae expressing the 224 fusion protein. The expression of the lectin cytoplasmic domain apparently inhibited adherence but not the cytolytic event occurring after adherence.
Phagocytosis and Serum Resistance Are Unaltered in 224 Expressing Amebae
E. histolytica has been shown to phagocytose a number of cells and particles including starch grains, bacteria, protozoa, and erythrocytes (Trissl et al., 1978). Because the lectin mediates adherence of erythrocytes to amebae, we tested whether phagocytosis would be affected by the induction of the 224 fusion protein. To ascertain the ability of 224-expressing amebae to phagocytose human erythrocytes, a phagocytosis assay was performed on amebae induced to express 224 for 24 h. Amebic phagocytosis of erythrocytes was unchanged on induction of 224 expression (amebae phagocytosing red blood cells was 48 ± 2% without induction and 51 ± 3% with induction) (our unpublished results).
Amebae activate the complement system, but are able to evade this early host defense mechanism. This evasion has been shown to be mediated by the ability of the lectin to bind complement components C8 and C9, thereby abrogating assembly of the membrane attack complex (Braga et al., 1992). Studies investigating the effect of 224 expression on the ability of amebae to evade lysis by complement did not demonstrate any consistent effect upon resistance to lysis by complement (our unpublished results). Thus, the altered phenotype in 224-expressing amebae appeared to be specific to the adherence function of the lectin, with no observed effects on cytolysis (independent of adherence), phagocytosis, or serum resistance.
Amebae Expressing the Lectin Cytoplasmic Domain Fusion Protein Are Less Virulent
We next wished to test whether the decreased adherence seen on induction of the 224 fusion protein would result in decreased virulence of E. histolytica in the gerbil model of amebic liver abscess. Gerbils were challenged by intrahepatic inoculation with 5 × 105 trophozoites. Gerbils challenged with amebae induced to express 224 and 324 received drinking water containing 2.5 mg/ml doxycycline from 1 d before challenge to the completion of the experiment. Amebic strains were induced to express the 224 and 324 fusion proteins 24 h before challenge. Gerbils challenged with amebae induced to express the 224 fusion protein containing the lectin cytoplasmic domain had abscesses that were 84% smaller than those of animals challenged with the uninduced 224 amebae (median abscess size of 23.6 mg without induction and 4.0 mg with 224 induction; p = 0.03). In contrast, challenge of the gerbils with amebae containing the 324 construct lacking the cytoplasmic domain demonstrated no significant change in liver abscess size on induction of the 324 fusion protein (14.0 mg without induction and 17.8 mg with 324 induction; p = 0.92). Amebae cultured from the abscesses maintained the expression of the 224 and 324 fusion proteins. We concluded that inducible expression of the 224 fusion protein containing the lectin cytoplasmic domain, but not the 324 fusion protein lacking the cytoplasmic domain, decreased the virulence of E. histolytica. These data are the first in vivo confirmation of the importance of the lectin, and specifically of adhesive regulation, in virulence.
Role of Integrin Motif in Regulation of Adherence
Finally, we wished to ascertain the role of lectin cytoplasmic domain amino acids T1253, I1254, T1255, and Y1261 in the decreased adherence phenotype seen on induction of the cytoplasmic tail 224 construct. We focused on this region of the cytoplasmic tail because of its identity to the region in the β2 integrin cytoplasmic tail known to be involved in regulation of integrin adhesiveness. Mutation of the corresponding residues in the β2 integrin cytoplasmic tail has been shown to have a deleterious effect on integrin adherence and cytoskeletal interaction (Hibbs et al., 1991; Peter and O’Toole, 1995). Induction of a mutated 224 construct containing a cytoplasmic domain Y/A1261 substitution (Figure 7A, mut1) resulted in a decrease in adherence equivalent to that seen on 224 induction. However, mutation of the entire motif (T/A1253, I/A1254, T/A 1255, and Y/A1261) in the 224 construct (mut2) resulted in loss of the decreased adherence phenotype (Figure 7). All three constructs, 224, mut1, and mut2, were expressed to equal levels in E. histolytica as assessed by Western blots with GFP antibodies (our unpublished results). These observations raise the possibility that the integrin motif present in the lectin cytoplasmic tail is involved in the regulation of lectin activity.
Figure 7.
Mutation of the lectin/integrin motif in the 224 fusion protein cytoplasmic tail abrogates the 224 phenotype. The cytoplasmic domain sequence in the 224 fusion protein was mutated to alanine at residue Y1261 (mut1) and at T1253, I1254, T 1255, and Y1261 (mut2). Adherence was measured in transfected amebae induced to express the 224, mut1, and mut2 fusion proteins for 24 h and compared with adherence of the uninduced amebae. For these experiments adherence was expressed as the percentage of adherence of the uninduced amebae, where an adherent ameba was counted as one containing at least three adherent CHO cells.
DISCUSSION
In this study, the inducible expression of an intracellular fusion protein containing the cytoplasmic tail of the E. histolytica lectin was demonstrated to decrease the extracellular adhesive activity of the wild-type lectin. This dominant negative effect on the wild-type lectin appeared to be at the level of regulation of lectin activity, because the heterodimeric structure and cell surface concentration of the wild-type lectin were unaltered. Mutation of the lectin cytoplasmic domain sequence with identity to the β2 integrin adhesion regulatory region in the 224 fusion protein resulted in loss of the dominant negative phenotype. These data suggest that the molecular mechanisms of regulation of adhesiveness by β2 integrins and the amebic lectin may be similar.
Amebae induced to express the dominant negative lectin mutant were markedly defective in the ability to form liver abscesses in animals, providing the first in vivo evidence of the role of the lectin, and its adhesive regulation, in virulence. Previous reports had implicated the Gal/GalNAc lectin in the in vitro adherence and killing of target cells. The innovations of stable and inducible expression in E. histolytica allowed for a genetic investigation into the role of the lectin in adherence and virulence. The data presented here showed that through inducible expression of lectin mutants, it was possible to begin to address the role of specific lectin domains in lectin function.
Only 224 protein-expressing amebae exhibited the decreased adherence phenotype, indicating that aberrant expression of the cytoplasmic tail (and not the transmembrane region present in the 324 fusion protein) of the lectin was responsible for the dominant negative capability of the lectin–GFP fusion protein. These data provided evidence that intracellular signals can elicit changes in the ligand-binding capability of the lectin. Interactions of the lectin cytoplasmic tail with other intracellular factors could be the manner in which this inside-out signaling is mediated in E. histolytica. Overexpression of the cytoplasmic tail which is present in the 224 construct could have acted to titrate out intracellular protein(s) essential for optimal adherence by the lectin.
Mutation of lectin cytoplasmic domain amino acids T1253, I1254, T1255, and Y1261 in the 224 construct resulted in loss of the dominant negative phenotype. We focused on this region of the cytoplasmic tail because of its identity with a region in the β2 integrin cytoplasmic tail known to be involved in regulation of integrin adhesiveness. Mutation of the corresponding residues in the β2 integrin cytoplasmic tail had been shown to have a deleterious effect on integrin adherence and cytoskeletal interaction (Hibbs et al., 1991; Peter and O’Toole, 1995). The 224 fusion protein containing a cytoplasmic domain Y/A1261 substitution (Figure 7A, mut1) had a dominant negative effect on adherence equivalent to the unmutated 224 protein. However, mutation of the entire motif (T/A1253, I/A1254, T/A 1255, and Y/A1261) in the 224 construct (mut2) resulted in loss of the decreased adherence phenotype, as was the case for the β2 integrin. Given these observations, it is tempting to propose a similar mechanism of adhesive regulation for the amebic lectin and the mammalian β2 integrin.
Regulation of integrin adherence involves essential, titratable cytoplasmic factors. Many of the cellular factors and events involved in regulating integrin adherence remain to be identified; however, several proteins have been described. CD98, a heterodimeric, integral membrane protein has been shown to be an essential, titratable factor involved in β1 integrin activation. Results indicated that the cytoplasmic tails of the β1 integrin and CD98 mediate the association of the two molecules (Fenczik et al., 1997). The protein cytohesin-1 has been shown to functionally and physically interact with the cytoplasmic domain of the integrin β2 subunit. This interaction plays a role in the regulation of α1β2 (LFA-1, CD11a/CD18) integrin-mediated adhesion. The amino acids in the β2 integrin domain that interact with cytohesin-1 have yet to be identified (Kolanus et al., 1996). Finally β3-endonexin interacts specifically with the β3 cytoplasmic domain; its overexpression has been demonstrated to increase platelet αIIββ3 affinity and adhesive function (Shattil et al., 1995).
The nature of the change within the integrin that activates its adhesive function is unknown, but working models have been proposed based on the current data and conserved sequence motifs of the integrins. The “piston,” “twist,” “zipper,” and “hinge” models involve the movement of one integrin subunit relative to the other, resulting in a conformational change in the extracellular domain (Williams et al., 1994). Clustering of integrins on the cell surface also has been shown to increase the avidity of the integrin-ligand interaction (Figdor et al., 1990; van Kooyk et al., 1994). Clustering results in a change in the extracellular conformation of the integrins which is attributable to the interaction of integrin cytoplasmic domains with cytoplasmic proteins (Lub et al., 1997). The ability of antilectin mAbs to activate the lectin and of cytoskeletal-disrupting agents to inhibit the lectin suggest similar mechanisms of integrin and lectin adhesive regulation.
Expression of the 224 protein had a dominant negative effect on adherence, but not on cytolysis of adherent amebae, phagocytosis of human erythrocytes, or avoidance of complement lysis. The lectin has been implicated in all of these processes. Given these data, amebic adherence, cytolysis, phagocytosis, and serum resistance appear to be distinct events. The lack of a dominant negative effect of the 224 fusion protein on all of the lectin-mediated functions may reflect participation of other members of the hgl family (such as hgl2) or a lack of participation of the hgl1 cytoplasmic domain in these events.
Inducible expression of dominant negative lectin mutants allowed for the first time the validation of a virulence factor, and its regulation, in E. histolytica through reverse genetics. The role of the cytoplasmic domain in the altered regulation of the lectin is important because of its role in virulence and suggests similar mechanisms may be responsible for the regulation of adherence in the amebic lectin and mammalian β2 integrin. Future studies will be directed to the identification of the cytoplasmic factor(s) which regulates the lectin’s activity.
ACKNOWLEDGMENTS
We are grateful to Thomas Parsons, Robert Kadner, Robert Bloodgood, Carol Otey, and Douglas DeSimone for helpful discussion and advice. We thank Upinder Singh for help with primer extension mapping of RNA. This research was supported by National Institutes of Health grant AI-26649. W.A.P. is a Burroughs Wellcome Scholar in Molecular Parasitology.
REFERENCES
- Braga LL, Ninomiya H, McCoy JJ, Eacker S, Wiedmer T, Pham C, Wood S, Sims PJ, Petri WA., Jr Inhibition of the complement membrane attack complex by the galactose-specific adhesion of Entamoeba histolytica. J Clin Invest. 1992;90:1131–1137. doi: 10.1172/JCI115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard GD, Bilke R. Adherence of pathogenic and non-pathogenic Entamoeba histolytica strains to neutrophils. Parasitol Res. 1992;78:146–153. doi: 10.1007/BF00931657. [DOI] [PubMed] [Google Scholar]
- Cai TQ, Wright SD. Energetics of leukocyte integrin activation. J Biol Chem. 1995;270:14358–14365. doi: 10.1074/jbc.270.24.14358. [DOI] [PubMed] [Google Scholar]
- Chadee K, Johnson ML, Orozco E, Petri WA, Ravdin JI. Binding and internalization of rat colonic mucins by the Gal/GalNAc adherence lectin of Entamoeba histolytica. J Infect Dis. 1988;158:398–406. doi: 10.1093/infdis/158.2.398. [DOI] [PubMed] [Google Scholar]
- Chadee K, Meerovitch E. Entamoeba histolytica: early progressive pathology in the cecum of the gerbil (Meriones unguiculatus) Am J Trop Med Hyg. 1985;34:283–291. doi: 10.4269/ajtmh.1985.34.283. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Diamond LS, Harlow DR, Cunnick C. A new medium for axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- Figdor CG, vanKooyk Y, Keizer GD. On the mode of action of LFA-1. Immunol Today. 1990;11:277–280. doi: 10.1016/0167-5699(90)90112-m. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Jakes S, Stacker SA, Wallace RW, Springer TA. The cytoplasmic domain of the integrin lymphocyte function-associated antigen 1β subunit: sites required for binding to intercellular adhesion molecule 1 and the phorbol ester-stimulated phosphorylation site. J Exp Med. 1991;174:1227–1238. doi: 10.1084/jem.174.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. αLβ2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Lub M, van Vliet SJ, Oomen SPMA, Pieters RA, Robinson M, Figdor CG, van Kooyk Y. Cytoplasmic tails of the β1, β2, and β7 integrins differentially regulate LFA-1 function in K562 cells. Mol Biol Cell. 1997;8:719–728. doi: 10.1091/mbc.8.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BJ, Chung CY, Dodson JM, Ashley LS, Braga LL, Snodgrass TL. Neutralizing monoclonal antibody epitopes of the Entamoeba histolytica galactose adhesin map to the cysteine-rich extracellular domain of the 170-kilodalton subunit. Infect Immun. 1993;61:1772–1778. doi: 10.1128/iai.61.5.1772-1778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BJ, Torian BE, Vedvick TS, Petri WA., Jr Sequence of a cysteine-rich galactose-specific lectin of Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:3248–3252. doi: 10.1073/pnas.88.8.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JJ, Weaver AM, Petri WA., Jr Use of monoclonal anti-light subunit antibodies to study the structure and function of the Entamoeba histolytica Gal/GalNAc adherence lectin. Glycoconj J. 1994;11:432–436. doi: 10.1007/BF00731279. [DOI] [PubMed] [Google Scholar]
- Peter K, O’Toole TE. Modulation of cell adhesion by changes in αLβ2 (LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeletal interaction. J Exp Med. 1995;181:315–326. doi: 10.1084/jem.181.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA., Jr Amebiasis and the Entamoeba histolytica Gal/GalNAc lectin: from lab bench to bedside. J Invest Med. 1996;44:24–35. [PubMed] [Google Scholar]
- Petri WA, Jr, Chapman MD, Snodgrass T, Mann BJ, Broman J, Ravdin JI. Subunit structure of the galactose and N-acetyl-d-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J Biol Chem. 1989;264:3007–3012. [PubMed] [Google Scholar]
- Petri WA, Jr, Smith RD, Schlesinger PH, Murphy CF, Ravdin JI. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Invest. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA, Jr, Snodgrass TL, Jackson TFHG, Gathiram V, Simjee AE, Chadee K, Chapman MA. Monoclonal antibodies directed against the galactose-binding lectin of Entamoeba histolytica enhance adherence. J Immunol. 1990;144:4803–4809. [PubMed] [Google Scholar]
- Ramakrishnan G, Ragland BD, Purdy JE, Mann BJ. Physical mapping and expression of gene families encoding the N-acetyl-d-galactosamine adherence lectin of Entamoeba histolytica. Mol Microbiol. 1996;19:91–100. doi: 10.1046/j.1365-2958.1996.356885.x. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G, Vines RR, Mann BJ, Petri WA., Jr A tetracycline-inducible gene expression system in Entamoeba histolytica. Mol Biochem Parasitol. 1997;84:93–100. doi: 10.1016/s0166-6851(96)02784-3. [DOI] [PubMed] [Google Scholar]
- Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. J Clin Invest. 1981;68:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin JI, John JE, Johnston LI, Innes DI, Guerrant RL. Adherence of Entamoeba histolytica trophozoites to rat and human colonic mucosa. Infect Immun. 1985;48:292–297. doi: 10.1128/iai.48.2.292-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, O’Toole T, Eigenthaler M, Thon V, Williams M, Babior BM, Ginsberg MH. β3-endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin β3 subunit. J Cell Biol. 1995;131:807–816. doi: 10.1083/jcb.131.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U, Rogers JB, Mann BJ, Petri WA., Jr Transcription initiation is controlled by three core promoter elements in the protozoan parasite Entamoeba histolytica. Proc Natl Acad Sci USA. 1997;94:8812–8817. doi: 10.1073/pnas.94.16.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl D, Martinez-Palomo A, de la Torre M, de la Hoz R, de Suarez EP. Surface properties of Entamoeba: increased rates of human erythrocyte phagocytosis in pathogenic strains. J Exp Med. 1978;148:1137–1145. doi: 10.1084/jem.148.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y, Weder P, Heije K, Figdor CG. Extracellular Ca2+ modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J Cell Biol. 1994;124:1061–1070. doi: 10.1083/jcb.124.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO/PAHO/UNESCO report of a consultation of experts on amoebiasis. Week Epidem Rep WHO. 1997;72:97–99. [Google Scholar]
- Williams MJ, Hughes PE, O’Toole TE, Ginsberg MH. The inner world of cell adhesion: integrin cytoplasmic domains. Trends Cell Biol. 1994;4:109–112. doi: 10.1016/0962-8924(94)90059-0. [DOI] [PubMed] [Google Scholar]