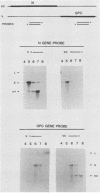
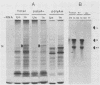
Abstract
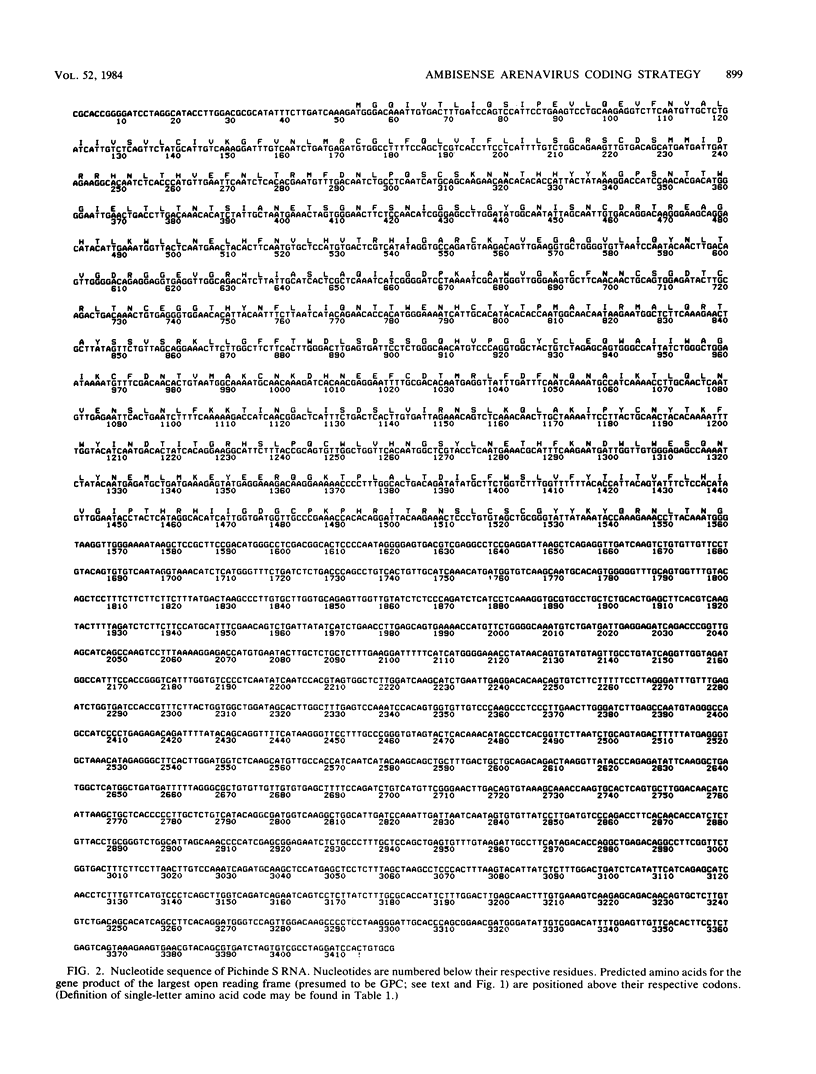
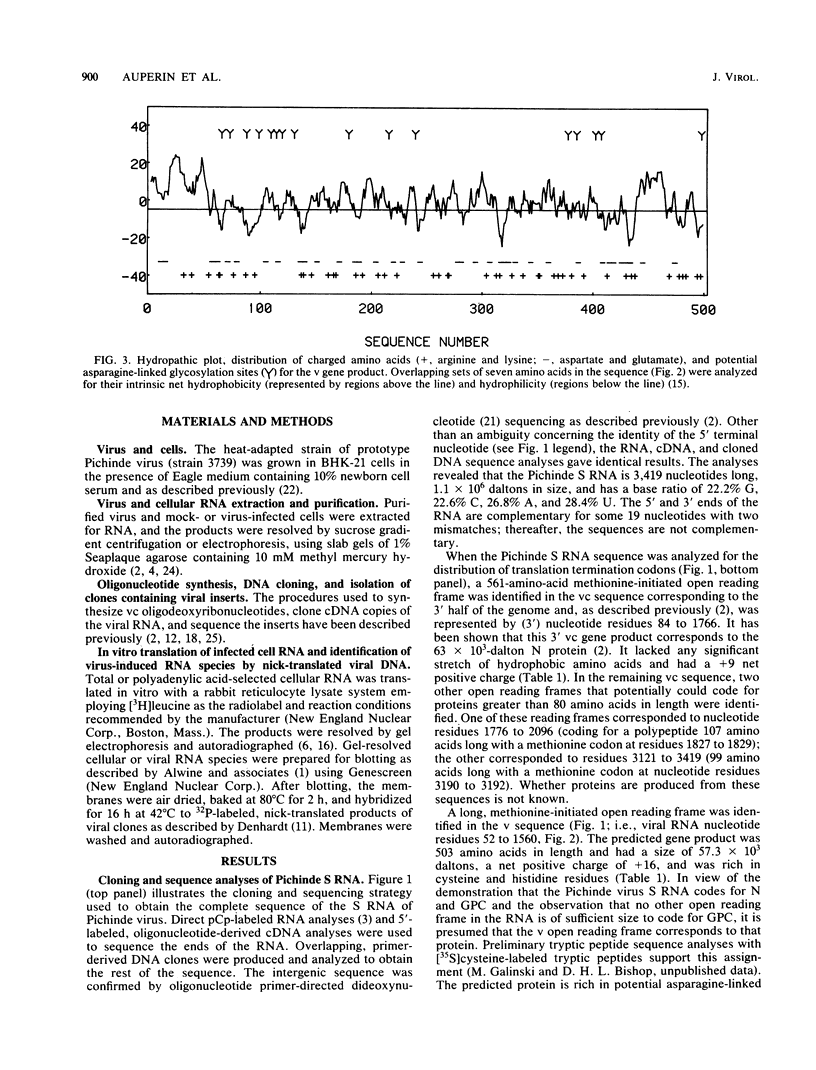
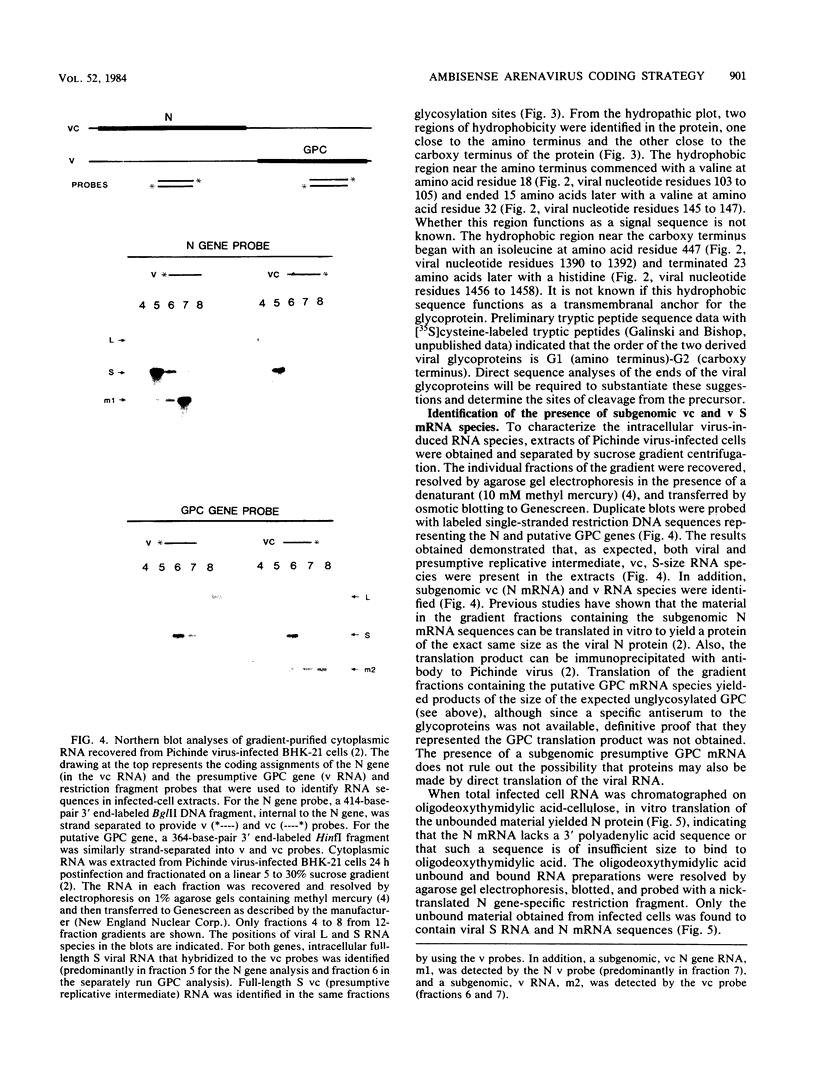
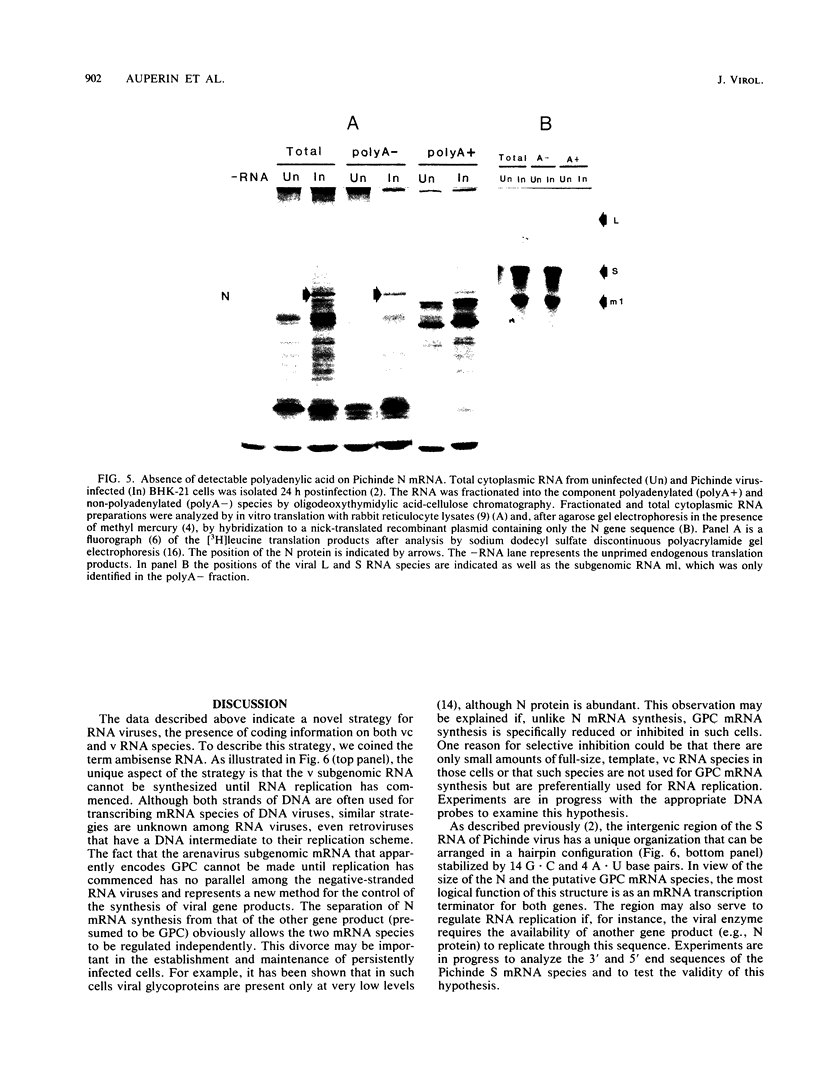
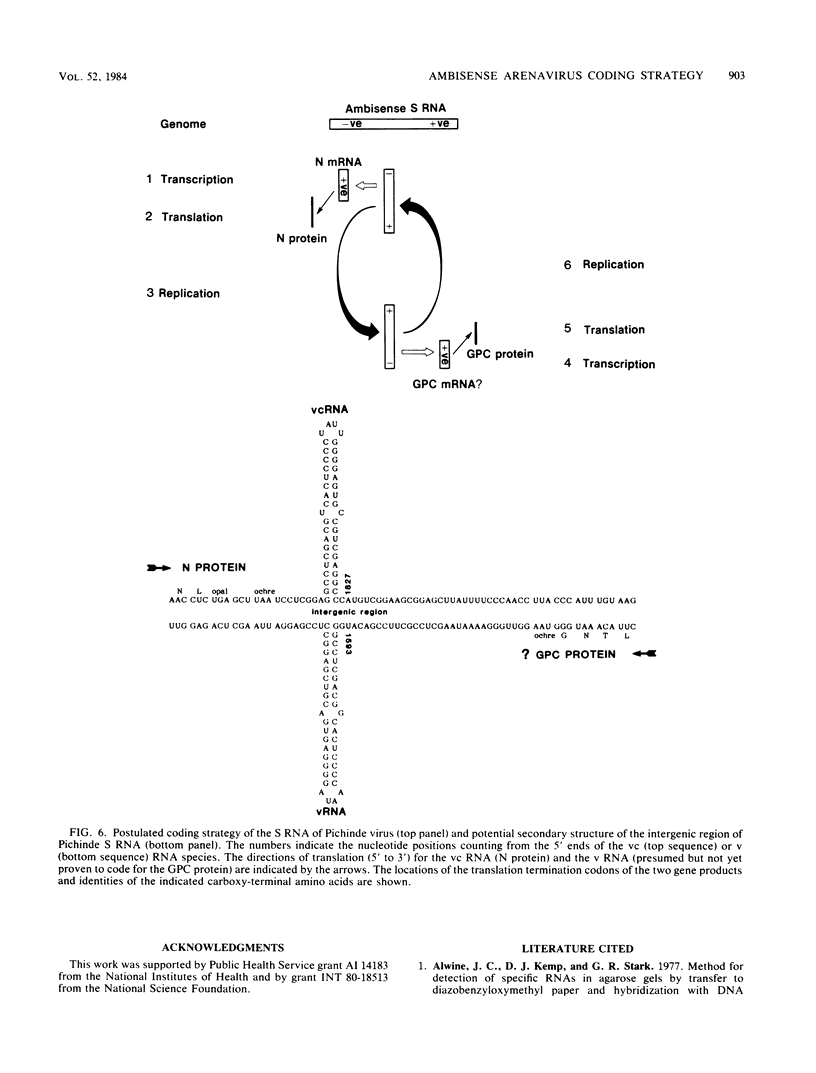
Analyses of the complete sequence of the 1.1 X 10(6)-dalton, small (S) RNA of the arenavirus Pichinde and virus-induced cellular RNA species have revealed that the viral nucleoprotein, N, is coded in a subgenomic, non-polyadenylated, virus-complementary mRNA corresponding to the 3' half of the viral RNA (Auperin et al., Virology 134:208-219, 1984). By contrast, a second S-coded product, presumably the viral glycoprotein precursor (GPC), is coded in a subgenomic, virus-sense mRNA corresponding to the 5' half of the RNA. Between the two genes is a unique RNA sequence that can be arranged in a hairpin configuration and may function as a transcription terminator for both genes. The term ambisense RNA is coined to describe this novel coding strategy of a viral RNA. The unique feature of the strategy is that the presumptive GPC mRNA and its translation product cannot be made until viral RNA replication has commenced. In addition, it allows the two subgenomic mRNA species to be regulated independently from each other or from other viral mRNA species. The implications of this strategy on possible mechanisms for the induction and maintenance of viral persistence, an important attribute of arenavirus infections, are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D. D., Galinski M., Bishop D. H. The sequences of the N protein gene and intergenic region of the S RNA of pichinde arenavirus. Virology. 1984 Apr 15;134(1):208–219. doi: 10.1016/0042-6822(84)90286-1. [DOI] [PubMed] [Google Scholar]
- Auperin D., Dimock K., Cash P., Rawls W. E., Leung W. C., Bishop D. H. Analyses of the genomes of prototype pichinde arenavirus and a virulent derivative of Pichinde Munchique: evidence for sequence conservation at the 3' termini of their viral RNA species. Virology. 1982 Jan 15;116(1):363–367. doi: 10.1016/0042-6822(82)90429-9. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Gould K. G., Akashi H., Clerx-van Haaster C. M. The complete sequence and coding content of snowshoe hare bunyavirus small (S) viral RNA species. Nucleic Acids Res. 1982 Jun 25;10(12):3703–3713. doi: 10.1093/nar/10.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopeptides. Virology. 1979 Nov;99(1):111–120. doi: 10.1016/0042-6822(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Fuller F. J., Marcus P. I., Hightower L. E., Ball L. A. Synthesis and processing of Sindbis virus nonstructural proteins in vitro. Virology. 1982 Apr 30;118(2):363–379. doi: 10.1016/0042-6822(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Dalton A. J., Rowe W. P., Smith G. H., Wilsnack R. E., Pugh W. E. Morphological and cytochemical studies on lymphocytic choriomeningitis virus. J Virol. 1968 Dec;2(12):1465–1478. doi: 10.1128/jvi.2.12.1465-1478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gait M. J., Singh M., Sheppard R. C., Edge M. D., Greene A. R., Heathcliffe G. R., Atkinson T. C., Newton C. R., Markham A. F. Rapid synthesis of oligodeoxyribonucleotides. IV. Improved solid phase synthesis of oligodeoxyribonucleotides through phosphotriester intermediates. Nucleic Acids Res. 1980 Mar 11;8(5):1081–1096. doi: 10.1093/nar/8.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez H. B., Compans R. W. Defective interfering Tacaribe virus and persistently infected cells. Virology. 1980 Nov;107(1):229–239. doi: 10.1016/0042-6822(80)90288-3. [DOI] [PubMed] [Google Scholar]
- Harnish D. G., Dimock K., Bishop D. H., Rawls W. E. Gene mapping in Pichinde virus: assignment of viral polypeptides to genomic L and S RNAs. J Virol. 1983 May;46(2):638–641. doi: 10.1128/jvi.46.2.638-641.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ramsingh A. I., Dimock K., Rawls W. E., Leung W. C. Size estimation of Pichinde virus RNA by gel electrophoresis under denaturing conditions. Intervirology. 1980;14(1):31–36. doi: 10.1159/000149159. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Bishop D. H. Recombination between temperature-sensitive mutants of the arenavirus Pichinde. J Virol. 1977 Nov;24(2):712–715. doi: 10.1128/jvi.24.2.712-715.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Cash P., Jahrling P., Eddy G., Bishop D. H. Arenavirus recombination: the formation of recombinants between prototype pichinde and pichinde munchique viruses and evidence that arenavirus S RNA codes for N polypeptide. Virology. 1980 Oct 30;106(2):250–260. doi: 10.1016/0042-6822(80)90248-2. [DOI] [PubMed] [Google Scholar]
- Vezza A. C., Clewley J. P., Gard G. P., Abraham N. Z., Compans R. W., Bishop D. H. Virion RNA species of the arenaviruses Pichinde, Tacaribe, and Tamiami. J Virol. 1978 May;26(2):485–497. doi: 10.1128/jvi.26.2.485-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S., Gait M. J., Brownlee G. G. The use of synthetic oligodeoxynucleotide primers in cloning and sequencing segment of 8 influenza virus (A/PR/8/34). Nucleic Acids Res. 1981 Jan 24;9(2):237–245. doi: 10.1093/nar/9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]