Abstract
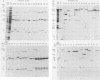
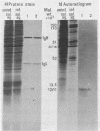
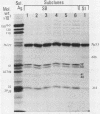
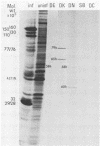
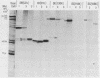
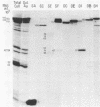
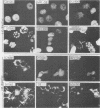
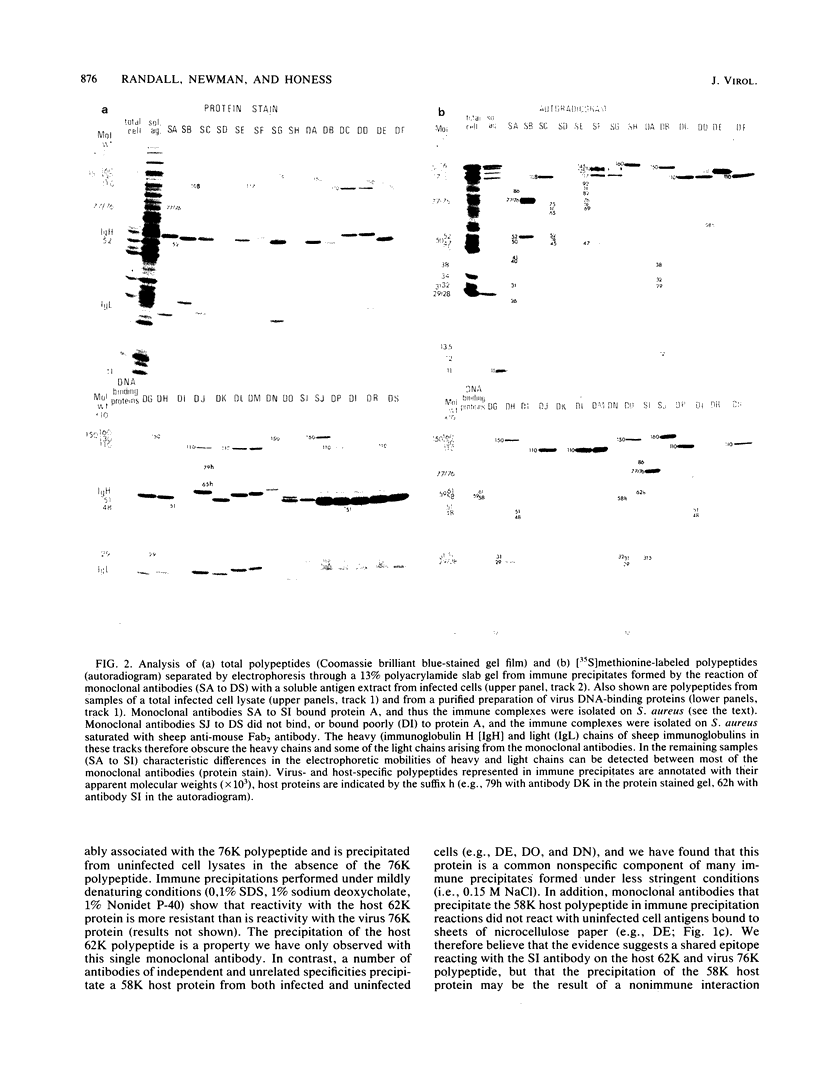
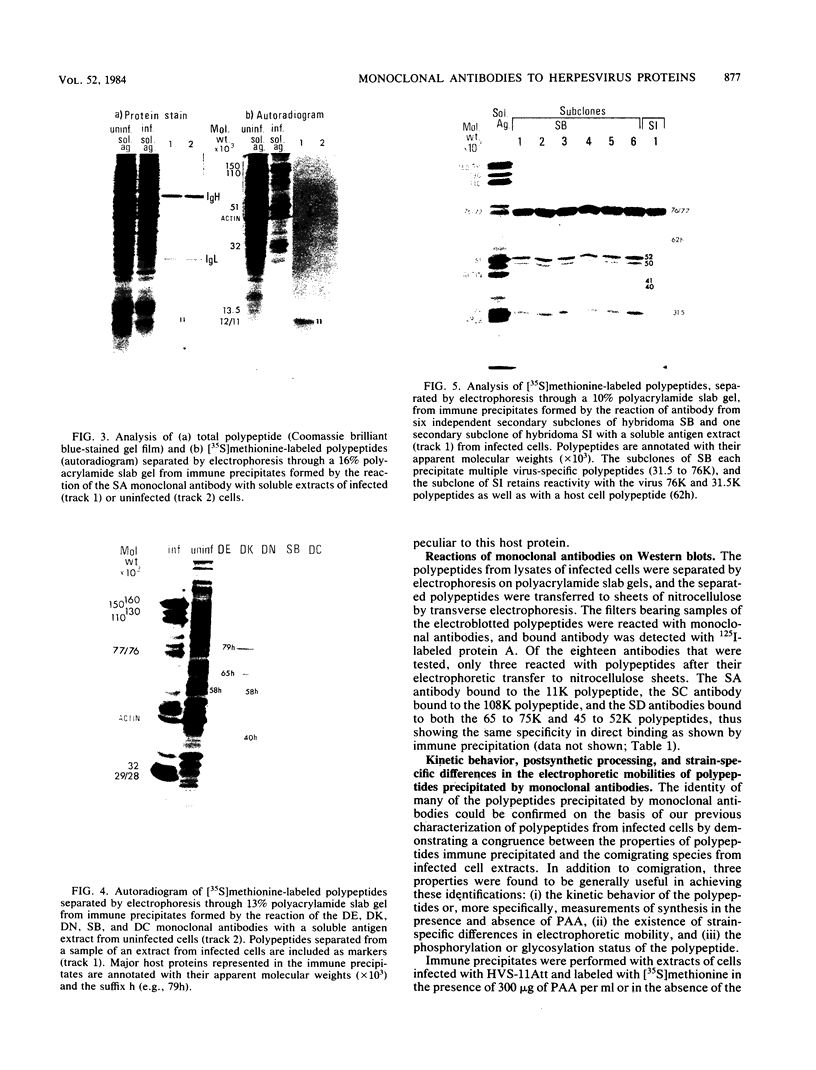
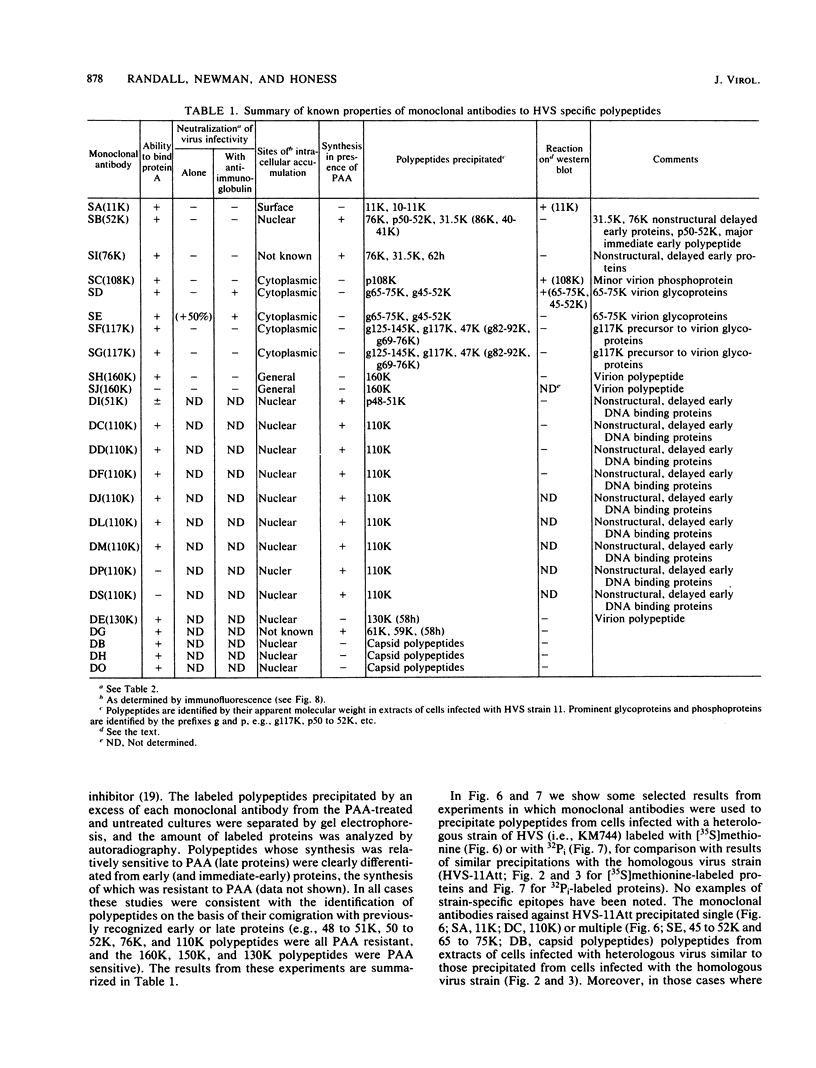
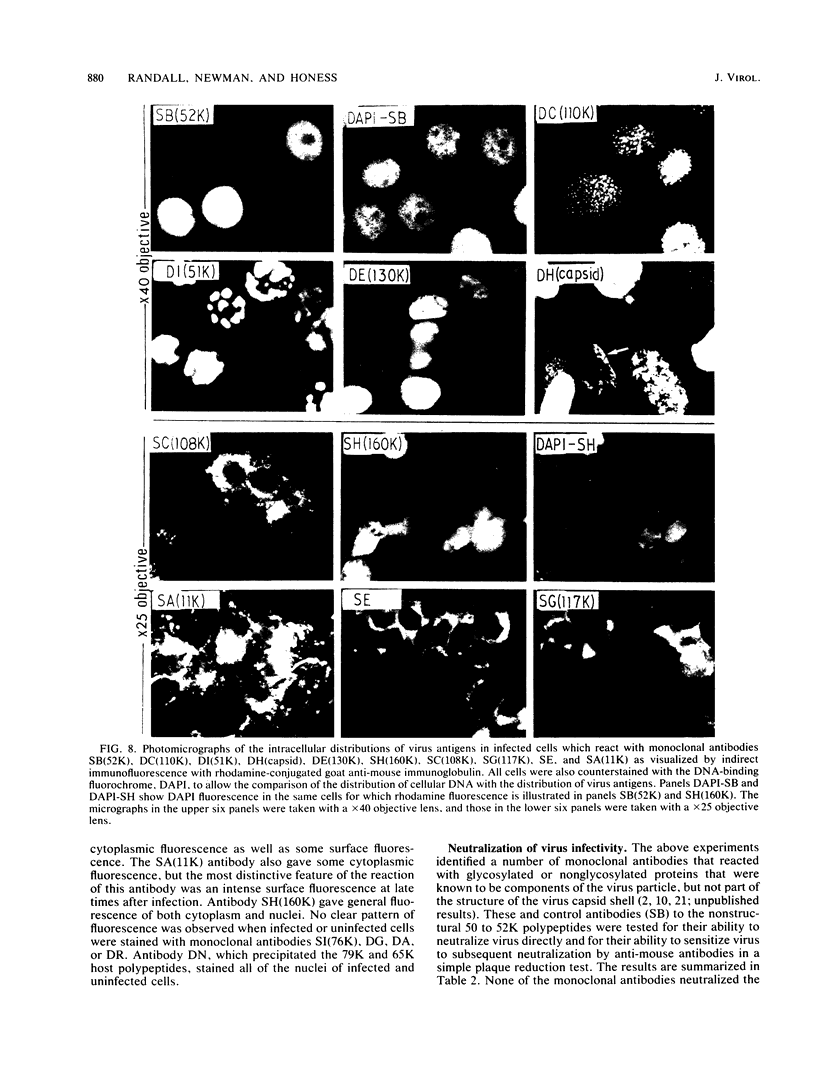
An improved screening procedure was applied to identify hybridomas secreting antibodies to herpesvirus saimiri-specified polypeptides among the products of fusions between SP2/0 myeloma cells and spleen cells from mice immunized with purified virus particles or virus-specific DNA-binding proteins. Twenty-four monoclonal antibodies were isolated with specificities for 13 different virus-specified polypeptides (or complexes of polypeptides), including the major capsid protein of the virus (150K), the 160K and 130K structural proteins, a 108K structural phosphoprotein, structural glycoproteins, the nonstructural early 76K protein, early nonstructural DNA-binding proteins of 48 to 51K and 110K and the major immediate-early protein of 52K. Antibody to the virus 76K protein precipitated a host protein of 62K, and a number of antibodies specific for host proteins were also isolated. Antibody to the 52K immediate-early polypeptide precipitated the delayed-early 76K protein, whereas the antibody to the 76K protein did not precipitate the 52K polypeptide. These observations suggest the presence of epitopes common to virus and host proteins and an antigenic site common to an immediate-early and a delayed-early virus protein. The antibodies were used to examine the sites of intracellular accumulation of virus polypeptides, the formation of complexes of structural proteins, and the postsynthetic processing of virus proteins. The present collection of monoclonal antibodies provides a set of reagents with specificities for members of each of the major kinetically or functionally distinct classes of virus gene products.
Full text
PDF

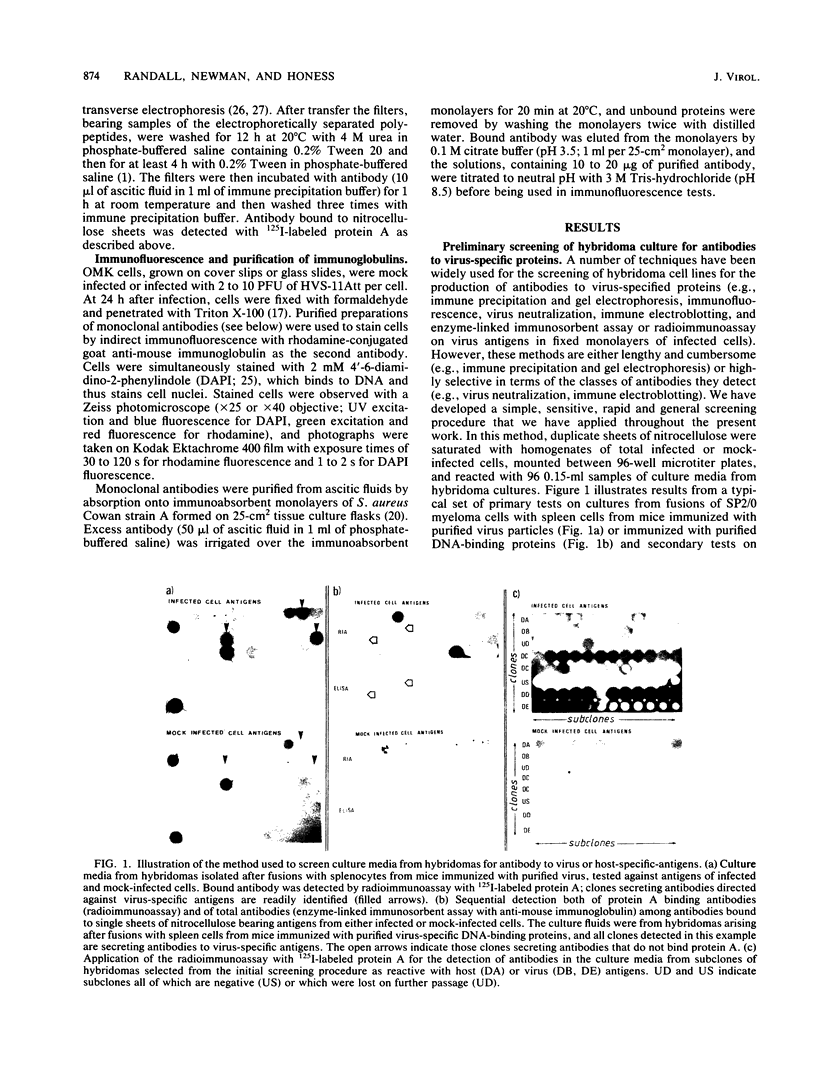





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Blair E. D., Honess R. W. DNA-binding proteins specified by herpesvirus saimiri. J Gen Virol. 1983 Dec;64(Pt 12):2697–2715. doi: 10.1099/0022-1317-64-12-2697. [DOI] [PubMed] [Google Scholar]
- Crawford L., Leppard K., Lane D., Harlow E. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J Virol. 1982 May;42(2):612–620. doi: 10.1128/jvi.42.2.612-620.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Honess R. W., O'Hare P., Young D. Comparison of thymidine kinase activities indiced in cells productively infected with herpesvirus saimiri and herpes simplex virus. J Gen Virol. 1982 Feb;58(Pt 2):237–249. doi: 10.1099/0022-1317-58-2-237. [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Chin G., Hayward G. S. Characterization of cytomegalovirus immediate-early genes. I. Nonpermissive rodent cells overproduce the IE94K protein form CMV (Colburn). Virology. 1982 Sep;121(2):393–403. doi: 10.1016/0042-6822(82)90177-5. [DOI] [PubMed] [Google Scholar]
- Keil G., Fleckenstein B., Bodemer W. Structural proteins of Herpesvirus saimiri. J Virol. 1983 Sep;47(3):463–470. doi: 10.1128/jvi.47.3.463-470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Mautner V., Willcox H. N. Adenovirus antigens: a model system in mice for subunit vaccination. J Gen Virol. 1974 Dec;25(3):325–336. doi: 10.1099/0022-1317-25-3-325. [DOI] [PubMed] [Google Scholar]
- Modrow S., Wolf H. Herpesvirus saimiri-induced proteins in lytically infected cells. I. Time-ordered synthesis. J Gen Virol. 1983 Jan;64(Pt 1):37–46. doi: 10.1099/0022-1317-64-1-37. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Sefton B. M., Hunter T., Walter G., Singer S. J. Immunofluorescent localization of the transforming protein of Rous sarcoma virus with antibodies against a synthetic src peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5322–5326. doi: 10.1073/pnas.79.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Honess R. W. Evidence for a herpesvirus saimiri-specified DNA polymerase activity which is aphidicolin-resistant and phosphonoacetate-sensitive. J Gen Virol. 1983 May;64(Pt 5):1013–1024. doi: 10.1099/0022-1317-64-5-1013. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Honess R. W. Identification of a subset of herpesvirus saimiri polypeptides synthesized in the absence of virus DNA replication. J Virol. 1983 Apr;46(1):279–283. doi: 10.1128/jvi.46.1.279-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W., O'Hare P. Proteins specified by herpesvirus saimiri: identification and properties of virus-specific polypeptides in productively infected cells. J Gen Virol. 1983 Jan;64(Pt 1):19–35. doi: 10.1099/0022-1317-64-1-19. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W. Proteins of herpesvirus saimiri: identification of two virus polypeptides released into the culture medium of productively infected cells. J Gen Virol. 1980 Dec;51(Pt 2):445–449. doi: 10.1099/0022-1317-51-2-445. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W. Proteins specified by Herpesvirus saimiri: purification and properties of a single polypeptide which elicits virus-neutralizing antibody. J Gen Virol. 1982 Jan;58(Pt 1):149–161. doi: 10.1099/0022-1317-58-1-149. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Newman C., Honess R. W. A single major immediate-early virus gene product is synthesized in cells productively infected with herpesvirus saimiri. J Gen Virol. 1984 Jul;65(Pt 7):1215–1219. doi: 10.1099/0022-1317-65-7-1215. [DOI] [PubMed] [Google Scholar]
- Randall R. E. Preparation and uses of immunoabsorbent monolayers in the purification of virus proteins and separation of cells on the basis of their cell surface antigens. J Immunol Methods. 1983 May 27;60(1-2):147–165. doi: 10.1016/0022-1759(83)90343-5. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., McGeoch D. J. A 3' co-terminal family of mRNAs from the herpes simplex virus type 1 short region: two overlapping reading frames encode unrelated polypeptide one of which has highly reiterated amino acid sequence. Nucleic Acids Res. 1984 Mar 12;12(5):2473–2487. doi: 10.1093/nar/12.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Precious B. Nucleic acid-binding properties of adenovirus structural polypeptides. J Gen Virol. 1982 Nov;63(Pt 1):69–79. doi: 10.1099/0022-1317-63-1-69. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Falk L. A., Deinhardt F. Attenuation of herpesvirus saimiri for marmosets after successive passage in cell culture at 39 degrees C. J Natl Cancer Inst. 1975 Nov;55(5):1243–1246. doi: 10.1093/jnci/55.5.1243. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]