Abstract
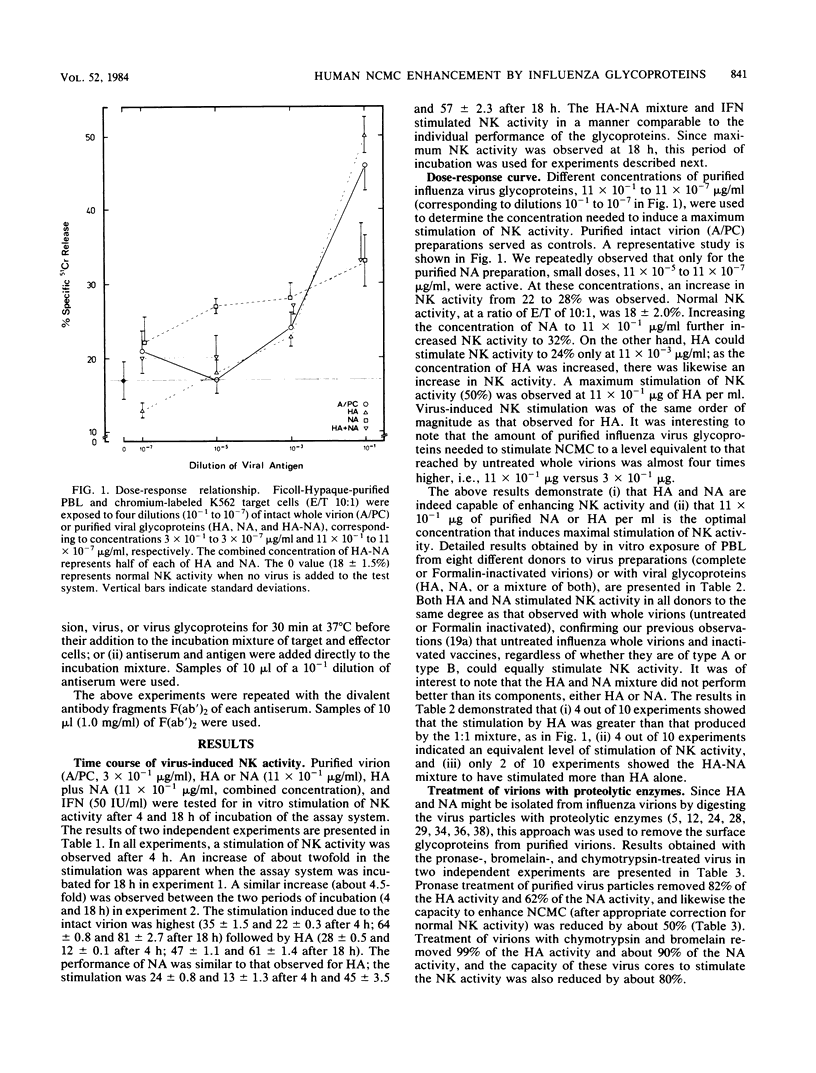
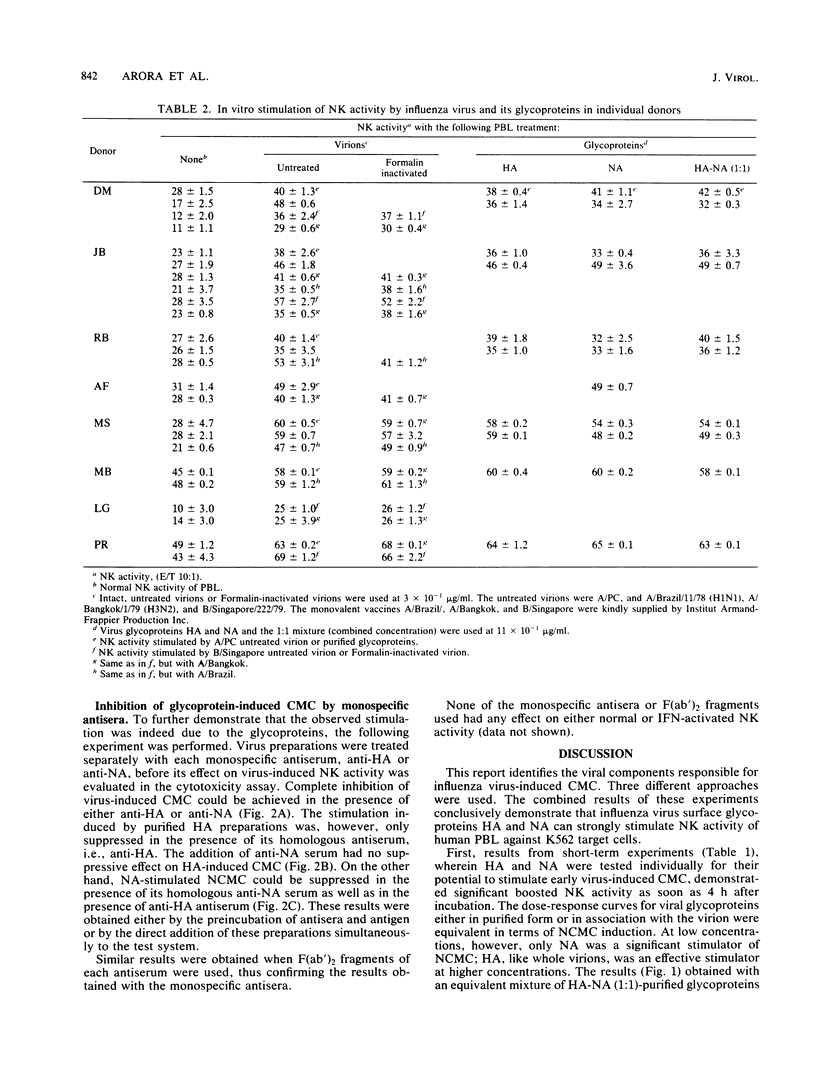
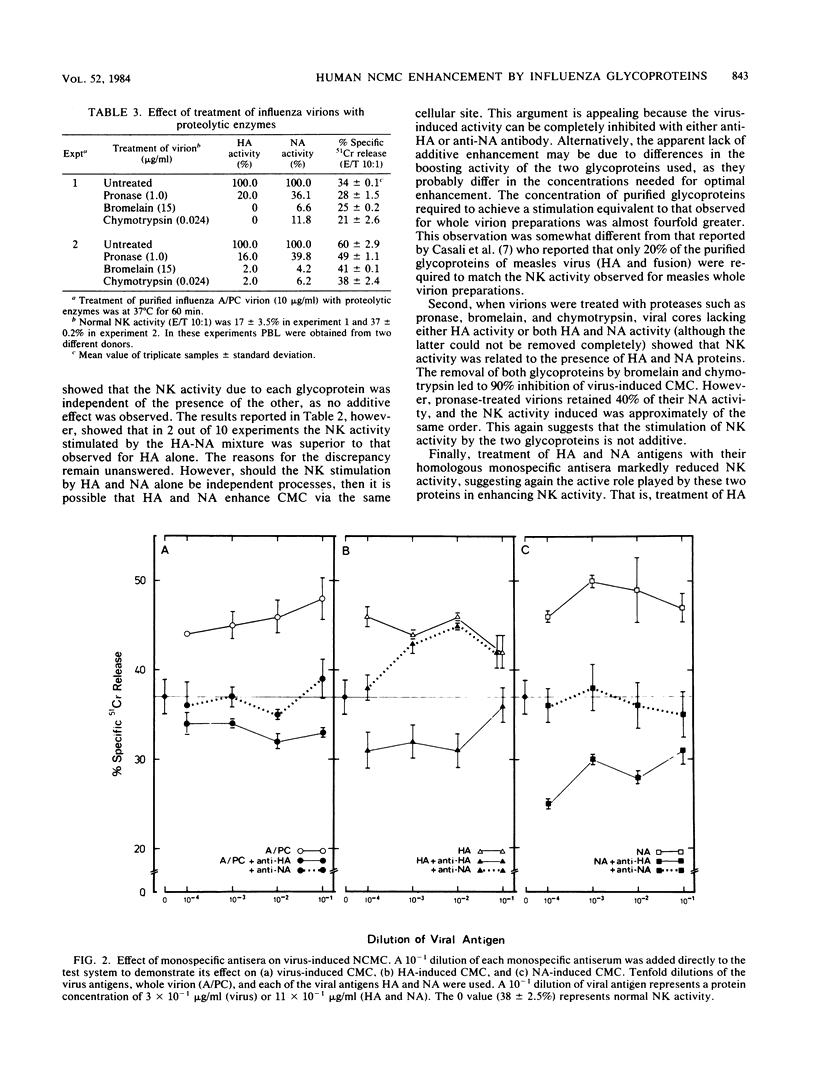
The role of the glycoproteins of influenza virus, hemagglutinin (HA), and neuraminidase (NA) in the in vitro stimulation of natural cell-mediated cytotoxicity (NCMC) or natural killer activity of human peripheral blood lymphocytes was evaluated with radiolabeled K562 cells as target cells in an overnight chromium release assay. Three different approaches were used. (i) Purified viral proteins were obtained by extraction with Nonidet P-40, separation on a sucrose gradient, and further purification by affinity chromatography. Ficoll-Hypaque-purified peripheral blood lymphocytes exposed to HA or NA individually or to a mixture of both significantly increased NCMC (32 to 50%). (ii) Treatment of HA and NA with their respective homologous antisera or F(ab')2 antibody abrogated the stimulation of NCMC by these glycoproteins. (iii) Virions treated with proteolytic enzymes resulted in viral cores lacking either HA or NA or both activities. Compared to whole virions, viral cores devoid of HA activity only induced a 50% increase in NCMC, whereas viral cores lacking HA activity and with traces of NA activity stimulated only 10% of the NCMC. These results suggest that influenza virus-induced cell-mediated cytotoxicity is largely due to its glycoproteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsheikhly A., Orvell C., Härfast B., Andersson T., Perlmann P., Norrby E. Sendai-virus-induced cell-mediated cytotoxicity in vitro. The role of viral glycoproteins in cell-mediated cytotoxicity. Scand J Immunol. 1983 Feb;17(2):129–138. doi: 10.1111/j.1365-3083.1983.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Arora D. J., Hill-Schubert J., Vincent L. The presence of two neuraminidases in an influenza virus. Can J Microbiol. 1980 Feb;26(2):243–249. doi: 10.1139/m80-037. [DOI] [PubMed] [Google Scholar]
- Arora D. J., Pavilanis V., Robert P. Two-step centrifugation method. A simplification of the density-gradient procedure for the purification of influenza virus. Can J Microbiol. 1973 May;19(5):633–638. doi: 10.1139/m73-104. [DOI] [PubMed] [Google Scholar]
- Bishop G. A., Glorioso J. C., Schwartz S. A. Role of interferon in human natural killer activity against target cells infected with HSV-1. J Immunol. 1983 Oct;131(4):1849–1853. [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Casali P., Sissons J. G., Buchmeier M. J., Oldstone M. B. In vitro generation of human cytotoxic lymphocytes by virus. Viral glycoproteins induce nonspecific cell-mediated cytotoxicity without release of interferon. J Exp Med. 1981 Sep 1;154(3):840–855. doi: 10.1084/jem.154.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Koren H. S., Jensen P. J. Natural killing can be independent of interferon generated in vitro. Cell Immunol. 1981 Jul 15;62(1):220–225. doi: 10.1016/0008-8749(81)90315-4. [DOI] [PubMed] [Google Scholar]
- Couch R. B., Kasel J. A. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Zoon K., Stanton G. J., Timonen T., Herberman R. B. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–1234. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis A. Some newly recognized aspects of resistance against and recovery from influenza. Arch Virol. 1982;73(3-4):207–217. doi: 10.1007/BF01318075. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Meager A., Beare A. S., Qi Y. H., Riley D., Schwarz G., Schild G. C., Rook A. H. Interferon induction and increased natural killer-cell activity in influenza infections in man. Lancet. 1981 Oct 24;2(8252):891–893. doi: 10.1016/s0140-6736(81)91390-8. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Meager A. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J Exp Med. 1981 Nov 1;154(5):1279–1289. doi: 10.1084/jem.154.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. A., Evans R., Kirkpatrick D., Lopez C. Heterogeneity of human NK cells: comparison of effectors that lyse HSV-1-infected fibroblasts and K562 erythroleukemia targets. J Immunol. 1983 Apr;130(4):1663–1667. [PubMed] [Google Scholar]
- Fitzgerald P. A., von Wussow P., Lopez C. Role of interferon in natural kill of HSV-1-infected fibroblasts. J Immunol. 1982 Aug;129(2):819–823. [PubMed] [Google Scholar]
- Haller O. Inborn resistance of ice to orthomyxoviruses. Curr Top Microbiol Immunol. 1981;92:25–52. doi: 10.1007/978-3-642-68069-4_3. [DOI] [PubMed] [Google Scholar]
- Härfast B., Orvell C., Alsheikhly A., Andersson T., Perlmann P., Norrby E. The role of viral glycoproteins in mumps-virus-dependent lymphocyte-mediated cytotoxicity in vitro. Scand J Immunol. 1980;11(4):391–400. doi: 10.1111/j.1365-3083.1980.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Justewicz D. M., Lecomte J., Mandeville R. Blocking of influenza virus-induced cell-mediated cytotoxicity by hemagglutinin-specific monoclonal antibody. J Infect Dis. 1984 Sep;150(3):348–357. doi: 10.1093/infdis/150.3.348. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D., Laver W. G., Schulman J. L., Webster R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968 Apr;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandeville R., Sombo F. M., Rocheleau N. Natural cell-mediated cytotoxicity in normal human peripheral blood lymphocytes and its in vitro boosting with BCG. Cancer Immunol Immunother. 1983;15(1):17–22. doi: 10.1007/BF00199456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nermut M. V. Further investigation on the fine structure of influenza virus. J Gen Virol. 1972 Dec;17(3):317–331. doi: 10.1099/0022-1317-17-3-317. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S. Determinants of immunity to influenza infection in man. Br Med Bull. 1979 Jan;35(1):69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- Santoli D., Koprowski H. Mechanisms of activation of human natural killer cells against tumor and virus-infected cells. Immunol Rev. 1979;44:125–163. doi: 10.1111/j.1600-065x.1979.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Seto JT BRZENIEK R., Rott R. Isolation of a low molecular weight sialidase (neuraminidase) from influenza virus. Biochim Biophys Acta. 1966 Feb 14;113(2):402–404. doi: 10.1016/s0926-6593(66)80081-4. [DOI] [PubMed] [Google Scholar]
- Stein-Streilein J., Bennett M., Mann D., Kumar V. Natural killer cells in mouse lung: surface phenotype, target preference, and response to local influenza virus infection. J Immunol. 1983 Dec;131(6):2699–2704. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D., Dee R. R., Knowles B. B. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation. J Exp Med. 1978 May 1;147(5):1299–1313. doi: 10.1084/jem.147.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Laver W. G. Selection and analysis of antigenic variants of the neuraminidase of N2 influenza viruses with monoclonal antibodies. Virology. 1982 Feb;117(1):93–104. doi: 10.1016/0042-6822(82)90510-4. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–106. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Autologous herpes simplex virus-infected cells are lysed by human natural killer cells. J Immunol. 1983 Oct;131(4):2011–2016. [PubMed] [Google Scholar]



