Abstract
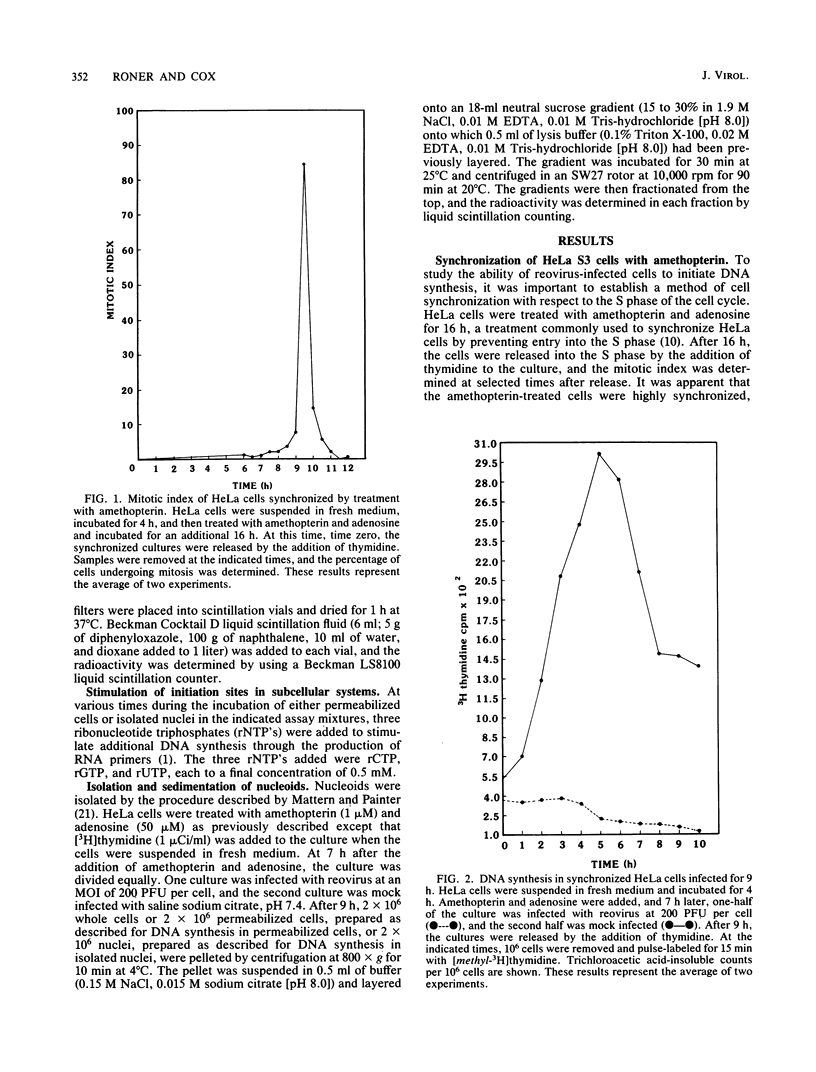
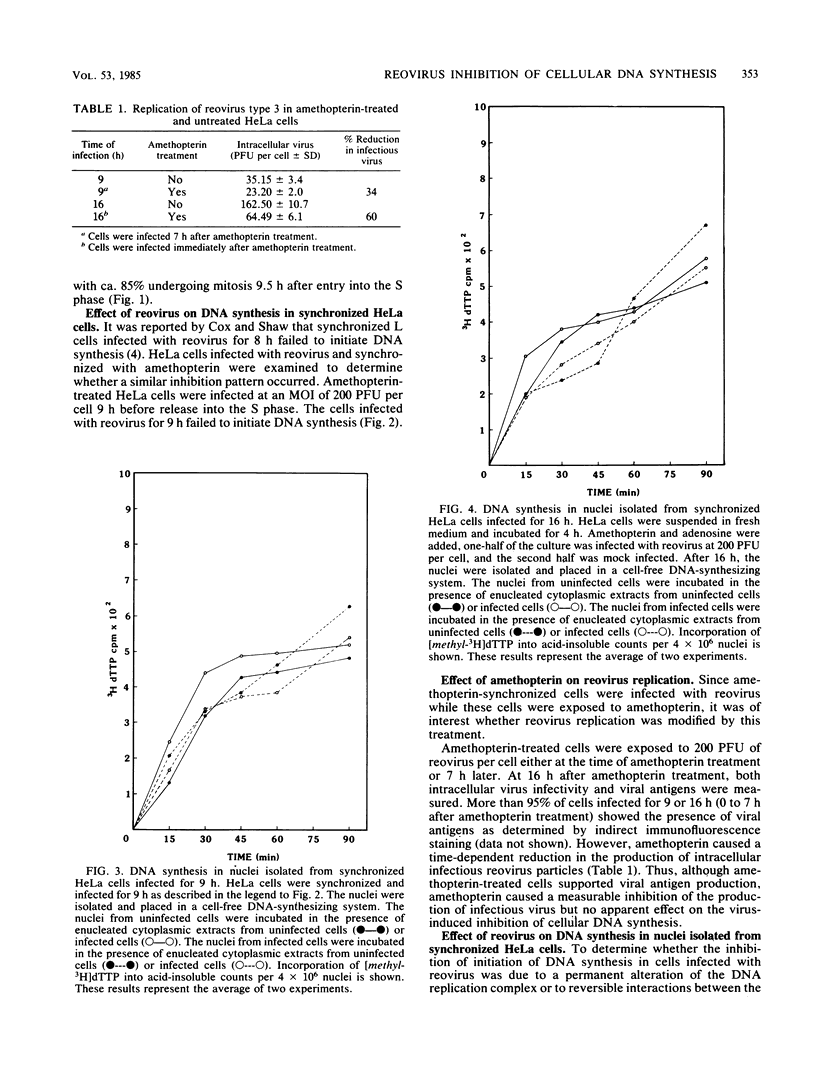
Synchronized HeLa cells, primed for entry into the synthesis phase by amethopterin, were prevented from initiating DNA synthesis 9 h after infection with reovirus type 3. However, nuclei isolated from synchronized cells infected with reovirus for 9 or 16 h demonstrated a restored ability to synthesize DNA. The addition of enucleated cytoplasmic extracts from infected or uninfected cells did not affect this restored capacity for synthesis. The addition of ribonucleotide triphosphates to nuclei isolated from infected cells stimulated additional DNA synthesis, suggesting that these nuclei were competent to initiate new rounds of DNA replication. Permeabilization of infected cells did not restore the ability of these cells to synthesize DNA. Nucleoids isolated from intact or permeabilized cells, infected for 9 or 16 h displayed an increased rate of sedimentation when compared with nucleoids isolated from uninfected cells. Nucleoids isolated from the nuclei of infected cells demonstrated a rate of sedimentation similar to that of nucleoids isolated from the nuclei of uninfected cells. The inhibition of initiation of cellular DNA synthesis by reovirus type 3 appears not to have been due to a permanent alteration of the replication complex, but this inhibition could be reversed by the removal of that complex from factors unique to the structural or metabolic integrity of the infected cell.
Full text
PDF

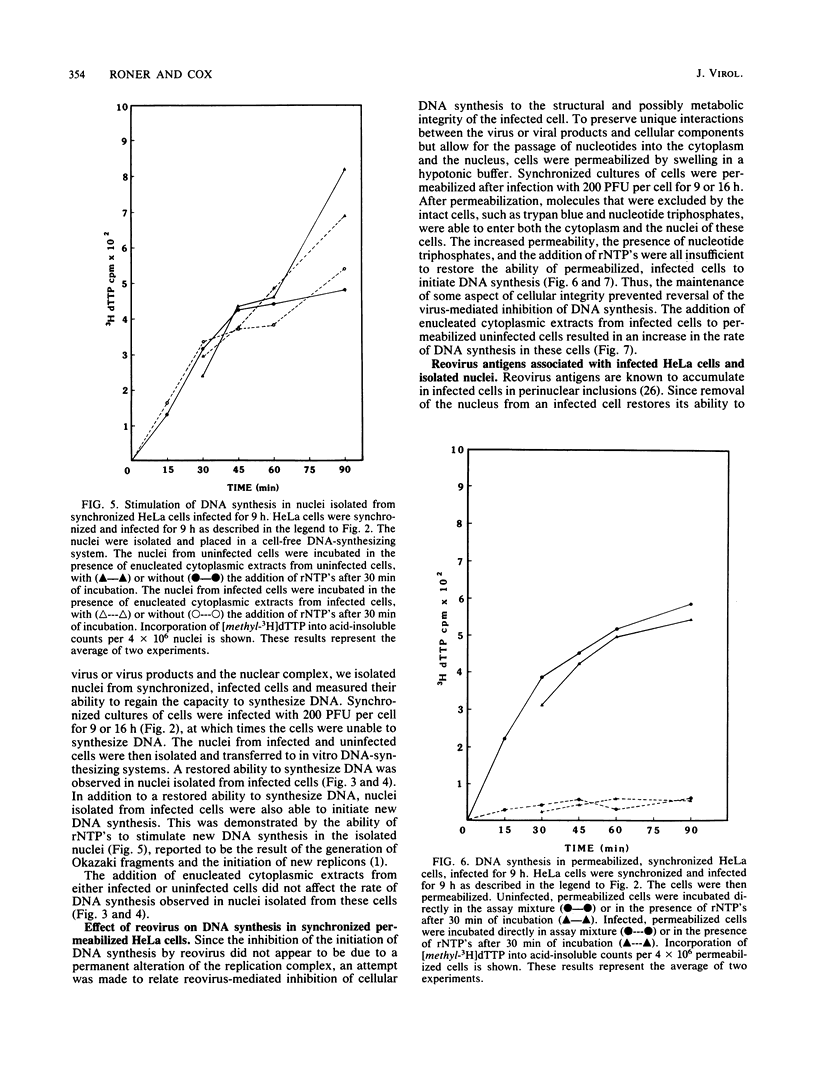
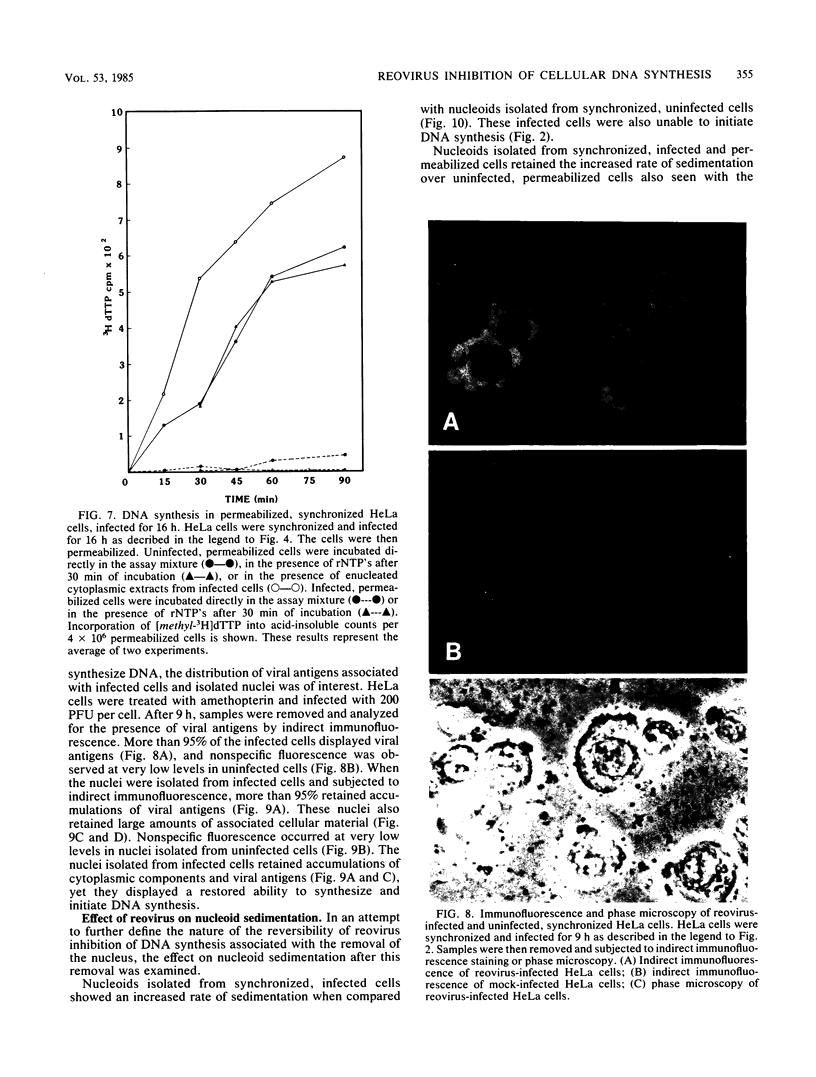
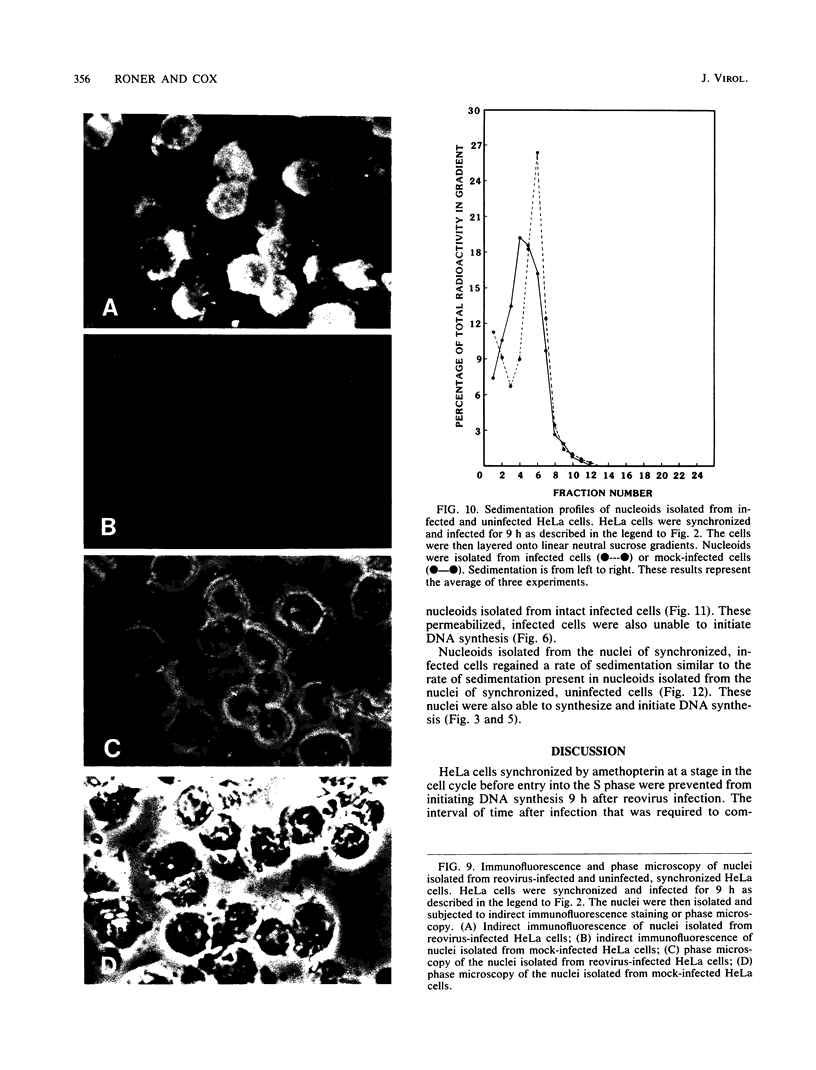
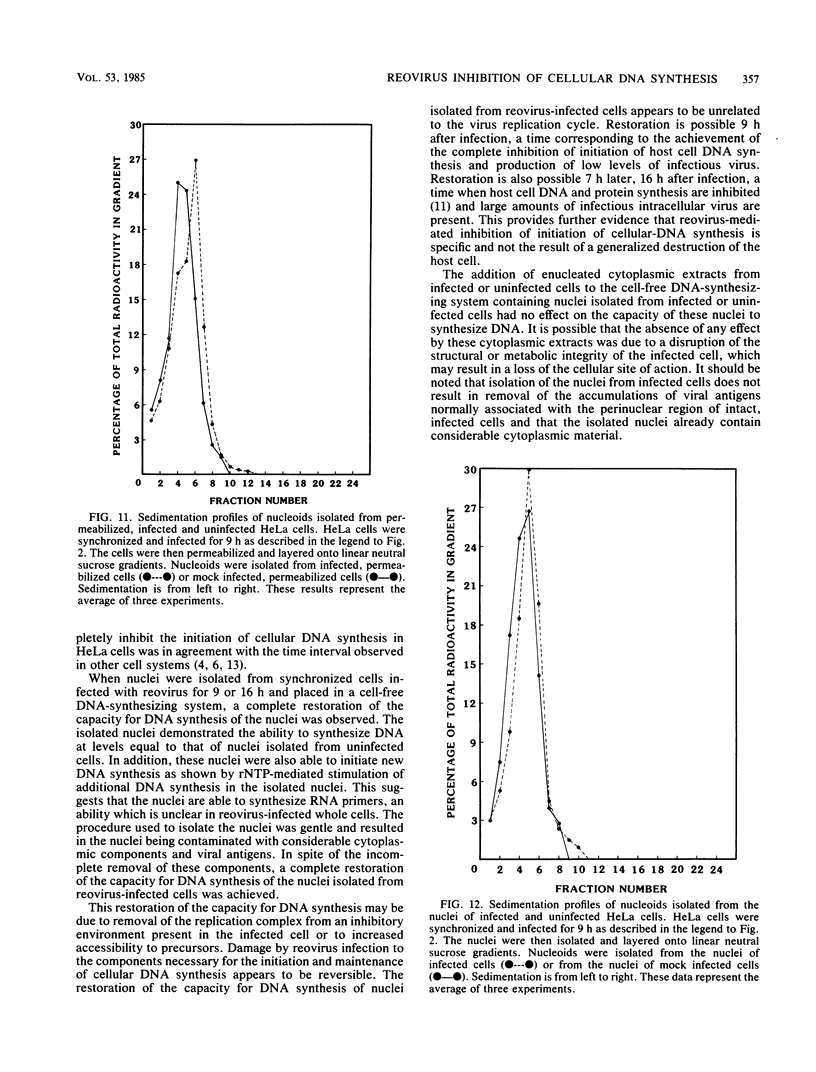


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brun G., Weissbach A. Initiation of HeLa cell DNA synthesis in a subnuclear system. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5931–5935. doi: 10.1073/pnas.75.12.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaly N., Johnstone M., Hand R. Alterations in nuclear structure and function in reovirus-infected cells. Clin Invest Med. 1979;2(4):141–152. [PubMed] [Google Scholar]
- Cox D. C., Clinkscales W. Infectious reovirus subviral particles: virus replication, cellular cytopathology, and DNA synthesis. Virology. 1976 Oct 1;74(1):259–261. doi: 10.1016/0042-6822(76)90152-5. [DOI] [PubMed] [Google Scholar]
- Cox D. C., Shaw J. E. Inhibition of the initiation of cellular DNA synthesis after reovirus infection. J Virol. 1974 Mar;13(3):760–761. doi: 10.1128/jvi.13.3.760-761.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. R., Stanish S. M., Cox D. C. Differential sensitivity of normal and transformed human cells to reovirus infection. J Virol. 1978 Nov;28(2):444–449. doi: 10.1128/jvi.28.2.444-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. The step in cellular DNA synthesis blocked by reovirus infection. Virology. 1969 Dec;39(4):935–938. doi: 10.1016/0042-6822(69)90032-4. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Pennington T. H. Virus development in enucleate cells: echovirus, poliovirus, pseudorabies virus, reovirus, respiratory syncytial virus and Semliki Forest virus. J Gen Virol. 1975 Feb;26(2):183–196. doi: 10.1099/0022-1317-26-2-183. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. A nuclear system for DNA replication from synchronized HeLa cells. Biochim Biophys Acta. 1968 Jul 23;161(2):455–468. doi: 10.1016/0005-2787(68)90122-6. [DOI] [PubMed] [Google Scholar]
- Friedman D. L. On the mechanism of DNA replication in isolated nuclei from HeLa cells. Biochim Biophys Acta. 1974 Jul 24;353(4):447–462. doi: 10.1016/0005-2787(74)90051-3. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I., DALES S., FRANKLIN R. M. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. doi: 10.1016/0042-6822(62)90139-3. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I. MACROMOLECULAR SYNTHESIS IN REOVIRUS-INFECTED L CELLS. Biochim Biophys Acta. 1963 Aug 20;72:651–653. [PubMed] [Google Scholar]
- Gautschi J. R., Burkhalter M., Reinhard P. Semiconservative DNA replication in vitro. II. Replicative intermediates of mouse P-815 cells. Biochim Biophys Acta. 1977 Feb 16;474(4):512–523. doi: 10.1016/0005-2787(77)90072-7. [DOI] [PubMed] [Google Scholar]
- Hand R., Kasupski G. DNA and histone synthesis in reovirus-infected cells. J Gen Virol. 1978 Jun;39(3):437–448. doi: 10.1099/0022-1317-39-3-437. [DOI] [PubMed] [Google Scholar]
- Hand R., Tamm I. Initiation of DNA replication in mammalian cells and its inhibition by reovirus infection. J Mol Biol. 1974 Jan 15;82(2):175–183. doi: 10.1016/0022-2836(74)90339-8. [DOI] [PubMed] [Google Scholar]
- Hand R., Tamm I. Reovirus: effect of noninfective viral components on cellular deoxyribonucleic acid synthesis. J Virol. 1973 Feb;11(2):223–231. doi: 10.1128/jvi.11.2.223-231.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. V., Stieber J. F., Mueller G. C. Dna synthesis in isolated HeLa nuclei. A system for continuation of replication in vivo. Eur J Biochem. 1973 Apr;34(2):383–394. doi: 10.1111/j.1432-1033.1973.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Krokan H., Bjorklid E., Prydz H. DNA synthesis in isolated HeLa cell nuclei. Optimalization of the system and characterization of the product. Biochemistry. 1975 Sep 23;14(19):4227–4232. doi: 10.1021/bi00690a012. [DOI] [PubMed] [Google Scholar]
- Krokan H., Cooke L., Prydz H. DNA synthesis in isolated HeLa cell nuclei. Evidence for in vitro initiation of synthesis of small pieces of DNA and their subsequent ligation. Biochemistry. 1975 Sep 23;14(19):4233–4237. doi: 10.1021/bi00690a013. [DOI] [PubMed] [Google Scholar]
- Lai M. H., Wérenne J. J., Joklik W. K. The preparation of reovirus top component and its effect on host DNA and protein synthesis. Virology. 1973 Jul;54(1):237–244. doi: 10.1016/0042-6822(73)90133-5. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Painter R. B. Dependence of mammalian DNA replication on DNA supercoiling. I. Effects of ethidium bromide on DNA synthesis in permeable Chinese hamster ovary cells. Biochim Biophys Acta. 1979 Jul 26;563(2):293–305. doi: 10.1016/0005-2787(79)90048-0. [DOI] [PubMed] [Google Scholar]
- Reinhard P., Burkhalter M., Gautschi J. R. Semiconservative DNA replication in vitro. I. Properties of two systems derived from mouse P-815 cells by permeabilization or lysis with Brij-58. Biochim Biophys Acta. 1977 Feb 16;474(4):500–511. doi: 10.1016/0005-2787(77)90071-5. [DOI] [PubMed] [Google Scholar]
- SPENDLOVE R. S., LENNETTE E. H., KNIGHT C. O., CHIN J. N. DEVELOPMENT OF VIRAL ANTIGEN AND INFECTIOUS VIRUS IN HELA CELLS INFECTED WITH REOVIRUS. J Immunol. 1963 Apr;90:548–553. [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular DNA synthesis: role of the S1 gene. J Virol. 1981 Apr;38(1):389–392. doi: 10.1128/jvi.38.1.389-392.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. E., Cox D. C. Early inhibition of cellular DNA synthesis by high multiplicities of infectious and UV-inactivated Reovirus. J Virol. 1973 Oct;12(4):704–710. doi: 10.1128/jvi.12.4.704-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton I. H., Kasupski G. J., Jr, Oblin C., Hand R. DNA binding of a nonstructural reovirus protein. Can J Biochem. 1981 Feb;59(2):122–130. doi: 10.1139/o81-018. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Ramig R. F., Mustoe T. A., Fields B. N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978 May 15;86(2):581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]