Abstract
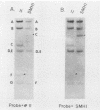
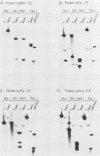
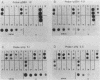
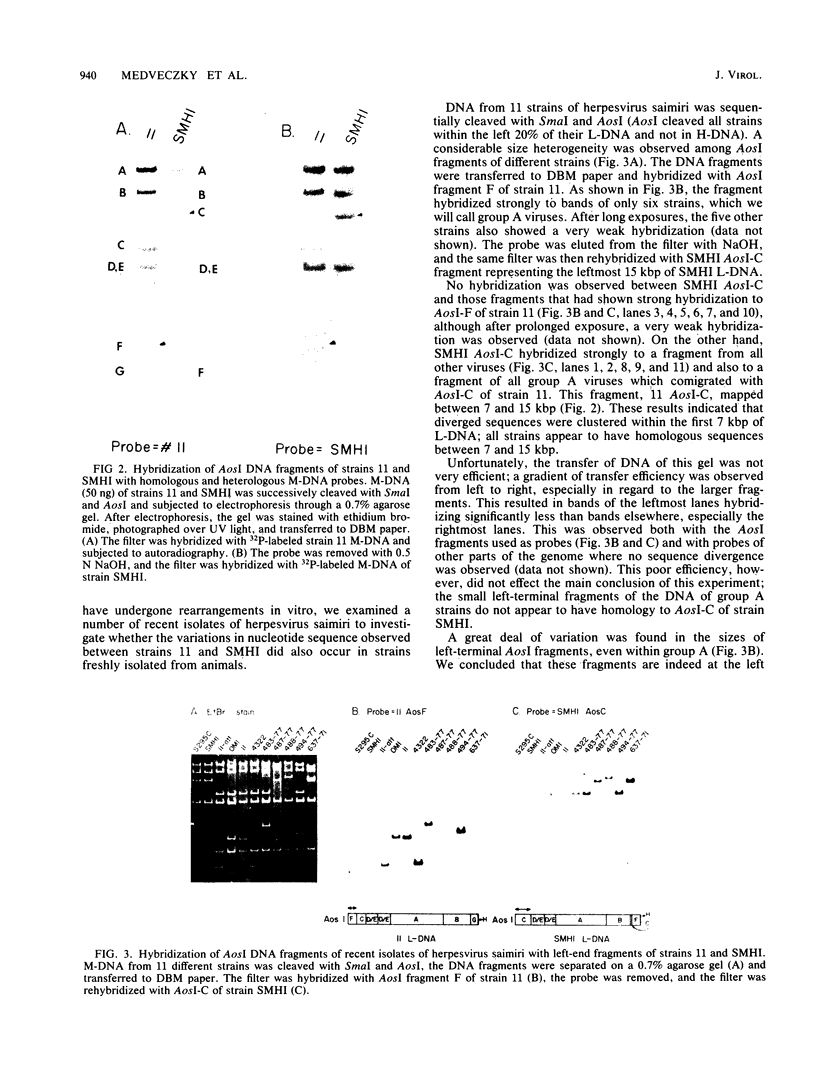
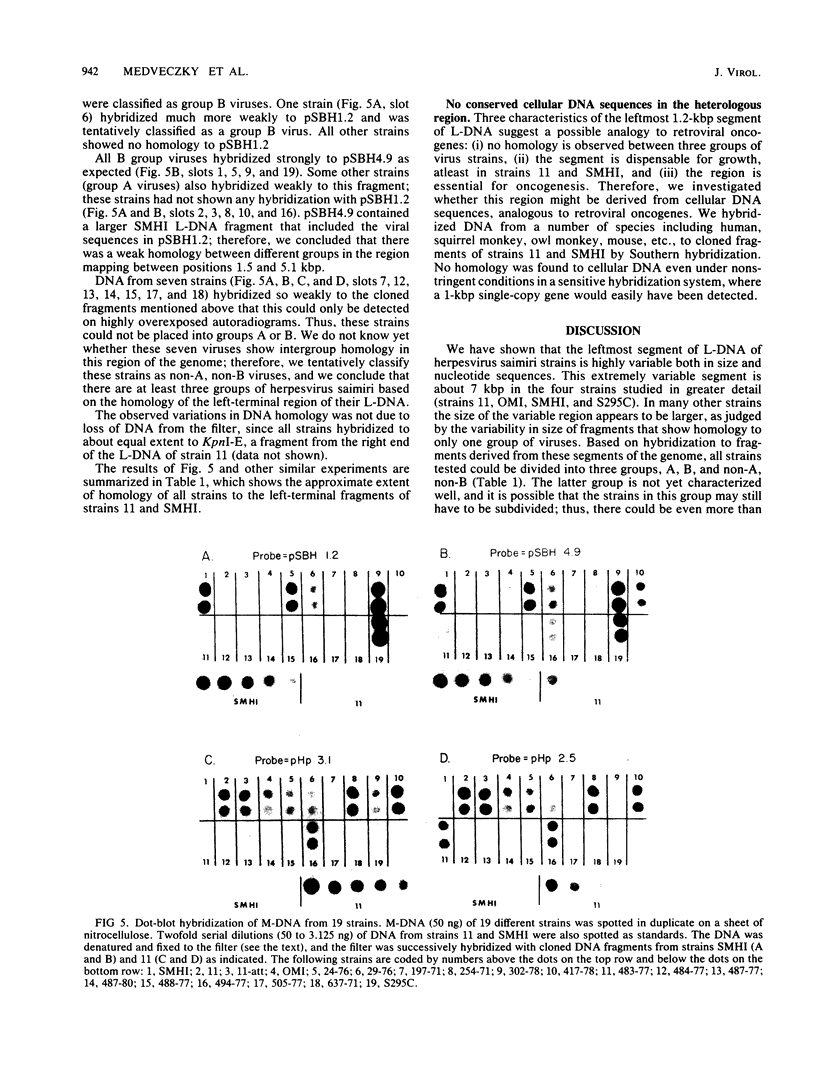
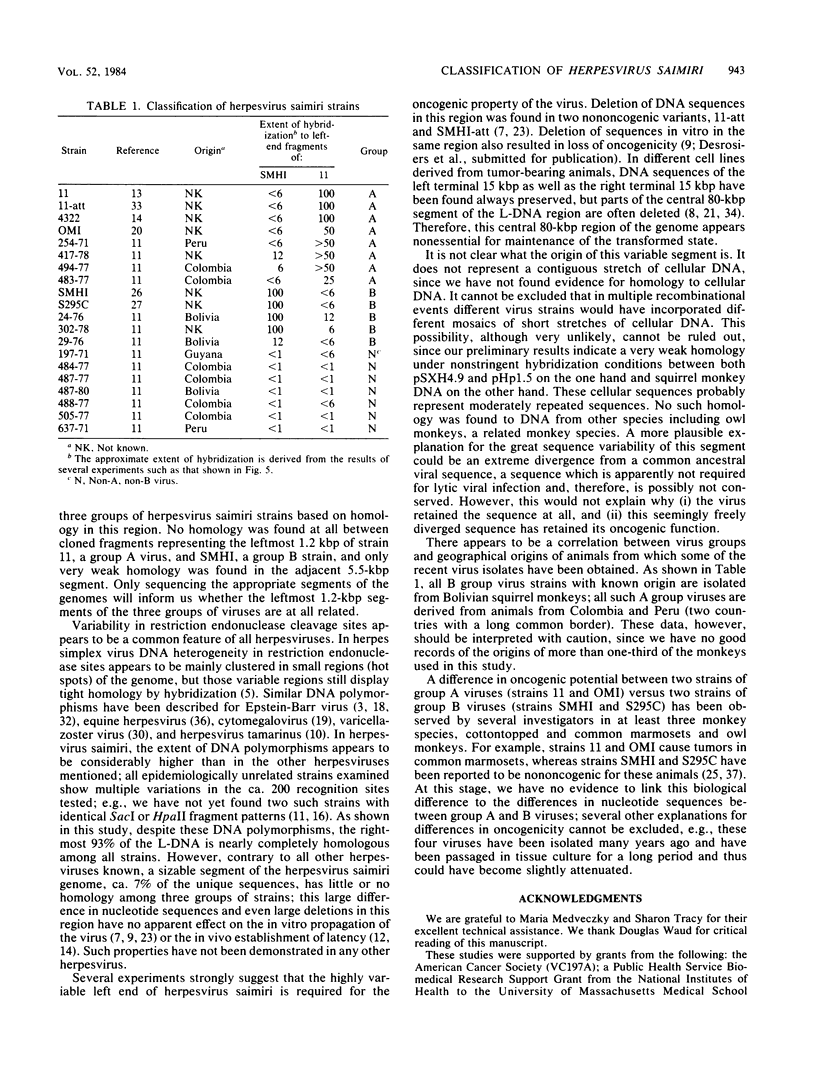
The leftmost 7 kilobase pairs of unique sequence L-DNA of herpesvirus saimiri was found to be highly variable among different strains as determined by restriction endonuclease analysis and blot hybridization. This region in one group of viruses (group A) showed only very weak hybridization with the DNA of two other groups. Similarly, a fragment of group B hybridized to DNA of its own group much more strongly than to group A. No homology was detected within a 1.2-kilobase-pair region between strain 11 (group A virus) and strain SMHI (group B) even under reduced stringency, and the adjacent 5.5-kilobase-pair segment of the region showed only a very weak intergroup hybridization. DNA of a third group of viruses (non-A, non-B) did not hybridize significantly with cloned fragments representing the leftmost 7-kilobase-pair region of either group A or group B. Since sequences in the highly variable region are required for the oncogenicity of the virus, these results raise interesting questions regarding the origin and function of this region of the genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman T. G., Roizman B., Adams G., Stover B. H. Restriction endonuclease fingerprinting of herpes simplex virus DNA: a novel epidemiological tool applied to a nosocomial outbreak. J Infect Dis. 1978 Oct;138(4):488–498. doi: 10.1093/infdis/138.4.488. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Meléndez L. V., Hunt R. D., King N. W., Anver M., Fraser C. E., Barahona H., Baggs R. B. Herpesvirus saimiri: VII. Induction of malignant lymphoma in New Zealand white rabbits. J Natl Cancer Inst. 1974 Dec;53(6):1803–1807. [PubMed] [Google Scholar]
- Desrosiers R. C., Burghoff R. L., Bakker A., Kamine J. Construction of replication-competent Herpesvirus saimiri deletion mutants. J Virol. 1984 Feb;49(2):343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A. Herpesvirus saimiri strain variability. J Virol. 1982 Jul;43(1):352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A., Jr Herpesvirus tamarinus and its relation to herpes simplex virus. J Gen Virol. 1981 Sep;56(Pt 1):119–130. doi: 10.1099/0022-1317-56-1-119. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. Herpesvirus saimiri DNA in tumor cells--deleted sequences and sequence rearrangements. J Virol. 1981 Aug;39(2):497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J Natl Cancer Inst. 1972 May;48(5):1499–1505. [PubMed] [Google Scholar]
- Falk L., Wright J., Deinhardt F., Wolfe L., Schaffer P., Benyesh-Melnick M. Experimental infection of squirrel and marmoset monkeys with attenuated Herpesvirus saimiri. Cancer Res. 1976 Feb;36(2 Pt 2):707–710. [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Huong S. M., Tegtmeier G. E., Alford C. Cytomegalovirus: genetic variation of viral genomes. Ann N Y Acad Sci. 1980;354:332–346. doi: 10.1111/j.1749-6632.1980.tb27976.x. [DOI] [PubMed] [Google Scholar]
- Hunt R. D., Garcia F. G., Barahona H. H., King N. W., Fraser C. E., Meléndez L. V. Spontaneous Herpesvirus saimiri lymphoma in an owl monkey. J Infect Dis. 1973 Jun;127(6):723–725. doi: 10.1093/infdis/127.6.723. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Mulder C., Burghoff R. L., Fleckenstein B., Desrosiers R. C. Deletion of DNA sequence in a nononcogenic variant of Herpesvirus saimiri. J Virol. 1984 May;50(2):662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs R., Fleckenstein B. Purification of Herpesvirus saimiri DNA. Bibl Haematol. 1973;39:457–461. doi: 10.1159/000427876. [DOI] [PubMed] [Google Scholar]
- Laufs R., Fleckenstein B. Susceptibility to Herpesvirus saimiri and antibody development in old and new world monkeys. Med Microbiol Immunol. 1973 Mar 8;158(3):227–236. doi: 10.1007/BF02120558. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Rangan S. R., Martin L. N., Enright F. M., Allen W. P. Herpesvirus saimiri-induced malignant lymphoma in rabbits. J Natl Cancer Inst. 1976 Jul;57(1):151–156. doi: 10.1093/jnci/57.1.151. [DOI] [PubMed] [Google Scholar]
- Richards J. C., Hyman R. W., Rapp F. Analysis of the DNAs from seven varicella-zoster virus isolates. J Virol. 1979 Dec;32(3):812–821. doi: 10.1128/jvi.32.3.812-821.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rymo L., Lindahl T., Adams A. Sites of sequence variability in Epstein-Barr virus DNA from different sources. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2794–2798. doi: 10.1073/pnas.76.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Falk L. A., Deinhardt F. Attenuation of herpesvirus saimiri for marmosets after successive passage in cell culture at 39 degrees C. J Natl Cancer Inst. 1975 Nov;55(5):1243–1246. doi: 10.1093/jnci/55.5.1243. [DOI] [PubMed] [Google Scholar]
- Schirm S., Müller I., Desrosiers R. C., Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984 Mar;49(3):938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Simpson T., Roizman B. Differentiation of respiratory and abortigenic isolates of equine herpesvirus 1 by restriction endonucleases. Science. 1981 Oct 30;214(4520):562–564. doi: 10.1126/science.6270790. [DOI] [PubMed] [Google Scholar]
- Wright J., Falk L. A., Wolfe L. G., Ogden J., Deinhardt F. Susceptibility of common marmosets (Callithrix jacchus) to oncogenic and attenuated strains of Herpesvirus saimiri. J Natl Cancer Inst. 1977 Nov;59(5):1475–1478. [PubMed] [Google Scholar]