Abstract
We report that cyclin D3/cdk4 kinase activity is regulated by p27kip1 in BALB/c 3T3 cells. The association of p27kip1 was found to result in inhibition of cyclin D3 activity as measured by immune complex kinase assays utilizing cyclin D3-specific antibodies. The ternary p27kip1/cyclin D3/cdk4 complexes do exhibit kinase activity when measured in immune complex kinase assays utilizing p27kip1-specific antibodies. The association of p27kip1 with cyclin D3 was highest in quiescent cells and declined upon mitogenic stimulation, concomitantly with declines in the total level of p27kip1 protein. The decline in this association could be elicited by PDGF treatment alone; this was not sufficient, however, for activation of cyclin D3 activity, which also required the presence of factors in platelet-poor plasma in the culturing medium. Unlike cyclin D3 activity, which was detected only in growing cells, p27kip1 kinase activity was present throughout the cell cycle. Since we found that the p27kip1 activity was dependent on cyclin D3 and cdk4, we compared the substrate specificity of the active ternary complex containing p27kip1 and the active cyclin D3 lacking p27kip1 by tryptic phosphopeptide mapping of GST-Rb phosphorylated in vitro and also by comparing the relative phosphorylation activity toward a panel of peptide substrates. We found that ternary p27kip1/cyclin D3/cdk4 complexes exhibited a different specificity than the active binary cyclin D3/cdk4 complexes, suggesting that p27kip1 has the capacity to both inhibit cyclin D/cdk4 activity as well as to modulate cyclin D3/cdk4 activity by altering its substrate preference.
INTRODUCTION
Two families of proteins play major roles in the regulation of the cell cycle (Roberts, 1993; Morgan, 1995; Sherr, 1996; Wuarin and Nurse, 1996). One family, the cdk, exerts control on downstream processes by phosphorylating selected proteins, such as Rb family members, on serine and threonine residues (Morgan, 1995). The other family consists of specialized regulatory proteins, cyclins, that bind to cdk molecules and modulate their activity to phosphorylate appropriate target proteins (Swenson et al., 1986; Hagan et al., 1988; Booher et al., 1989; Draetta et al., 1989; Hadwiger et al., 1989; Moreno et al., 1989). The catalytic activity of monomeric cdk subunits is almost undetectable, and the pathway to its formation requires, as a first step, the binding of a cyclin subunit (Solomon et al., 1990). While the resulting binary cyclin/cdk complex is functionally competent, its activity is still subject to both positive and negative control. Maximal activity is attained only after phosphorylation of a conserved threonine residue covering the ATP-binding pocket on the catalytic subunit (Ducommun et al., 1991; Gould et al., 1991). This phosphorylation is performed by a constitutive cellular kinase activity, cdk-activating kinase (CAK), itself a cyclin/cdk complex (Poon et al., 1993; Solomon et al., 1993; Fisher and Morgan, 1994). In addition, cdk activity is also subject to negative control by two mechanisms: binding of inhibitory subunits and phosphorylation on an amino-terminal tyrosine residue (Picard et al., 1989; Pondaven et al., 1990). The cdk-inhibitory proteins are collectively called CKIs (Elledge and Harper, 1994; Sherr and Roberts, 1995), of which seven have been identified to date.
Both primary sequence homology and cdk target specificity indicate CKIs fall into two groups, the Kip/Cip family and the Ink4 family. Unlike the members of the Ink4 family, which are able to inhibit only cdk4/cdk6 activity, the members of the Kip/Cip family are able to exhibit inhibitory activity with all cdk complexes (Xiong et al., 1993; Harper et al., 1995; Matsuoka et al., 1995). This latter group is currently composed of three members: p2 1Cip (Harper et al., 1993; Xiong et al., 1993), p27kip1 (Hengst et al., 1994; Polyak et al., 1994b; Toyoshima and Hunter, 1994), and p57 (Lee et al., 1995; Matsuoka et al., 1995). The earliest known inhibitors, p21Cip1 and p27kip1, are also the most extensively studied and have been linked to growth arrest brought about through a number of different environmental signals. Conditions that promote DNA damage or otherwise cause an induction of p53 have almost invariably been demonstrated to result in the induction of p21Cip1 (el-Deiry et al., 1993; Dulic et al., 1994), whereas conditions such as high cell density, TGF-β growth inhibition, and serum withdrawal have been associated with induction of p27kip1 (Polyak et al., 1994a; Toyoshima and Hunter, 1994; Agrawal et al., 1995). Sequence comparisons between p27kip1 and p21Cip1 revealed a region of identity in the N-terminal region of each protein that contains the motif responsible for its inhibitory activity (Nakanishi et al., 1995). In addition to direct inhibition of catalytic activity, association of p27kip1 with either cdk2 or cdk4 complexes can block their CAK-mediated phosphorylation (Matsuoka et al., 1994). Overexpression of p27kip1 was found to cause G1 arrest (Polyak et al., 1994b; Toyoshima and Hunter, 1994), and antisense inhibition of p27kip1 expression prevented cell cycle exit in response to mitogen depletion (Coats et al., 1996; Rivard et al., 1996), consistent with its proposed role as a negative regulator of cell cycle progression in vivo. Unlike p21Cip1, p27kip1 protein levels either remain relatively constant in cycling cells or are high in quiescent cells and decreased in growing cells (Nourse et al., 1994; Toyoshima and Hunter, 1994; Agrawal et al., 1995). Thus, in some cell types, p27kip1 is an essential component of the pathway that connects mitogenic signals to the cell cycle at the restriction point and may be the primary CKI protein responsible for producing an inhibitory threshold for activation of cyclin/cdk complexes in quiescent cells.
It is widely agreed that cyclin D1 plays an important role in sequestering p27kip1 during the G1 phase, allowing cyclin E/cdk2 to become active and thus permitting entry into S phase (Polyak et al., 1994a). Although cdk activities are observed during G1, the role of p27kip1 inhibition of cyclin D/cdk4 activity in cell cycle regulation remains to be clarified. Recently published data have led to several different conclusions concerning the effect of p27kip1 on D type cyclin activity including: 1) p27kip1-associated cyclin D/cdk4 is active (Reynisdottir and Massague, 1997), 2) p27kip1-associated cyclin D complex is not active (Polyak et al., 1994b; Toyoshima and Hunter, 1994; LaBaer et al., 1997), and 3) p27kip1 must be removed before the cyclin D/cdk can be activated by CAK (Kato et al., 1997). Moreover, it has recently been demonstrated that while p27kip1 readily inhibits cyclin/cdk2 activity, it requires multiple p27kip1 molecules to inhibit an active cyclin D/cdk4 complex. In contrast to its roles as an inhibitor of cdk, p27kip1-immune complexes isolated from MANCA cells, a human Burkitts lymphoma cell line, were reported to contain an Rb kinase activity (Soos et al., 1996). The p27kip1-specific activity was incapable of phosphorylating histone H1 and was removed by depletion of cdk6, characteristics suggesting that it was a cyclin D-dependent activity. Furthermore, p27kip1 complexes prepared from primary mouse keratinocytes stimulated to undergo differentiation contained an Rb kinase activity that was dependent on cyclin D3/cdk4 (Hauser et al., 1997). Clearly, the mechanisms whereby cyclin D/cdk complexes are activated and the role that p27kip1 may play on cyclin D/cdk activity, such as to inhibit this activity or to bring about a Rb kinase in association with cyclin D/cdk4/p27kip1 complexes, has not been elucidated.
The physiological role of p27kip1, in most cases, has been linked to inhibition of cyclin E/cdk2, and the association of p27kip1 with cyclin D/cdk4 is believed to be required only to lower the inhibitory threshold for cyclin E/cdk2 activation. This idea is strongly supported by numerous studies but does not exclude the possibility that a ternary cyclin D/cdk4/p27kip1 complex may be physiologically distinct from a binary complex lacking p27kip. Since it has been previously demonstrated that cyclin D3/cdk4 activity also appears during G1 traverse in BALB/c 3T3 fibroblasts (Dong et al., 1998) and that cyclin D3 is bound to p27kip1 complexes in quiescent cells (Agrawal et al., 1996), we have examined the possibility that cyclin D3 activity may be subject to inhibition by p27kip1 in this cell line. Our results indicate that p27kip1 is removed from cyclin D3 complexes after mitogenic stimulation with PDGF, which may be necessary but is not sufficient for cyclin D3/cdk4 activation. In addition, we have found that the association of p27kip1 with cyclin D3 in quiescent cells also gives rise to a kinase activity that may be detected in p27kip1-immune complexes. By comparing the phosphorylation specificity of ternary p27kip1/cyclin D3/cdk4 complexes and binary complexes lacking p27kip1, we demonstrate that the addition of p27kip1 results in an alteration of substrate specificity.
MATERIALS AND METHODS
Cell Culture
BALB/c 3T3 fibroblasts (clone A31) were maintained in an incubator with a humidified atmosphere (5% CO2, 95% air) at 37°C. Experimental cultures were grown to confluence in 100-mm Petri dishes using DMEM supplemented with 10% calf serum (CS), 4 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Quiescent cultures were stimulated 2–3 d after density-arrest by the addition of fresh medium (DMEM) containing 10 ng/ml PDGF-BB, and 10% CS (Agrawal et al., 1995; Winston et al., 1996).
Reagents, Recombinant Plasmids, and Antibodies
Biological buffers, detergents, and chemicals (except for those indicated below) were from Sigma Chemical (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). Cell culture medium, antibiotics, and protein A-agarose beads were from Life Technologies (Gaithersburg, MD), and PDGF-BB was from BioSource (Camarillo, CA). Nitrocellulose paper and reagents for SDS-PAGE were from Bio-Rad (Hercules, CA). Rabbit anti-mouse HRP, ECL reagents, and radioisotopes were from Amersham (Arlington Heights, IL). Autoradiographic film was from Eastman Kodak (Rochester, NY). A plasmid encoding GST-Rb 379–928 was obtained from Doug Cress (H. Lee Moffitt Cancer Center and Research Institute). The cyclin D1 (72–13G) and D3 (C-16) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The cdk4 antibody was raised against C-terminal polypeptide of mouse cdk4. The cyclin E antibody was raised against C-terminal polypeptide of mouse cyclin E. The p27kip1 antibody was raised against His6–kip fusion protein (Agrawal et al., 1996).
Preparation of Mouse Primary Fibroblasts (MPFs)
p27+/− mice were the gift of Dr. Andrew Koff (Memorial Sloan-Kettering Cancer Center, New York, NY) (Kiyokawa et al., 1996). Further intercross of the p27+/− mice produced both p27−/− and p27+/− offspring. To prepare primary dermal fibroblasts, the bottom layer of skin was obtained from newborn mice, transferred to fresh sterile PBS containing 0.1% trypsin, and stirred in a small beaker for 30 min at room temperature. The PBS containing the subcutaneous tissue was filtrated through three layers of sterile abrasive cloth. The cells in the filtrate were grown in DMEM supplemented with 10% CS.
Preparation of Cell Extracts
Cultures were rinsed twice in ice-cold PBS, harvested by scraping, and collected by centrifugation at 12,000 × g for 6 s. The pellets were resuspended in lysis buffer (50 mM HEPES [pH 7.5], 100 mM NaCl, 2 mM EDTA, 0.5% NP-40, 10% Glycerol, 0.1 mM sodium orthovanadate, 0.1 mM PMSF, 2.5 μg/ml leupeptin, and 1 mM DTT), vortexed vigorously, and incubated on ice for 30 min. After centrifugation at 14,000 × g for 3 min, the supernatant was transferred into fresh tubes, and then stored at −70°C.
Immunological Assays
Immunoprecipitation was performed using 40 μg of total proteins from cell extracts. Specific antisera were incubated with protein A-agarose beads in 250 μl of the lysis buffer described above at 4°C for 2 h while shaking. The antibody-linked beads were washed once with lysis buffer, mixed with appropriate cell extracts, and incubated for 2 h at 4°C. For subsequent immunoblotting analysis, complexes bound to protein A-agarose were washed twice with lysis buffer, resuspended in 15 μl of SDS-PAGE loading buffer, heated to 95°C for 5 min, separated by SDS-PAGE, and transferred to a nitrocellulose membrane. The blots were incubated with the primary antibody for 1 h followed by a 1-h incubation with a secondary antibody. The bound antibodies were visualized using the ECL detection system (Amersham) according to the manufacturer’s instructions.
In Vitro Kinase Assay Using Purified Fusion Proteins
Specific antiserum (2 μl) and 30 μl of 25% protein A-agarose beads were added to 250 μl of lysis buffer, and the mixture was rocked for 2 h at 4°C, followed by the addition of 40 μg of total protein from cell extracts and an additional 2 h of mixing. Immune complexes were collected by centrifugation at 14,000 × g for 1 min and washed twice with lysis buffer, once with 2 × kinase reaction buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 5 mM MnCl2, 10 mM DTT), resuspended in 8 μl of 1× kinase reaction buffer containing 10 μCi [γ-32P]-ATP, 10 μM cold ATP, and 1 μg of GST-pRb. The mixture was incubated at 30°C for 30 min, resuspended in 10 μl of SDS-PAGE loading buffer, heated to 95°C for 5 min, and separated on an 11% SDS-polyacrylamide gel. Phosphorylated proteins were visualized by exposure to Kodak XAR-5 x-ray film for 30 min to 10 h and quantitated by exposure on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
In Vitro Kinase Assay with Synthetic Peptide Substrates
For in vitro kinase assay using synthetic peptides, substrate peptides derived from the pRb sequence and previously demonstrated to reflect cdk4 phosphorylation sites were synthesized and purified by HPLC at Genemed Synthesis (South San Francisco, CA). The nomenclature utilized in this report corresponds to that previously reported (Kitagawa et al., 1996).
Immune complexes were obtained as described above and resuspended in 8 μl of 1× kinase reaction buffer containing 10 μCi [γ-32P]-ATP, 10 μM cold ATP, and 1 μg of synthetic peptide. The mixture was incubated at 30°C for 30 min, added with 10 μl of 2× loading buffer, heated to 95°C for 5 min, and separated on a 16.5% T/6% C tricine-SDS-polyacrylamide gel. Tricine-SDS-PAGE was carried out essentially as described (Schagger et al., 1990). A three-layer gel (stacking, 4%; spacing, 10%; and separating, 16.5%) was constructed, and phosphorylated protein was loaded and electrophoresed at 140 V until the tracking dye was within 3 cm of the bottom of the gel. The dye line was cut, and the remainder of the gel was fixed in a buffer containing 10% methanol and 10% acetic acid for 30 min. Phosphorylated protein was visualized by exposure to Kodak XAR-5 x-ray film in a Life Technologies magnetic film cassette for 2–24 h and quantitated by exposure on a PhosphorImager (Molecular Dynamics).
Tryptic Phosphopeptide Mapping
To examine the phosphorylation specificity of various immune complexes, GST-Rb was phosphorylated as described above in vitro. After 32P-labeled samples were separated on an 11% SDS-polyacrylamide gel, the gel was dried, and the protein bands were cut out from individual lanes using a single-edge razor blade. The pieces of gel were placed in a microcentrifuge tube and incubated in 400 μl freshly prepared 50 mM ammonium bicarbonate, pH 7.4, for 5 min at room temperature. After removal of the paper backing, the pieces of gel were ground until they could pass through a disposable tip of a 200-μl adjustable pipette. To elute radioactive proteins from gel, 40 μl β-mercaptoethanol and 8 μl 10% SDS were added to the gel pieces, followed by boiling for 3 min and incubating for 90 min at room temperature on a shaker. After centrifugation, the supernatant was transferred to a new tube. Protein was recovered by adding 20 μg boiled RNase A and 250 μl ice-cold 100% trichloroacetic acid to the supernatant, followed by incubating the mixture for 1 h on ice. The precipitates were washed twice with 96% ice-cold ethanol, air-dried, resuspended in 50 μl of 50 mM ammonium bicarbonate, pH 7.9, and digested for 10 h with 10 μg trypsin (Boehringer Mannheim, Mannheim, Germany).
Peptides were separated on cellulose TLC plates (Merck, Darmstadt, Germany) using electrophoresis in formic acid, glacial acetic acid, and deionized water (50:156:1794/2 l) as first dimension and chromatography in butanol, pyridine, glacial acetic acid, and deionized water (75:50:15:60/200 ml) as second dimension. The plates were allowed to dry in a fume hood overnight, and the phosphopeptides were visualized by exposure to a phosphoimaging screen.
RESULTS
Cyclin D3 Activity Is Accompanied by a Reduced Association with p27kip1
It has been demonstrated previously that cyclin D3/cdk4 kinase activity appears after density-arrested BALB/c 3T3 cultures are stimulated to undergo cell cycle traverse, 12–18 h after mitogenic stimulation (Dong et al., 1998). We determined whether this activity was present in continuously cycling cells and its fate upon density-dependent growth inhibition (Figure 1A). It is clear from these data that cyclin D3 kinase activity is present in growing cells and down-regulated upon growth inhibition and then reappears after mitogenic stimulation. The cyclin D3 protein level, however, did not fluctuate in a proportional manner and appeared to remain relatively constant throughout the course of these growth transitions (Figure 1B). This observation suggested the cyclin D3-associated activity was, therefore, controlled at a level other than cyclin accumulation. Although it has been established that the level of cdk4 protein also remains constant in this cell line, the levels of p27kip1 are known to increase during density-dependent growth arrest (Figure 1C and Agrawal et al., 1995, 1996), suggesting that the association of p27kip1 with cyclin D3/cdk4 may have contributed to the loss of activity observed in Figure 1A.
Figure 1.
Cyclin D3-dependent kinase activities are inversely correlated with the level of p27kip1 in a cell cycle-dependent manner. To examine whether cyclin D3 activity was regulated during growth transitions, we utilized asynchronous cultures of BALB/c 3T3 cells in growth medium (DMEM) supplemented with 10% CS. Cultures were harvested at 24-h intervals during the growth cycle until density arrest was attained (lanes 1–4). After growth arrest, quiescent cells were mitogenically stimulated with growth medium supplemented with 10% CS + 10 ng/ml PDGF and harvested 18 h later (lane 5). (A) Portions of each cell extract, containing 40 μg of protein, were subjected to immunoprecipitation with cyclin D3 antibody-linked beads. Immunoprecipitates were used to measure in vitro kinase activity using GST-Rb as a substrate. Phosphorylated GST-Rb was separated on 11% SDS-PAGE and visualized by autoradiography. Parallel samples of each cell extract, containing 40 μg of protein, were subjected to Western blot analysis and probed with cyclin D3 antibodies (panel B) and p27kip1 antibodies (panel C). Proteins were detected using the ECL system as described in MATERIALS AND METHODS.
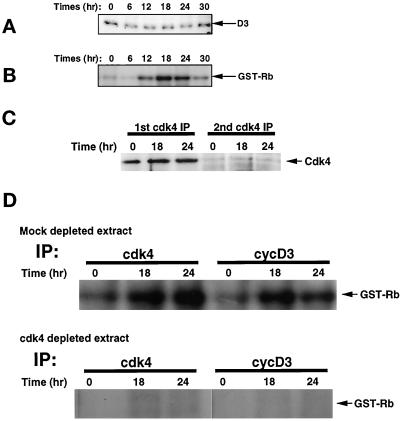
We also analyzed the level of cyclin D3 protein and its associated cdk and CKI subunits more closely in populations synchronized by density arrest and then mitogenically stimulated. As with the experiment shown in Figure 1, synchronized populations did not exhibit a significant change in the level of cyclin D3 protein during cell cycle traverse (Figure 2A), although the level of cyclin D3 activity was significantly elevated between 12 and 18 h (Figure 2B). This pattern is in striking contrast to that observed for cyclin D1 kinase activity, which increases concomitantly with an increase in cyclin D1 protein during serum-dependent cell cycle traverse (Agrawal et al., 1996; Winston et al., 1996). To examine the possibility that cyclin D3 activity was dependent on cdk4, the catalytic subunit responsible for cyclin D1 activity in murine fibroblasts, we prepared cell extracts that were depleted of cdk4 (Figure 2C). We then compared the mock depleted and cdk4-depleted extracts for the presence of cdk4 kinase activity and cyclin D3 kinase activity (Figure 2D). The data revealed that the cyclin D3 activity was quantitatively removed by immunodepletion of cdk4, demonstrating that the activity observed in Figure 2B reflected cyclin D3/cdk4 complexes.
Figure 2.
Cyclin D3 level and cyclin D3-dependent kinase activity throughout the cell cycle. Quiescent, density- arrested cells were mitogenically stimulated by the addition of fresh DMEM supplemented with 10% CS and 10 ng/ml of PDGF-BB. Cell cultures were harvested at the times indicated. (A) Portions of each cell extract containing 40 μg of total proteins were subjected to Western blot analysis and probed with anti-cyclin D3 antibody. (B) Forty micrograms of protein from each cell extract were utilized for immunoprecipitation with cyclin D3 antibody-linked beads. Immunoprecipitates were used to measure in vitro kinase activity using GST-Rb as a substrate. Phosphorylated GST-Rb was separated on 11% SDS-PAGE and visualized by autoradiography. (C) Depletion of cdk4 was accomplished by immunoprecipitation of cdk4 from 40 μg of protein derived from cell extracts prepared from quiescent cells (t = 0) or cells that were mitogenically stimulated for 18 or 24 h (indicated above individual lanes). The depleted extracts were subjected to a second immunoprecipitation with anti-cdk4, and the products of both rounds of immunoprecipitation were separated by SDS-PAGE and examined for the presence of cdk4 to determine the portion of cdk4 that was removed by this treatment. (D) Cyclin D3-associated and cdk4 kinase activities were determined in cdk4-depleted or mock-depleted extracts by incubating the immune complexes (indicated above individual lanes) with GST-Rb as a substrate. Products were separated by SDS-PAGE, and phosphorylated proteins were detected by autoradiography.
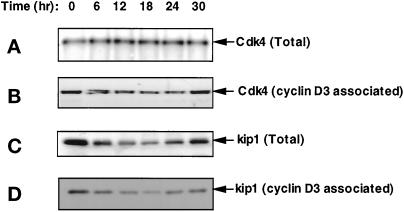
It has recently been demonstrated, however, that ectopic expression of both cyclin D1 and cdk4 does not result in the formation of an active complex primarily as a result of a failure of the subunits to associate in the absence of serum factors (Cheng et al., 1998). To examine the possibility that the lack of cyclin D3-associated activity was due to the absence of a catalytic subunit, the in vivo association of cyclin D3 with cdk4 was evaluated in extracts derived from quiescent cells and those mitogenically stimulated for various lengths of time. Figure 3A reveals that the amount of cdk4 protein remained constant throughout the course of the experiment. In addition, examination of cyclin D3-immune complexes for cdk4 protein revealed the amount of cdk4 associated with cyclin D3 also remained proportional throughout the cell cycle (Figure 3B), indicating that the increased cyclin D3-associated activity was not the result of increased amount of binary complexes. Since the total level of p27kip1 was inversely proportional to the level of cyclin D3 activity, we also determined the amount of p27kip1 associated with cyclin D3. The results, shown in Figure 3, C and D, revealed that cyclin D3-associated p27kip1 declined upon mitogenic stimulation, reaching a minimum in the interval in which cyclin D3 activity appeared. Thus, the amount of p27kip1 associated with cyclin D3 was inversely proportional to the cyclin D3 Rb kinase activity. Taken together, the data in Figures 1–3 support the hypothesis that p27kip1 plays a role in the regulation of the activity of cyclin D3/cdk4.
Figure 3.
The level of total and cyclin D3- associated cdk4 does not fluctuate during cell cycle traverse, whereas the level of p27kip1 does. Quiescent, density-arrested cultures were mitogenically stimulated by the addition of fresh DMEM supplemented with 10% CS and 10 ng/ml of PDGF-BB and harvested at the times indicated. Cell extracts containing 40 μg of total proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with cdk4 antibody (panel A), and p27kip1 antibody (panel C). Parallel samples were subjected to immunoprecipitation with cyclin D3 antibody-linked beads. Immunoprecipitates were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with cdk4 antibody (panel B) or p27kip1 antibody (panel D), respectively.
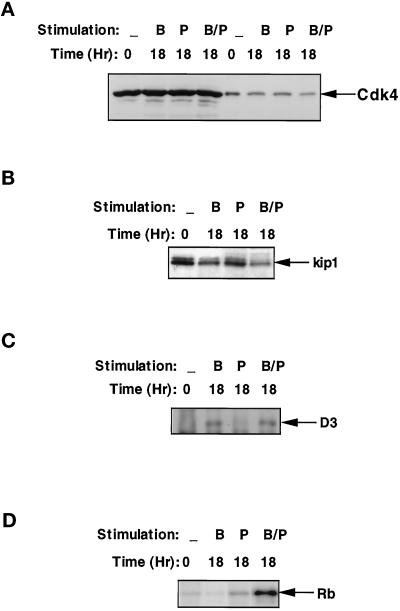
PDGF does not bring about cell cycle traverse of BALB/c-3T3 cells unless growth factors found in platelet-poor plasma (PPP) are also present; however, PDGF does bring about a reduction in the amount of p27kip1 (Agrawal et al., 1996; Winston et al., 1996). To determine whether PDGF was sufficient to cause a reduction in the amount of p27kip1 associated with cyclin D3, we examined cultures treated for 18 h with PDGF, 10% PPP, or PDGF + 10% PPP for the level of cdk4 and p27kip1. All three treatments resulted in cultures that exhibited similar levels of total cdk4 protein and cyclin D3-associated cdk4 (Figure 4A), clearly indicating the absence of activity was not the result of a failure to assemble cyclin D3/cdk4 complexes. In addition, cultures stimulated with PDGF, either in the presence or in the absence of PPP, exhibited a reduced level of p27kip1 protein (Figure 4B). Consistent with this reduction, the portion of cyclin D3 protein that was not complexed to p27kip1, as determined by Western blotting of cell extracts that had been immunodepleted of p27kip1 (Figure 4C), differed dramatically between quiescent and mitogenically stimulated cells. In extracts prepared from quiescent cells and cells treated with PPP alone, cyclin D3 was quantitatively removed by immunodepletion of p27kip1, while similar levels of cyclin D3 were present in p27kip1-immunodepleted extracts prepared from cells treated with PDGF or with PDGF + 10% PPP. Cyclin D3 activity, however, was observed only in cultures treated with PDGF in the presence of PPP (Figure 4D), supporting the hypothesis that the removal of p27kip1 from the cyclin D3 complex is required, but not sufficient, for activation of the cyclin D3/cdk4 Rb kinase. Thus, the induction of cyclin D3- associated Rb kinase activity during the mitogenic stimulation of BALB/c 3T3 cells appears to result from a two-step activation. The first step is the removal of p27kip1 from the ternary p27kip1/cyclin D3/cdk4 complex, which is under the control of PDGF. The second step is the activation of the cyclin D/cdk4 complex, which is dependent on the presence of factors in PPP or regulated by traverse of the cell cycle.
Figure 4.
p27kip1 is not the only factor that can affect the activity of cyclin D3-dependent kinase. Density-arrested BALB/c 3T3 cultures were stimulated with DMEM supplemented with 10 ng/ml PDGF alone (lanes denoted with “B”), 10% PPP alone (lanes denoted with “P”), or 10 ng/ml PDGF and 10% PPP (lanes denoted with “B/P”) for 18 h. Cell extracts were prepared from each culture, and portions containing 40 μg of protein were utilized for all the following experiments: (A) Determination of the level of cdk4 protein. The total amount of cdk4 was determined with cell extracts (lanes 1–4), while the cyclin D3-bound cdk4 was determined by separating cyclin D3-immune complexes prepared from parallel samples (lanes 5–8). (B) Determination of the level of total p27kip1 protein. (C) Determination of the amount of cyclin D3 that was not complexed with p27kip1. Cell extracts were first immunodepleted for p27kip1, and the resulting supernatents were subjected to immunoprecipitation with cyclin D3 antibodies. The cyclin D3-immune complexes were separated by SDS-PAGE and immunoblotted with a cyclin D3 antibody. (D) Rb kinase activity associated with cyclin D3-immune complexes. Cyclin D3-immune complexes were prepared and utilized for in vitro kinase assays with a GST-Rb substrate. Phosphorylated proteins were separated on SDS-PAGE and visualized by autoradiography.
p27kip1-associated Rb Kinase Remains Elevated Throughout the Cell Cycle
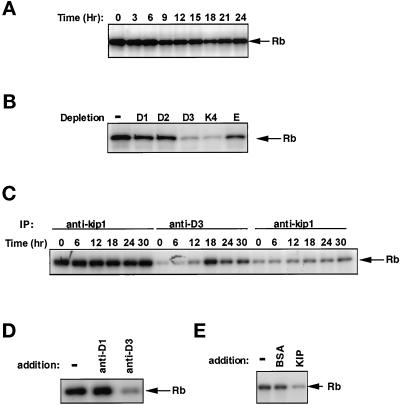
Recently, p27kip1-immune complexes were shown to have Rb-associated kinase activity in hematopoetic (Soos et al., 1996) and epithelial (Hauser et al., 1997) cells. We detected p27kip1-specific kinase activity in BALB/c 3T3 fibroblasts extracts by assaying p27kip1-immune complexes for phosphotransferase activity with GST-Rb as substrate. As shown in Figure 5A, a p27kip1 Rb kinase was present in quiescent cells and continued to be detectable in mitogenically stimulated cells, although this activity appeared to decrease somewhat after 9–12 h of stimulation. To determine the cyclin on which p27kip1 kinase activity was dependent, we immunodepleted either cyclin D1, D2, D3, cdk4, or cyclin E and tested the immunodepleted extracts for the presence of p27kip1 Rb kinase activity (Figure 5B). These experiments revealed that the p27kip1 Rb kinase was most dependent on cyclin D3 and cdk4 (Figure 5B).
Figure 5.
p27kip1-associated kinase activity in BALB/c 3T3 fibroblasts. Quiescent, density-arrested cells were mitogenically stimulated by the addition of fresh DMEM supplemented with 10% CS and 10 ng/ml PDGF-BB, and then harvested at the times indicated. (A) Cell extracts containing 40 μg of total proteins were subjected to immunoprecipitation with p27kip1 antibody-linked beads. The resulting immune complexes were tested for the presence of kinase activity in vitro with a GST-Rb substrate. Phosphorylated GST-Rb was separated on 11% SDS-PAGE and visualized by autoradiography. (B) Equal portions, containing 40 μg protein, of an extract prepared from quiescent cells were subjected to immunodepletion with cyclin D1, D2, and D3, Cdk4, or cyclin E antibody-linked beads (indicated above individual lanes). The immunodepleted cell extracts were subjected to immunoprecipitation with p27kip1 antibody-linked beads, and immunoprecipitates were used to measure in vitro kinase activity using GST-Rb as a substrate. Phosphorylated GST-Rb was separated on 11% SDS-PAGE and visualized by autoradiography. (C) Cell extracts were subjected to immunoprecipitation with p27kip1 antibody (anti-kip, left) and cyclin D3 antibody (anti-D3). After centrifugation, the supernatants without cyclin D3 were subject to second immunoprecipitation with p27kip1 antibody (anti-kip, right). Immunoprecipitates were used to measure in vitro kinase activity using GST-Rb as a substrate. Phosphorylated GST-Rb was separated on 11% SDS-PAGE and visualized by autoradiography. (D) Immune complexes prepared with p27kip1 antibody and a quiescent cell extract were tested for their response to the addition of antibodies specific for cyclin D1 and cyclin D3. Immunoprecipiates were incubated in the presence or absence of 1 μl of monoclonal cylin D1 or D3 antibodies (as indicated above individual lanes) for 30 min in 250 μl of the lysis buffer, washed twice with the lysis buffer, and used to measure in vitro kinase activity using GST-Rb as a substrate. Phosphorylated GST-Rb was separated on 11% SDS-PAGE and visualized by autoradiography. (E) The cyclin D3-associated Rb kinase activity is sensitive to inhibition by p27kip1. Cyclin D3-immune complexes, prepared from stimulated cell extracts, were incubated at 37°C without any addition, with the addition of 100 ng BSA, or the addition of 100 ng recombinant His6-p27kip1. After incubation, each sample was washed once in lysis buffer and then washed once in kinase buffer before GST-Rb kinase activity was measured as described above.
To examine whether the dependency of p27kip1 kinase activity on cyclin D3 was periodic, p27kip1 activity was determined with or without prior cyclin D3 depletion in extracts prepared from quiescent cells and cells mitogenically stimulated for various times up to 18 h. Each extract was divided into two portions: the first utilized to measure p27kip1 Rb kinase activity (Figure 5C, lanes 1–6) and the second to measure cyclin D3 kinase activity (Figure 5C, lanes 7–12). In addition, the second group of extracts was assayed for p27kip1 activity after removal of cyclin D3 (Figure 5C, lanes 13–18). In accord with the results shown above, the Rb kinase activity associated with p27kip1 remained relatively constant, while the cyclin D3 Rb kinase activity was not observed until 12 h of mitogenic stimulation. In addition, the p27kip1 activity was uniformly removed by cyclin D3 immunodepletion throughout the cell cycle. Note that the dependence of p27kip1 activity on cyclin D3/cdk4 was observed (lanes 13–15) even though no cyclin D3 activity could be detected in cells until 12 h after stimulation (lane 13/14).
From the results presented above, it is clear that untreated cells and cells treated with PPP contained a cyclin D3 population that is entirely bound to p27kip1 since immunodepletion with p27kip1-specific antibody completely removed cyclin D3 (Figure 4C). However, cells treated with PDGF in the presence or absence of PPP had, in addition, a distinct pool of cyclin D3 that was not associated with p27kip1. Since cyclin D3 immunodepletion resulted in the removal of p27kip1-associated kinase activity, the p27kip1 Rb kinase must represent the former pool. The second pool of cyclin D3, not associated with p27kip1, is responsible for the cyclin D3 activity, since cyclin D3 activity can be recovered from p27kip1-depleted extracts prepared from stimulated cells. Because the p27kip1 Rb kinase is present and dependent on cyclin D3 in quiescent cells, when cyclin D3 kinase cannot be detected (Figure 5A), we determined whether the addition of cyclin D3 antibodies to p27kip1-immune complexes could mask the Rb kinase activity (Figure 5D). The results demonstrate that inhibition of Rb kinase activity resulted from the addition of an antibody to cyclin D3 but not from the addition of an antibody to cyclin D1 and suggest that the function of cyclin D3 in a ternary p27kip1/cyclin D3/cdk4 complex is extremely sensitive to the steric constraints imposed by antibody binding. The effect of anticyclin D3 antibody-directed inhibition was not specific for the antiserum that is shown, and similar results were also obtained with a monoclonal antibody to cyclin D3 (our unpublished observations). In addition, it is interesting to note that when recombinant p27kip1 was added to active cyclin D3/cdk4 complexes prepared from stimulated cells, the activity was greatly diminished (Figure 5E), demonstrating the active cyclin D3/cdk4 complex can be inhibited by p27kip1 in vitro.
P27KIP1 Alters the Cyclin D3/cdk4 Rb Substrate Specificity
Our data reveal that cyclin D3/ckd4-dependent kinase activity in mitogenically stimulated cells resided in two separate pools: one pool was not associated with p27kip1, and one pool clearly was associated with p27kip1. The cyclin D3/cdk4 activity that was not associated with p27kip1 could be readily immunoprecipitated and measured with an antibody to cyclin D3. The second pool of cyclin D3/cdk4 was associated with p27kip1 and did not display Rb kinase activity when immunoprecipitated or treated with antibodies to cyclin D3; however, this complex did display Rb kinase activity when prepared with an antibody to p27kip1. The data in Figure 5D indicated that the association of p27kip1 with the cyclin D3/cdk4 complex resulted in a structural alteration such that the presence of cyclin D3 antibodies inhibited kinase activity. To investigate whether p27kip1 association may have functional consequences, we sought to determine whether the association of p27kip1 with the cyclin D3/Cdk4 complex altered the in vitro substrate specificity with an Rb substrate.
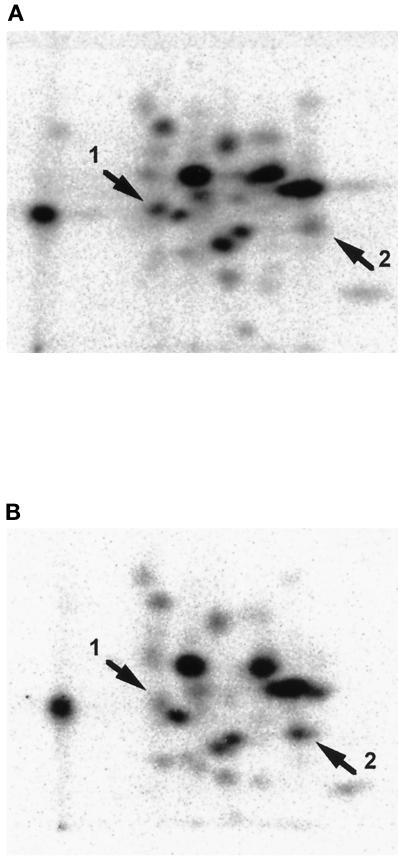
Extracts prepared from density-arrested cells and from cells that had been mitogenically stimulated for 18 h were used to isolate p27kip1- and cyclin D3-immune complexes, respectively, and these complexes were used to phosphorylate Rb in vitro. The phosphorylated Rb product from each reaction was analyzed by tryptic phosphopeptide mapping to compare the sites of phosphorylation. Figure 6 shows a comparison of the phosphopeptide maps obtained with p27kip1-phosphorylated Rb (Figure 6A) and the cyclin D3-phosphorylated Rb (Figure 6B). The arrows in each panel indicate peptides that are differentially phosphorylated by the two complexes. Arrow 1 indicates a phosphopeptide that is greatly diminished in the phosphopeptide map from cyclin D3/cdk4-phosphorylated Rb, while arrow 2 indicates a phosphopeptide present in cyclin D3/cdk4-phosphorylated Rb (arrow 2) that is diminished in the Rb phosphorylated by the p27kip1/cyclin D3/cdk4 activity (Figure 6A). These data clearly demonstrate that the association of p27kip1 with the cyclin D3/cdk4 altered the phosphorylation specificity, as determined by a change in the relative level of phosphorylation at two sites. The observation that one peptide was preferentially phosphorylated by each kinase complex indicated that the alteration was indeed related to specificity and not simply a consequence of reduced activity. It is unlikely that the difference in the specificity could be explained by the presence of a minor contaminating kinase species since immunodepletion of cdk4 results in the removal of both p27kip1-associated (Figure 5B) and cyclin D3-associated (Figure 2C) activity. The differences in the phosphorylation specificity between p27kip1/cyclin D3/cdk4 and cyclin D3/cdk4 were studied further in a second type of assay utilizing peptides with sequences derived from Rb protein. Figure 7 shows the phosphorylation of these individual peptides by the two different complexes. In the active complex containing p27kip1, peptide G is utilized as a substrate more than twice as efficiently as peptide J, while in the active cyclin D3 complex (without p27kip1), peptides G and J are equally active as substrates.
Figure 6.
Tryptic phosphopeptide analysis of substrate specificities of p27kip1-associated kinase and cyclin D3-associated kinase. Immune complexes prepared with p27kip1 antibody and a quiescent cell extract (A) and immune complexes prepared with cyclin D3 antibody and a stimulated cell extract (B) were utilized for in vitro phosphorylation of GST-Rb. Phosphorylated GST-Rb was purified with SDS-PAGE, digested with trypsin, separated on cellulose TLC plates, and visualized by autoradiography, as described in MATERIALS AND METHODS. To allow quantitative comparison of phosphopeptide maps, exposures were also performed with a Molecular Dynamics PhosphorImager. Arrowheads indicate peptides that were differentially phosphorylated by the two immune complexes.
Figure 7.
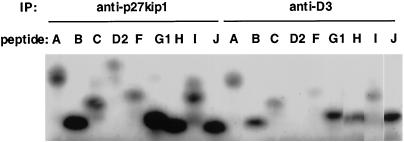
Phosphorylation of synthetic peptides, derived from Rb sequences, by cyclin D3/cdk4 and cyclin D3/cdk4/p27kip1. Immune complexes prepared as outlined in the legend for Figure 6 were prepared and utilized to phosphorylate synthetic peptides in vitro. The peptide nomenclature corresponds to that reported previously (Kitagawa et al., 1996). Phosphorylated peptides were separated on a 16.5% T/6% C Tricine-SDS-polyacrylamide gel and visualized by autoradiography as described in MATERIALS AND METHODS. To allow quantitative comparisons, dried gels were also exposed on a Molecular Dynamics PhosphorImager, and the resulting images were analyzed with ImageQuant software.
Restoration of in Vitro p27kip1Rb Kinase Activity in Fibroblasts Derived from p27kip1−/− Mice Using Recombinant p27kip1
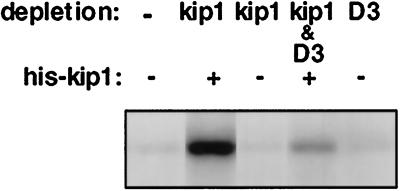
To test whether p27kip1 complexes containing cyclin D3/cdk4 could be formed by the addition of recombinant p27kip1 protein, we utilized dermal fibroblasts isolated from newborn mice with a kip−/− phenotype (Kiyokawa et al., 1996). Density-arrested cultures were mitogenically stimulated with fresh medium containing 10% CS and PDGF (10 ng/ml). Extracts prepared from stimulated p27kip1−/− cells were cleared of possible p27kip1 cross-reacting materials and were then mixed with recombinant p27kip1 protein. After a 30-min incubation at 37°C, the exogenous p27kip1 protein was recovered by immunoprecipitation and assayed for p27kip1 Rb kinase activity. The data shown in Figure 8 demonstrate that incubation of p27kip1 in a cell extract containing active cyclin D3 complexes can result in the formation of a p27kip1 Rb kinase activity in a cyclin D3-dependent manner.
Figure 8.
Restoration of p27kip1 kinase activity with recombinant p27kip1. Dermal fibroblasts were isolated from newborn mice with a kip−/− phenotype (Koff et al., 1996). Density-arrested cultures were stimulated with fresh medium containing 10% CS serum and 10 ng/ml PDGF for 20 h. Cell extracts containing 40 μg of protein were subjected to immunodepletion with p27kip1, cyclin D3, p27kip1 plus cyclin D3 antibodies, or used without any further treatment. Immunodepleted cell extracts were incubated in the presence or absence of recombinant kip protein for 30 min at 30°C and immunoprecipitated with p27kip1 antibody-linked beads. Immunoprecipitates were analyzed for in vitro kinase activity using GST-Rb as a substrate. Phosphorylated proteins were separated by SDS-PAGE and visualized by autoradiography.
DISCUSSION
The data presented in this report demonstrate the cell cycle regulation of cdk4 activity by cyclin D3. The control of cdk4 activity through cyclin D1 has been extensively studied (Xiong et al., 1991; Matsushime et al., 1992, 1994; Won et al., 1992; Baldin et al., 1993; Quelle et al., 1993; Winston and Pledger, 1993); however, there is considerably less work on its control through the additional d-type cyclins D2 and D3, which are also present in a variety of cell types (Kato and Sherr, 1993; Meyerson and Harlow, 1994; Polyak et al., 1994a; Hauser et al., 1997). We have shown that cyclin D3 protein was present in both growing and nongrowing BALB/c 3T3 cells, although D3-associated Rb kinase activity was only detectable in growing cells. This observation provides a marked contrast with cyclin D1, which is absent in quiescent cells and accumulates upon entering the growth cycle, correlating with increased activity (Agrawal et al., 1996; Winston et al., 1996). By examining cyclin D3 complexes for the presence of catalytic and inhibitory subunits, we have demonstrated that the periodic activity can be explained, in part, by the accumulation of p27kip1 in quiescent cells that binds quantitatively to cyclin D3/cdk4 complexes. Once inactivated in this manner, removal of p27kip1 is not sufficient to regain activity, which is accomplished only under culture conditions that promote S-phase entry. Curiously, while the cyclin D3/cdk4 complexes isolated from quiescent cells do not exhibit in vitro kinase activity, the corresponding p27kip1-immune complexes do exhibit in vitro activity. Furthermore, this activity is dependent on cyclin D3/cdk4 and can thus be depleted with antibodies to either protein. These data suggested that p27kip1 may not only inhibit the cyclin D3/cdk4 complexes per se, but also modify the activity. This hypothesis was confirmed using both a recombinant Rb substrate as well as peptide substrates derived from Rb sequences known to be phosphorylated by cdk4. In both types of assays, p27kip1 complexes derived from quiescent cells and dependent on cyclin D3/cdk4 for activity were found to exhibit different phosphorylation specificities than the active cyclin D3/cdk4 complexes isolated from growing cells. Thus, these data are consistent with the notion that cyclin D3/cdk4 activity may serve dual functions during cell cycle traverse, the first in a binary form that is present during growth and a second function in a ternary complex with p27kip1 that modifies its substrate specificity.
Although periodic cyclin D1 activity has been demonstrated to primarily result from periodic cyclin D1 synthesis, it is clear from our data that cyclin D3 is present throughout the growth cycle in BALB/c 3T3 cells. Furthermore, we have found that the association of cyclin D3 with cdk4 also remains constant and thus can explain neither the loss of activity in quiescent cells nor the appearance of activity upon subsequent mitogenic stimulation. In contrast, the periodic association of p27kip1 with cyclin D3 renders this cdk inhibitor a likely candidate for mediating regulation of cyclin D3 activity. The Kip/Cip family of proteins was initially described as proteins capable of inhibiting both cdk2 and cdk4 (Polyak et al., 1994b; Toyoshima and Hunter, 1994). Recent studies, however, directed at a more detailed understanding of the role of p27kip1 in regulation of d-cyclin/cdk4 activity, have provided data that, if not conflicting, remain to be reconciled. In accord with our results, Kato et al. (1997) found that cyclin D1/cdk4 complexes found in serum-starved RAT-1 cells were inactive due to associated p27kip1, primarily resulting in complexes that did not undergo CAK-mediated cdk4 phosphorylation, a property that was previously described for p27kip1 in vitro (Matsuoka et al., 1994). Furthermore, while both p27kip1 and p21Cip1 are active in promoting the assembly of cyclin D/cdk4 complexes, those complexes formed by p21Cip1 resulted in complexes with kinase activity, whereas those formed by p27kip1 did not (LaBaer et al., 1997). The latter observation is again consistent with the notion that p27kip1 is a physiological inhibitor of cdk4. Other reports, however, have questioned the ability of p27kip1 to effect inhibition under physiological conditions, demonstrating active cyclin D/cdk4 activity in the presence of micromolar concentrations of p27kip1 (Blain et al., 1997). Together, these findings leave the role of p27kip1 in the regulation of cyclin D/cdk4 complexes unclear. Our experiments indicate that the amount of cyclin D3/cdk4 activity in BALB/c-3T3 cells is inversely correlated with amount of p27kip1 bound to cyclin D3/cdk4 (Figures 1 and 2), and we demonstrated that the inhibitory effect of p27kip1 could be observed in vitro by addition of recombinant p27kip1 protein to cyclin D3 complexes (Figure 5E). Our data, therefore, support the hypothesis that p27kip1 is a physiological cdk4 inhibitor, at least in the case of cyclin D3. We note that much of the work reporting the effect of p27kip1 on cdk4 activity has primarily utilized cyclin D1 and cyclin D2 (Polyak et al., 1994a; Blain et al., 1997; Kato et al., 1997; LaBaer et al., 1997; Reynisdottir and Massague, 1997). Thus, our results may also indicate a difference in the specificity of p27kip1 with respect to a particular cyclin subunit.
Previous studies showed that both PDGF and PPP are required for stimulation of BALB/c 3T3 cells to enter the cell cycle, and our results indicate that activation of cyclin D3/cdk4 requires both mitogenic components. While it has been established that PDGF is sufficient to result in a reduction of the total level of p27kip1 (Agrawal et al., 1996; Winston et al., 1996), we demonstrate here that PDGF stimulation also results in the removal of p27kip1 from cyclin D3 complexes. However, p27kip1-free cyclin D3/Cdk4 complexes generated by PDGF treatment alone were not active and, therefore, we concluded that a PPP-dependent factor(s) or cell cycle progression was required for activating the cyclin D3/cdk4 complex. This is consistent with the finding that CAK is required for activating cyclin D complexes after removal of p27kip1 (Kato et al., 1997). However, since CAK has been shown to have constitutive activity (Matsuoka et al., 1994), the lack of activity in the cyclin D3/cdk4 complexes that are free of p27kip1 suggests that another cell cycle-regulated event must be required to activity the cyclin D3/cdk4 complex at the appropriate time within the traverse of G1.
p27kip1-associated kinase activity that could phosphorylate pRb but not histone H1 was first described in MANCA cells, a hematopoetic B-cell line (Soos et al., 1996). The MANCA p27kip1 kinase activity appeared to be dependent on cdk6, since a cdk6-specific antibody was able to deplete 50% of the activity. Therefore, it was proposed that p27kip1-associated kinase activity was derived from cdk6 and that p27kip1 was incapable of inhibiting the cyclin D/cdk6. Since cdk6 has not been found in BALB/c 3T3 mouse fibroblasts and since there is a constant high p27kip1-associated Rb kinase activity in the cell throughout the cell cycle, we explored the derivation of the activity. In our experiments, more than 70% of the p27kip1 Rb kinase activity could be depleted by cyclin D3-specific and cdk4-specific antibodies, but was not affected by depletion with D1, D2, or E-specific antibodies (Figure 5B). Therefore, in BALB/c-3T3 fibroblasts, p27kip1-associated kinase activity is derived from cyclin D3/cdk4 and not cdk6. Interestingly, in quiescent cells we could measure kinase activity in p27kip1-immune complexes but not in cyclin D3-immune complexes. Further experiments indicated that the binding of a cyclin D3-specific antibody itself partially inhibited the kinase activity of the p27kip1 immunocomplex, suggesting that a conformational change may occur upon the binding of p27kip1 to cyclin D3/cdk4 (Figure 5D). It is possible that the sensitivity of the ternary complex to inhibition by cyclin D3 antibodies could also explain the loss of activity we observed after addition of recombinant p27kip1 protein to cyclin D3-immune complexes, although we note that several studies have demonstrated the inhibition of D-cyclin-dependent cdk4 activity in assays with purified proteins.
Because the p27kip1 Rb kinase activity did not change while the amount of ternary complexes decreased substantially (see Figures 3 and 4), we propose that only a portion of the complex (p27kip1/cyclin D3/cdk4) is an active kinase in quiescent cells. This decline in the amount of ternary complexes is most likely a reflection of the reduced level of p27kip1 that occurs after mitogenic stimulation since the level of cdk4 and cyclin D3 remained relatively constant under these conditions. We note that these data could also be explained by a decreased specific activity of ternary complexes present in quiescent cells as compared with ternary complexes present in mitogenically stimulated cells. This latter possibility would nonetheless require an additional modification to convert the lower-specific activity form to the higher-specific activity form. The reaction required to activate this ternary complex is not known and may represent a novel activation mechanism, since it is known that CAK is unable to phosphorylate cdk4 complexes containing p27kip1 (Matsuoka et al., 1994).
In light of the role of p27kip1 as a growth suppressor, it might be expected that a p27kip1-associated kinase would have different characteristics than a binary cyclin D3/cdk4 complex. To address whether such a difference might be detected as an alteration in substrate specificity, we performed tryptic phosphopeptide mapping of in vitro phosphorylated Rb and also compared catalytic efficiency with a panel of peptide substrates. We demonstrated that p27kip1-associated kinase has a different substrate specificity than that of cyclin D3-dependent kinase in experiments using either an Rb substrate or peptides as substrates (Figures 6 and 7). The results from both types of experiments demonstrated an alteration in phosphorylation patterns between p27kip1/cyclin D3/cdk4 and cyclin D3/cdk4 complexes, indicating that the binding of p27kip1 can result not only in inhibition of cdk activity but also modify the target specificity.
ACKNOWLEDGMENTS
This work was supported by National Cancer Institute/National Institutes of Health grant CA-67360 and the Cortner Couch Endowed Chair for Cancer Research. We would like to acknowledge the assistance of the H. Lee Moffitt Cancer Center Flow Cytometry Core and Molecular Imaging Core. We would also like to thank Rebecca Koransky for excellent editorial assistance.
REFERENCES
- Agrawal D, Dong F, Wang YZ, Kayda D, Pledger WJ. Regulation of cyclin E and p27kip during mitosis in BALB/c 3T3 cells. Cell Growth Differ. 1995;6:1199–1205. [PubMed] [Google Scholar]
- Agrawal D, Hauser P, McPherson F, Dong F, Garcia A, Pledger WJ. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol Cell Biol. 1996;16:4327–4336. doi: 10.1128/mcb.16.8.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- Booher RN, Alfa CE, Hyams JS, Beach DH. The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell. 1989;58:485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27(Kip1) regulated by mitogen-activated protein kinase kinase [In Process Citation] Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Dong F, Cress WD, Jr, Agrawal D, Pledger WJ. The role of cyclin D3-dependent kinase in the phosphorylation of p130 in mouse BALB/c 3T3 fibroblasts. J Biol Chem. 1998;273:6190–6195. doi: 10.1074/jbc.273.11.6190. [DOI] [PubMed] [Google Scholar]
- Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989;56:829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Ducommun B, Brambilla P, Felix MA, Franza BR, Jr, Karsenti E, Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991;10:3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Elledge SJ, Harper JW. Cdk inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Fisher RP, Morgan DO. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994;78:713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Gould KL, Moreno S, Owen DJ, Sazer S, Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991;10:3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger JA, Wittenberg C, Richardson HE, de Barros Lopes M, Reed SI. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci USA. 1989;86:6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I, Hayles J, Nurse P. Cloning and sequencing of the cyclin-related cdc13+ gene and a cytological study of its role in fission yeast mitosis. J Cell Sci. 1988;91:587–595. doi: 10.1242/jcs.91.4.587. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harper JW, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser PJ, Agrawal D, Flanagan M, Pledger WJ. The role of p27kip1 in the in vitro differentiation of murine keratinocytes. Cell Growth Differ. 1997;8:203–211. [PubMed] [Google Scholar]
- Hengst L, Dulic V, Slingerland JM, Lees E, Reed SI. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, A., Takahashi, H., Takahashi, Y., and Matsushime, H. (1997). Contact inhibition-induced inactivation of the cyclin D-dependent kinase in rat fibroblast cell line, 3Y1. Leukemia 11(suppl 3), 361–362. [PubMed]
- Kato JY, Sherr CJ. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci USA. 1993;90:11513–11517. doi: 10.1073/pnas.90.24.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, et al. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Kato JY, Fisher RP, Morgan DO, Sherr CJ. Activation of cyclin-dependent kinase 4 (cdk4) by mouse MO15-associated kinase. Mol Cell Biol. 1994;14:7265–7275. doi: 10.1128/mcb.14.11.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ. p57kip2, a structurally distinct member of the p21cip1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, Sherr CJ. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Robetorye RS, Adami GR, Pereira-Smith OM, Smith JR. Identification of the active region of the DNA synthesis inhibitory gene p21Sdi1/CIP1/WAF1. EMBO J. 1995;14:555–563. doi: 10.1002/j.1460-2075.1995.tb07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- Picard A, Capony JP, Brautigan DL, Doree M. Involvement of protein phosphatases 1 and 2A in the control of M phase-promoting factor activity in starfish. J Cell Biol. 1989;109:3347–3354. doi: 10.1083/jcb.109.6.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994a;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994b;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Pondaven P, Meijer L, Beach D. Activation of M-phase-specific histone H1 kinase by modification of the phosphorylation of its p34cdc2 and cyclin components. Genes Dev. 1990;4:9–17. doi: 10.1101/gad.4.1.9. [DOI] [PubMed] [Google Scholar]
- Poon RY, Yamashita K, Adamczewski JP, Hunt T, Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993;12:3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Reynisdottir I, Massague J. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- Rivard N, L’Allemain G, Bartek J, Pouyssegur J. Abrogation of p27Kip1 by cDNA antisense suppresses quiescence (G0 state) in fibroblasts. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- Roberts JM. Turning DNA replication on and off. Curr Opin Cell Biol. 1993;5:201–206. doi: 10.1016/0955-0674(93)90103-w. [DOI] [PubMed] [Google Scholar]
- Schagger H, Hagen T, Roth B, Brandt U, Link TA, von Jagow G. Phospholipid specificity of bovine heart bc1 complex. Eur J Biochem. 1990;190:123–130. doi: 10.1111/j.1432-1033.1990.tb15554.x. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Solomon MJ, Glotzer M, Lee TH, Philippe M, Kirschner MW. Cyclin activation of p34cdc2. Cell. 1990;63:1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Solomon MJ, Harper JW, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos TJ, Kiyokawa H, Yan JS, Rubin MS, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- Swenson KI, Farrell KM, Ruderman JV. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986;47:861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Winston J, Dong F, Pledger WJ. Differential modulation of G1 cyclins and the Cdk inhibitor p27kip1 by platelet-derived growth factor and plasma factors in density-arrested fibroblasts. J Biol Chem. 1996;271:11253–11260. doi: 10.1074/jbc.271.19.11253. [DOI] [PubMed] [Google Scholar]
- Winston JT, Pledger WJ. Growth factor regulation of cyclin D1 mRNA expression through protein synthesis-dependent and -independent mechanisms. Mol Biol Cell. 1993;4:1133–1144. doi: 10.1091/mbc.4.11.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won KA, Xiong Y, Beach D, Gilman MZ. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc Natl Acad Sci USA. 1992;89:9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin J, Nurse P. Regulating S phase: CDKs, licensing and proteolysis. Cell. 1996;85:785–787. doi: 10.1016/s0092-8674(00)81261-1. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65:691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]