Abstract
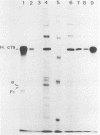
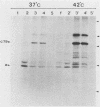
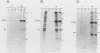
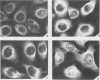
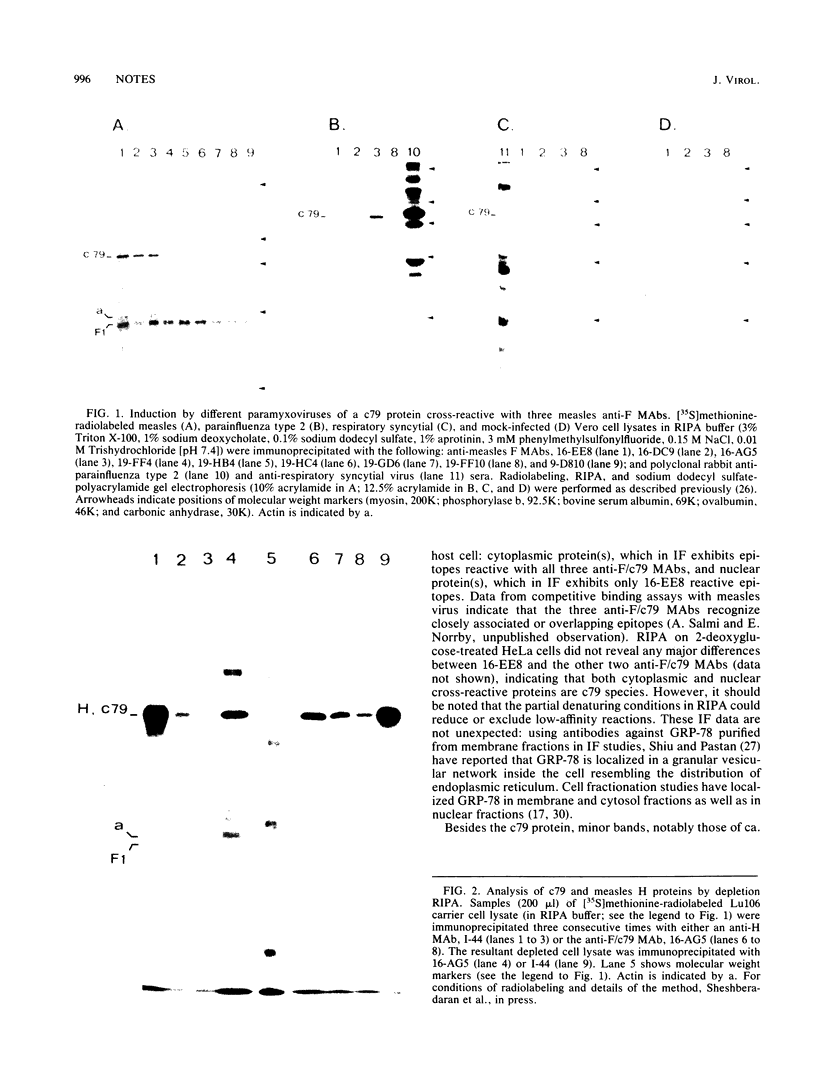
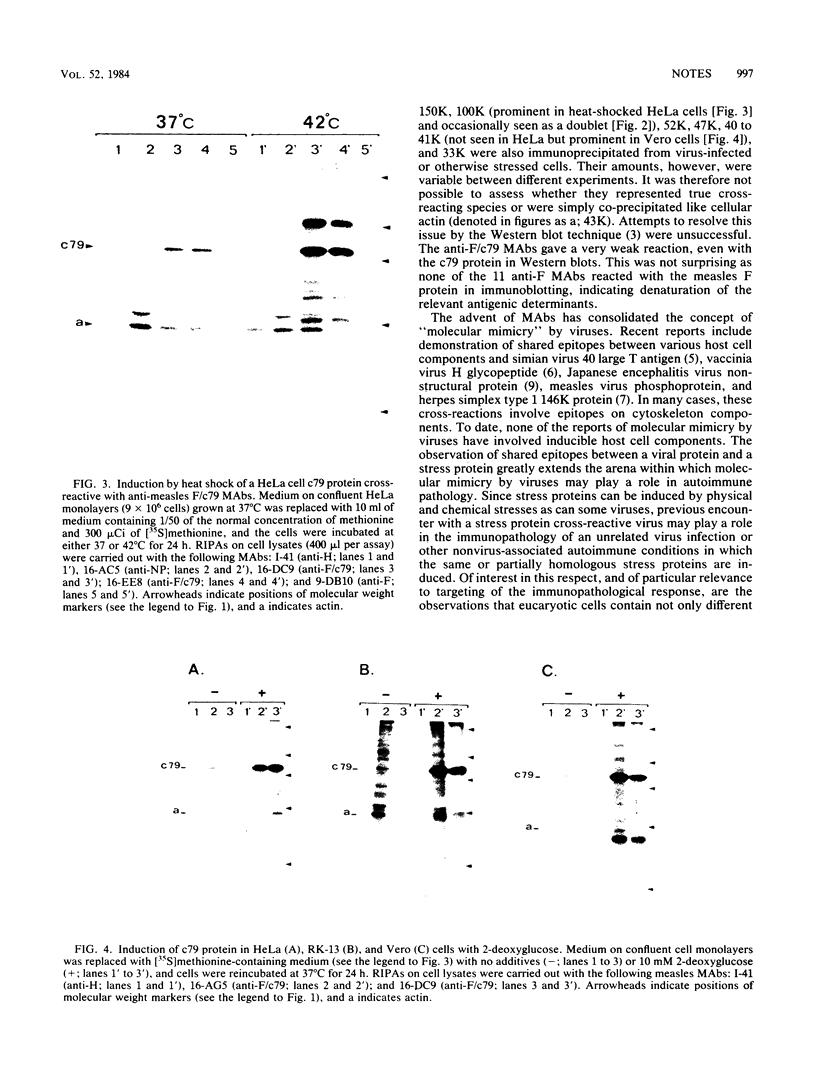
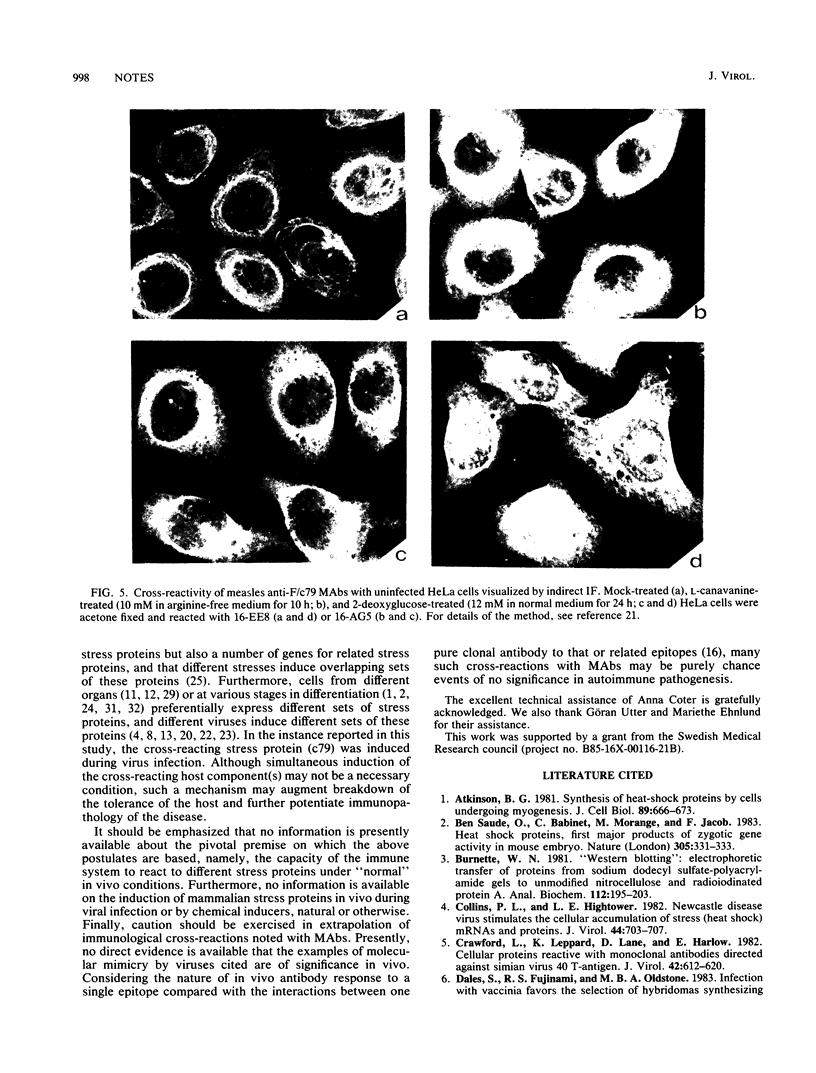
In a group of 11 monoclonal antibodies specifically reacting with the measles virus fusion protein, three antibodies also immunoprecipitated other proteins, in particular a 79,000-molecular-weight protein from virus-infected cells. The cross-reacting 79,000-molecular-weight protein was shown to be a virus-induced host stress protein. This protein could be induced by (i) different paramyxoviruses, (ii) heat shock of uninfected HeLa cells, and (iii) 2-deoxyglucose, tunicamycin, or L-canavanine treatment of different mammalian cell lines. Immunofluorescence of stressed HeLa cells localized the cross-reacting host protein(s) mainly in the cytoplasm. The significance of these results in relation to autoimmunity is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson B. G. Synthesis of heat-shock proteins by cells undergoing myogenesis. J Cell Biol. 1981 Jun;89(3):666–673. doi: 10.1083/jcb.89.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaude O., Babinet C., Morange M., Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983 Sep 22;305(5932):331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L., Leppard K., Lane D., Harlow E. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J Virol. 1982 May;42(2):612–620. doi: 10.1128/jvi.42.2.612-620.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Fujinami R. S., Oldstone M. B. Infection with vaccinia favors the selection of hybridomas synthesizing autoantibodies against intermediate filaments, one of them cross-reacting with the virus hemagglutinin. J Immunol. 1983 Sep;131(3):1546–1553. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B., Wroblewska Z., Frankel M. E., Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R. F., Ulug E. T., Bose H. R., Jr Induction of stress proteins in Sindbis virus- and vesicular stomatitis virus-infected cells. Virology. 1983 Sep;129(2):319–332. doi: 10.1016/0042-6822(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Gould E. A., Chanas A. C., Buckley A., Clegg C. S. Monoclonal immunoglobulin M antibody to Japanese encephalitis virus that can react with a nuclear antigen in mammalian cells. Infect Immun. 1983 Aug;41(2):774–779. doi: 10.1128/iai.41.2.774-779.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haire M. Fibrillar anti-cellular antibody associated with mumps and measles infection. Clin Exp Immunol. 1972 Nov;12(3):335–341. [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E., White F. P. Cellular responses to stress: comparison of a family of 71--73-kilodalton proteins rapidly synthesized in rat tissue slices and canavanine-treated cells in culture. J Cell Physiol. 1981 Aug;108(2):261–275. doi: 10.1002/jcp.1041080216. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Türler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983 Jan;3(1):1–8. doi: 10.1128/mcb.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H. Unlearning about latency. Med Microbiol Immunol. 1982;170(4):209–219. doi: 10.1007/BF02123311. [DOI] [PubMed] [Google Scholar]
- Kurki P., Virtanen I., Stenman S., Linder E. Characterization of human smooth muscle autoantibodies reacting with cytoplasmic intermediate filaments. Clin Immunol Immunopathol. 1978 Dec;11(4):379–387. doi: 10.1016/0090-1229(78)90165-4. [DOI] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984 Apr 10;259(7):4616–4621. [PubMed] [Google Scholar]
- Lidman K., Biberfeld G., Fagraeus A., Norberg R., Torstensson R., Utter G., Carlsson L., Luca J., Lindberg U. Anti-actin specificity of human smooth muscle antibodies in chronic active hepatitis. Clin Exp Immunol. 1976 May;24(2):266–272. [PMC free article] [PubMed] [Google Scholar]
- Linder E., Kurki P., Andersson L. C. Autoantibody to "intermediate filament" in infectious mononucleosis. Clin Immunol Immunopathol. 1979 Dec;14(4):411–417. doi: 10.1016/0090-1229(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Norrby E., Chen S. N., Togashi T., Shesberadaran H., Johnson K. P. Five measles virus antigens demonstrated by use of mouse hybridoma antibodies in productively infected tissue culture cells. Arch Virol. 1982;71(1):1–11. doi: 10.1007/BF01315171. [DOI] [PubMed] [Google Scholar]
- Notarianni E. L., Preston C. M. Activation of cellular stress protein genes by herpes simplex virus temperature-sensitive mutants which overproduce immediate early polypeptides. Virology. 1982 Nov;123(1):113–122. doi: 10.1016/0042-6822(82)90299-9. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E., Sivertsen A., Firtel R. A. An unusual transposon encoding heat shock inducible and developmentally regulated transcripts in Dictyostelium. Cell. 1983 Nov;35(1):243–251. doi: 10.1016/0092-8674(83)90227-1. [DOI] [PubMed] [Google Scholar]
- Sheshberadaran H., Chen S. N., Norrby E. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology. 1983 Jul 30;128(2):341–353. doi: 10.1016/0042-6822(83)90261-1. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Pastan I. H. Properties and purification of a glucose-regulated protein from chick embryo fibroblasts. Biochim Biophys Acta. 1979 Jan 25;576(1):141–150. doi: 10.1016/0005-2795(79)90493-8. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Yildiz A., Sotelo J., Osung O., Holborow E. J., Kanakoudi F., Small J. V. Viral infections and IgM autoantibodies to cytoplasmic intermediate filaments. Clin Exp Immunol. 1979 Jul;37(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- Voellmy R., Bromley P. A. Massive heat-shock polypeptide synthesis in late chicken embryos: convenient system for study of protein synthesis in highly differentiated organisms. Mol Cell Biol. 1982 May;2(5):479–483. doi: 10.1128/mcb.2.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala C. A., Salas-Prato M., Yan W. T., Banjo B., Perdue J. F. In cultured chick embryo fibroblasts the hexose transport components are not the 75 000 and 95 000 dalton polypeptides synthesized following glucose deprivation. Can J Biochem. 1980 Oct;58(10):1179–1188. doi: 10.1139/o80-158. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]
- Zuker C., Cappello J., Chisholm R. L., Lodish H. F. A repetitive Dictyostelium gene family that is induced during differentiation and by heat shock. Cell. 1983 Oct;34(3):997–1005. doi: 10.1016/0092-8674(83)90557-3. [DOI] [PubMed] [Google Scholar]