Abstract
A team of senior scientists was formed in 2006 to create a blueprint for the regeneration of whole human teeth along with all of the supporting structure of the dentition. The team included experts from diverse fields, each with a reputation for stellar accomplishment. Participants attacked the scientific issues of tooth regeneration but, more importantly, each agreed to work collaboratively with experts from other disciplines to form a learning organization. A commitment to learn from one another produced a unique interdisciplinary and multidisciplinary team. Inspired by the Kennedy space program to send a man to the moon, with its myriad of problems and solutions that no one discipline could solve, this tooth regeneration team devised an ambitious plan that sought to use stem cell biology, engineering, and computational biology to replicate the developmental program for odontogenesis. In this manner, team members envisioned a solution that consisted of known or knowable fundamentals. They proposed a laboratory-grown tooth rudiment that would be capable of executing the complete program for odontogenesis when transplanted to a suitable host, recreating all of the dental tissues, periodontal ligament, cementum, and alveolar bone associated with the canonical tooth. This plan was designed to bring regenerative medicine fully into the dental surgery suite, although a lack of funding has so far prevented the plan from being carried out.
Keywords: regenerative medicine, stem cells, multidisciplinary research, tooth regeneration, translational research
In the 1960s, President John F. Kennedy captured the American imagination in a speech he gave at Rice University: “We choose to go to the moon in this decade and do the other things, not because they are easy, but because they are hard.” In 2006, his words inspired a team of scientists to undertake a grand project to improve the oral health of the American people. Its goal was to regenerate a complete single rooted tooth (Figure 1) that included the bioceramic portions and all of the tissues integrating the tooth to the bone, using a biological regeneration approach that was to be backed up by a biomimetic alternative (Figure 2).
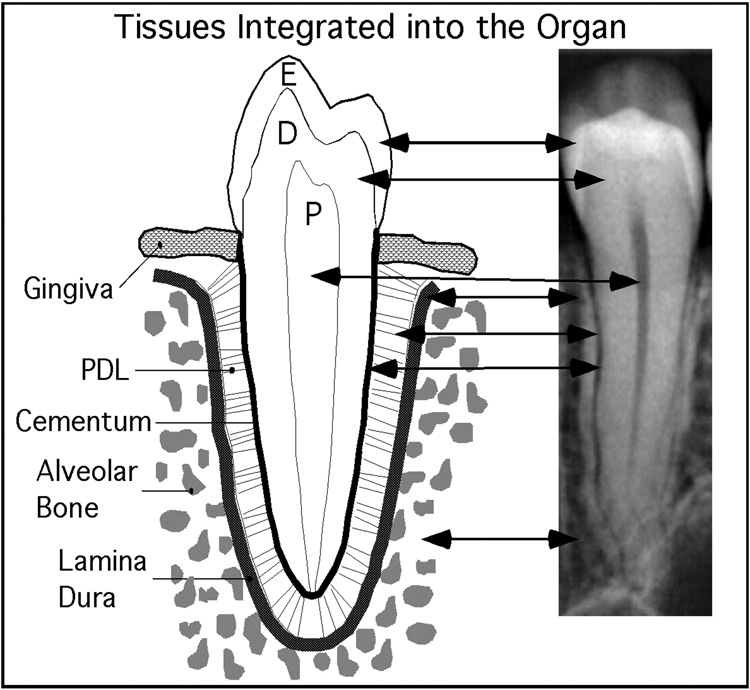
Figure 1. Canonical tissues integrated into the organ.
The target of the regeneration strategy was to create a single rooted tooth. This hybrid cartoon-radiograph illustrates the complexity of a single tooth.
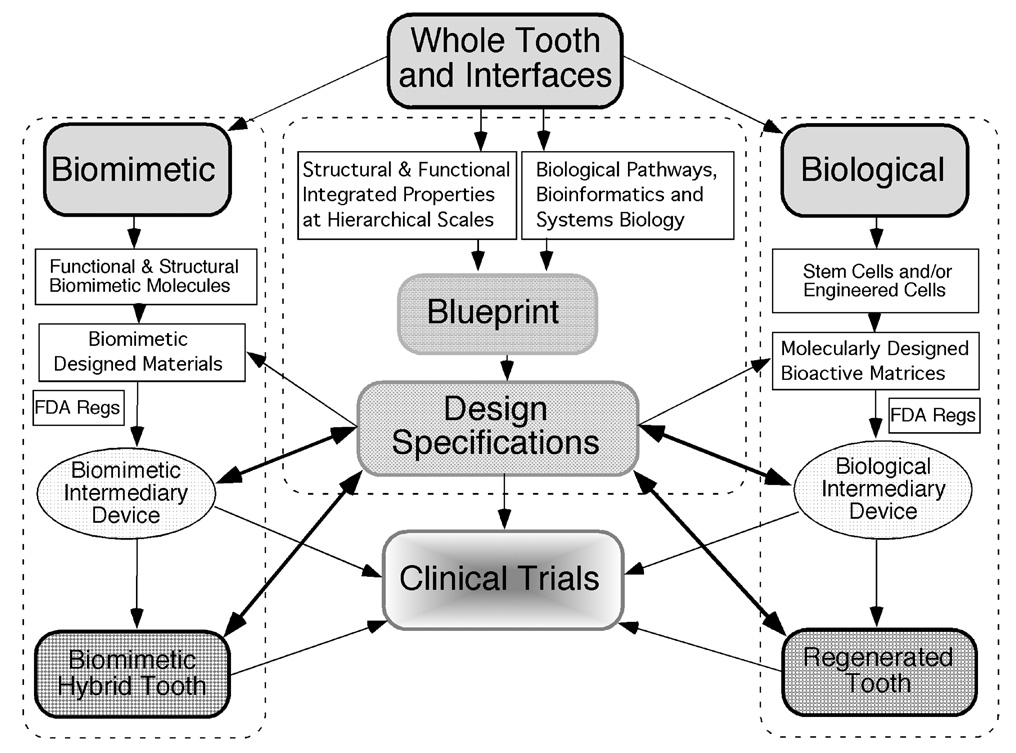
Figure 2. Overarching plan for development of a regenerated human tooth.
The central blueprint of the tooth would provide all of the production specifications obtained from measurements of canonical teeth and would be used to design a regenerated tooth and measure its fidelity. A biological approach using cell biology, developmental biology, and stem cell biology principles would be married to engineering design and bioactive matrices created by nanotechnology to provide the basis of regenerating a cap stage tooth that could be engrafted to a host. A biomimetic approach was envisioned as a complement to the biological approach and would provide an alternative should the biological approach meet irreconcilable roadblocks. Compliance with FDA regulations was deemed vital for the project in order to move it towards safety trials in animals and then into clinical trials with select patients.
A team of scientists was drawn from several universities and from multiple disciplines to accomplish this goal. First, we identified three parallel interdisciplinary research groups. One group developed a blueprint with structural and functional design specifications drawn from a human tooth, focusing on gene expression, functional organization of the matrix, and resulting biomineralized composite ceramic tissues. Next, the scientific teams mined, evaluated, and developed essential information needed for the blueprint and created high-fidelity models capable of evaluating the outcomes from the regeneration strategies by comparing the regenerated tooth to the data from the canonical example. The knowledge base gained from normal development and contained in the blueprint was then to be translated, using stem cells in conjunction with computational biology and engineering principles, into a regenerated whole tooth. The blueprint information would also be used to guide the fabrication of nature-inspired biomimetic equivalents of tooth components, aided by synthetic nanotechnology systems that would provide appropriate biologically relevant signals.
Our interdisciplinary team consisted of twenty-five principal scientists in the fields of developmental biology, stem cell biology, engineering, chemistry, structural biology, computational biology, tissue engineering, high performance teams, crisis management, systems biology, genomics, proteomics, oral surgery, wound repair, periodontology, material sciences, angiogenesis, the FDA regulatory system, and systems engineering.
In 2006, the National Institute of Dental and Craniofacial Research (NIDCR) released to the scientific community the following invitation: “The NIDCR invites Specialized Center-Cooperative Agreement (U54) applications to establish a National Center focused on Building a Tooth by Bridging Biology and Material Sciences (BTBBMS). The specific objective of this initiative is the complete characterization of teeth and their supporting tissues as an integrated biological and biomechanical system that can provide the engineering specifications and design principles required to create blueprints for the design of new teeth and their associated structures.”
RFA DE-07-009 went on to define the health problem that we sought to solve: “By the age of 50 years, the average American has lost approximately 8 teeth (DHHS Report, 2000). By age 65, approximately 35% of the population is edentulous. Treatment for tooth loss often involves dentures, whose long-term use results in bone resorption of the underlying jaw. Alternative treatments offered by oral surgeons include replacement of lost teeth with dental implants or rarely autologous tooth re-implantation. Approximately 74 million U.S. adults are potential candidates for dental implants. Dental implant procedures are expensive (ranging from $2,000 for a single tooth to $25,000 for an entire jaw), are at times uncomfortable, and frequently eventually fail. For example, the American Academy of Oral and Maxillofacial Surgeons defines implant success as 85–90% survival of the implant for only 5–10 years.”
Five prioritized goals shaped our team’s approach to whole tooth regeneration: 1) to regenerate functional tooth structures and supporting tissues; 2) to develop intermediary devices for repairing diseased oral tissues; 3) to develop stem cell lines from single teeth applicable to all regenerative medicine strategies; 4) to grow polydisciplinary teams with renewable knowledge and techniques to achieve these goals; and 5) to translate this knowledge and technical capability into devices that improve health, create educational opportunities, inspire new research pathways, create new clinical applications, and generate jobs in technology-rich environments.
Vision of a Solution: A Clinical Procedure for Tooth Regeneration
We looked to developmental biology, coupled with engineering, for the strategies needed to regenerate human teeth. We hypothesized that, by recapitulating the developmental signals from canonical teeth, naïve stem cells could be induced to odontogenic potential (Figure 3). Epithelial-mesenchymal interactions are the hallmark of tooth development. These interactions are characterized by the reciprocal exchange of signals between these two naïve germ layer tissues and result in the emergence of unique terminal phenotypes with their supporting cells (Figure 3A).1,2 The simplicity of back and forth signals was the leverage point for our regeneration strategy.
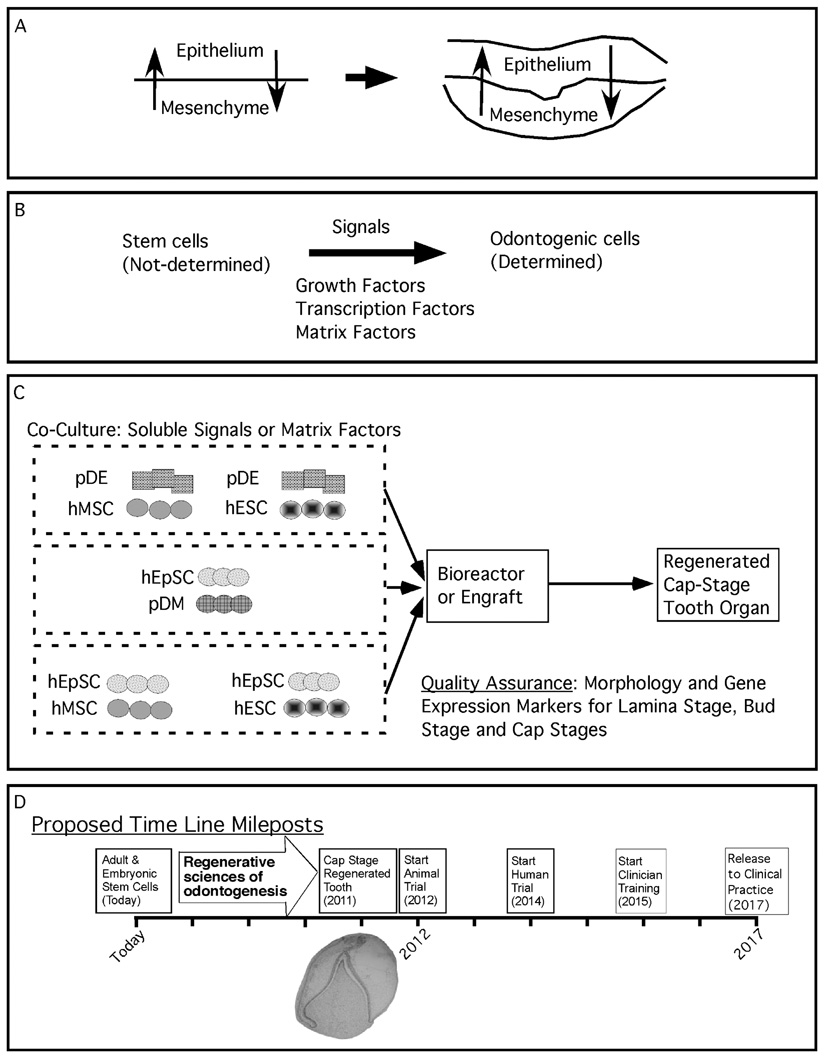
Figure 3. Rationale for regenerating a human tooth.
Panel A defines the reciprocal signals occurring between all epithelial-mesenchymal interacting tissues. Fundamental to organogenesis is the ability of one tissue to induce in another tissue the expression of previously quiescent genes that determines the induced tissue to acquire a new identity. Panel B describes the use of growth factors, transcription factors, and matrix factors to provide the signals inducing a naïve stem cell population to adopt odontogenic potential. Panel C is a strategy for screening a variety of stem cell populations, such as mesenchymal stem cells (MSC), embryonic stem cells (ESC), and epithelial stem cells (EpSC) for their ability to adopt odontogenic capacity under the influence of an odontogenic tissue, such as porcine dental epithelia (pDE) or porcine dental mesenchyme (pDM). Panel D is the time line required to bring the laboratory steps together for the in vitro manipulation of stem cell populations to regenerate a cap stage tooth bud and to initiate clinical training with release to the dental community within a decade.
Efforts to use such strategies have been previously identified.3–5 In the continuously developing rodent incisor, for example, the determination and differentiation of stem cells to terminal phenotypes and their unique signals have been well defined and provided some of the necessary molecular details needed for regeneration.6–9 A systems biology and bioinformatics approach was launched to define in high resolution all of the known gene products that define the canonical pathways for tooth development (Figure 2).
We also sought ways to estimate our ignorance by predicting the extent of the factors that were not yet known and then devising the means to fill in these gaps in knowledge.10–12 The team paid particular attention to the fate of the supporting tissues of the tooth, the periodontal ligament, cementoblasts, and supporting alveolar bone. The emphasis on regenerating the supporting tissue of the tooth was held as a central tenet to our design plans, because the team felt strongly that the key to successfully integrating a regenerated tooth would lie in regenerating the periodontium.13,14
Our strategy was to exploit stem cells, adult or embryonic (Figure 3B), in a milieu of growth factors that replicated those signals normally exchanged at each of the appropriate stages of odontogenesis. In addition, we planned the use of a nanofabricated bio-informative matrix for both cell support and informational content.15,16 It had already been experimentally shown that cell differentiation could be enhanced by using a supporting matrix that closely matched the material properties of the authentic matrix.17 Moreover, tailoring the stiffness of the supporting matrix could also be used to control proliferation of embedded cells in the matrix.18,19
Another function of the nanodesigned matrix was information content.20 For example, peptide amphiphiles containing laminin epitopes have been used for the induction of neurons from stem cell precursors. By mating soluble signaling factors with the proper physical clues, we planned to engineer an optimal environment for cell differentiation.21
Finally, we envisioned the need to engraft the regenerated cap stage to the host. For this, a series of peptide amphiphiles was designed that could provide a transiently protected environment that would suppress inflammatory responses predicted to be detrimental to engraftment, while providing a pro-angiogenic and pro-reinnervation environment that would support the continued development of the host-engrafted cap stage primordia.22
In our efforts to identify the type of stem cells that would exhibit ideal growth characteristics, as well as being competent for odontogenic induction, a number of stem cell precursors were to be screened (Figure 3C). A series of inductive signals was envisioned to be exchanged between members of the naïve stem cell population when they were challenged by their reciprocal odontogenic tissue (Figure 3A). The success of the interactions would be assessed by using gene expression parameters achieved by the stem cells after induction. This would be done by comparing the gene expression profiles obtained from these samples to those obtained from authentic developing teeth. These profiles were to be stored as a searchable computer database created as part of the molecular blueprint (Figure 1). Our goal was to ascertain if the gene expression profile from the induced stem cell population faithfully matched the gene expression profile from canonical teeth, thus ensuring that development was progressing unhindered. In this manner, we would replicate the signal cascade that normally occurs during tooth development, and these signals would yield an authentic copy of the dental tissue created from the naïve stem cell population.
Human embryonic stem cell lines (hESC) approved for use by the National Institutes of Health, adult mesenchymal stem cells (MSC), and human epithelial stem cells (hEpSC) would be surveyed with the goal of identifying the optimal stem cell population for production of the regenerated tooth.23–26 Sonoyama et al. have recently shown that the fabrication of a dental root stock engineered from a stem cell population could be engrafted into the jaw of a pig, where it functioned with a traditionally fabricated dental crown.23
With this carefully outlined strategy, the team forged a vision around the long-term scientific objective of a clinical procedure for tooth regeneration. We proposed to create a cap stage tooth organ fabricated in vitro that would be transplanted into a patient and subsequently grown autonomously into a fully formed adult tooth in vivo. The cap stage would be created by inducing odontogenic fate in stem cells using a marriage of engineering sciences with cell and developmental biology that would enable near-natural ideal-control of the biochemical and biomechanical environment of a cell culture. The underlying hypothesis was that recapitulating the quantitative and qualitative parameters of gene expression identified in the canonical developing tooth would be transferred to the stem cells and would result in a regenerated tooth that was a copy of the original. Toward this end, quality assurance points were introduced at selected stages of regeneration to ensure that the gene expression parameters being sought were achieved. A strategy for virtual, real-time, continuous quality assurance was transplanted from the engineering discipline, where it had been used for evaluating complex systems and had enabled significant improvement in compliance with design specifications.
The cap stage was chosen as the ideal point in tooth development for transplantation because it contains all the cell types and associated signals required to form the adult tooth, as well as the ability to induce the host to provide vasculature and innervation. We hypothesized that the odontogenic potential in the regenerated cap stage tooth would contribute to odontogenic induction to the host’s stem cells resident at the engraftment site. This would further promote the formation of a new periodontal support, including cementoblasts, periodontal ligaments, and alveolar bone. Some of these tissues would likely be provided by stem cell populations resident in the host. Under the influence of the regenerated tooth, these host stem cells would participate in the regenerative process, with the cap stage acting as an organizing center. In favor of our design, the cap stage tooth organ was also the simplest vehicle for transferring to the patient the outcome of all the in vitro assembly processes described herein.
This regenerative procedure would offer an alternative to the mechanical solution of synthetic dental implants. Also, by providing a biological solution to the problem of tooth loss, the procedure would transform the prognosis for edentulous patients as well as for patients with severely diseased teeth or diminished jawbone. We hypothesized that it is a realistic ultimate goal to carry out the majority, if not all, of the tooth regeneration by cell biology routes.
A tooth regenerated as a living organ would have important clinical advantages over a synthetic tooth, including a biological hermetic boundary between the gingivae and the root, participation in the immune system, innervation, sensory perception, and vascularization. The presence of a regenerated root, cementum, and periodontal ligament would confer to the regenerated tooth the ability to repair itself in response to injury and for the periodontium to remodel and adapt to the mechanical environment, whether arising from orthodontic procedures or mastication. The regenerated tooth would be a familiar organ to dentists, who could apply to it all of the advantages of modern dental therapeutics.
We therefore proposed a strategy of addressing all the challenges peculiar to biologic methods. We would use synthetic or biomimetic materials primarily to create an ideally controlled environment for cell development, and secondarily as an alternative if the biologic solutions met an obstacle that could not be overcome by this creative team of scientists and clinicians.
Vision of the Scientific Objectives for the First Five Years of the Program
We believed that an approved clinical procedure of tooth regeneration, via an implanted biomanufactured cap stage, could be realized by 2017, in a ten-year plan. In the first five years, we proposed short-term scientific objectives that could bring the procedure to the point of animal trials.
We projected that these objectives would likely also provide tangible scientific and clinical benefits as we progressed through the regeneration strategy in its entirety. Among these benefits would be the following: 1) establish a goal-driven management team leading a network of outstanding senior scientists and a generation of newly trained young investigators, who would unite the disciplines of cell biology, materials science, informatics, mechanics, dentistry, and system engineering into a single research community focused on tooth regeneration; 2) identify an uninterrupted signaling cascade predicted to induce odontogenesis in optimally chosen stem cell populations forming the cap stage tooth organ; 3) create a map of gene expression and cell signals associated with the progression of naïve stem cells to their odontogenic fate; 4) create a new science for controlling the biochemical and biomechanical environment of a cell culture to induce organogenesis (this science would include a proteome-based pharmacologic repertoire to manipulate cell signal pathways); nano-textured, information-rich matrices that would guide odontogenic fate; and fast new experimental techniques for non-destructive in situ monitoring of cell development; and 5) establish an understanding of the cell and molecular biology required for the regeneration of tooth roots and their adnexa necessary to ensure successful biological integration of the cap stage with the jawbone following transplantation.
These outcomes, when attained, would enrich the ongoing efforts to regenerate the periodontium, and would be readily transferred to an already well-developed technology base existing in the dental profession. To contribute to regenerative medicine in general, we hoped to recreate a generalized source of stem cells for future applications. In addition, we would create an assembly sequence for the manipulation of stem cells that could be adapted for other organs based on their use of common early development patterns, capabilities, and progression. Finally, just as the space program has yielded many inventions and technologies adapted to improving health, we would create deliverable intermediary biological devices adapted from the organ regeneration program that could address clinical patient needs. Such intermediary products would include the fabrication of the dentine enamel junction as a means of improving bonding between dental tissues and restorative ceramics and the creation of a biomimetic periodontal ligament based on the hydroxyapatite binding peptides, identified by Oren et al. and Ma et al.27,28
Steps in Regenerating a Tooth via a Cap-Stage Implant
Our plan required a number of progressive steps: 1) harvest adult stem cells (bone marrow stromal stem cells or tooth-derived postnatal stem cells) or employ NIH-approved human embryonic stem cells; 2) expand the cells in culture, with cell banking for future organ regeneration needs; 3) seed the cells into an intelligent peptide amphiphile-based scaffold that provides an optimized biochemical and biomechanical environment; 4) instruct the cells with spatially targeted, soluble molecular signals and/or induce with porcine sources of odontogenic tissue; 5) confirm that the gene expression profile of the cells demonstrates readiness for the next stage in the odontogenesis pathway; and 6) repeat these steps until the cells have expressed genes associated with the cap stage of odontogenesis.
Education of Health Care Providers and Regenerative Medicine Research Scientists
We planned an annual scientific meeting as part of our reporting obligation to the NIDCR funding agency. This meeting would organize workshops for members of the dental profession on themes emerging from our research project on tooth regeneration. This would foster regenerative medicine education in dentistry and support practicing dentists and graduate dental specialists with the skill sets needed to ensure successful translation of products from the research bench to the patient’s chair-side. In addition to educating the dental community, this project would embrace graduate students and postdoctoral fellows interested in working in this interdisciplinary research environment. Over the decade this project would be active, hundreds of investigators would be trained. With the integrative scientific knowledge gleaned from this project, they would then be able to grow the professional community in the use of regenerative dentistry.
Where We Stand Today on Tooth Regeneration
This is a story that does not yet have an ending. What remains remarkable about this story is that there is no single author: all efforts to date have been written by teams of investigators cross-fertilizing each other with newly won techniques and technologies that will push the process forward. Some chapters in this story have been written and evaluated by scientific, peer-review panels who have weighed in on the merits of our ideas. The story has had mixed outcomes up to now. The NIDCR has decided not to accept any of the applications submitted to it for further consideration of support. The “Request for Proposals” for tooth regeneration by the NIDCR is closed, having been a one-time solicitation.
Some aspects of tooth regeneration have evolved in the form of several intertwined research proposals recently submitted as part of the NIH Roadmap Initiative. A team of investigators from Harvard University plans to regenerate human teeth, as well as the pancreas and heart. The team I have described in this report wishes them every measure of success, as our dreams and their future accomplishments can measurably improve the health of the American people and guide worldwide regenerative medicine strategies. In the words of Kurt Vonnegut (1922–2007), “and so it goes.”
Acknowledgments
This work was supported by National Institute of Dental and Craniofacial Research P20 DE017460 as part of planning a research project for regenerating human teeth and their supporting tissues. The ideas and concepts reported herein are not the exclusive work of the author. Rather, the author is only reporting on a series of interactions with scientists and NIH/NIDCR administrators that shaped our effort to regenerate human teeth and their supporting structure. Any errors are the result of the author’s omission and not attributable to any of his colleagues. The following individuals contributed to the research program proposed to regenerate the tooth, though none of them was provided an opportunity to edit the author’s perceptions; this article represents a singular view of events. Individuals who contributed to the writing of the RFA response are (in alphabetical order): Dr. Yang Chai, University of Southern California; Dr. Yipin Chen, The Ohio State University; Dr. Brian Cox, Teledyne Scientific & Imaging LLC; Dr. Anthony Evan, University of California, Santa Barbara; Dr. Thomas Hart, National Institute for Dental and Craniofacial Research; Dr. Jukka Juenvall, University of Helsinki; Dr. Michael Kahn, University of Southern California; Dr. David Marshall, Teledyne Scientific & Imaging LLC; Dr. Robert McMeeking, University of California, Santa Barbara; Dr. Phillip Messersmith, Northwestern University; Dr. Ian Mitroff, University of Southern California; Dr. Martin Pera, University of Southern California; Dr. Frances Richmond, University of Southern California; Dr. Ram Samudrala, University of Washington; Dr. Mehmet Sarikaya, University of Washington; Dr. Songtao Shi, University of Southern California; Dr. Martha Somerman, University of Washington; Dr. Samuel I. Stupp, Northwestern University; Dr. Candan Tamerler-Behar, University of Washington and Istanbul Technical University; Dr. Irma Thesleff, University of Helsinki; Dr. Cun-Yu Wang, University of Michigan; Dr. Stephen Weiner, Weizmann Institute; and Dr. Yan Zhou, University of Southern California. In addition, Dr. Richard Maas, Harvard University, Dr. Thomas Diekwisch, University of Illinois, and Dr. Eleni Kousvelari, National Institute of Dental and Craniofacial Research, played fundamentally significant roles in shaping the program of research described herein.
REFERENCES
- 1.Maas R, Bei M. The genetic control of early tooth development. Crit Rev Oral Biol Med. 1997;8(1):4–39. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- 2.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67(2):111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 3.Chai Y, Slavkin HC. Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech. 2003;60(5):469–479. doi: 10.1002/jemt.10287. [DOI] [PubMed] [Google Scholar]
- 4.Thesleff I, Wang XP, Suomalainen M. Regulation of epithelial stem cells in tooth regeneration. C R Biologies. 2007;330(6–7):561–564. doi: 10.1016/j.crvi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe PT, Young CS. Test-tube teeth. Sci Am. 2005;293(2):34–41. doi: 10.1038/scientificamerican0805-34. [DOI] [PubMed] [Google Scholar]
- 6.Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147(1):105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thesleff I, Jarvinen E, Suomalainen M. Affecting tooth morphology and renewal by fine-tuning the signals mediating cell and tissue interactions. Novartis Found Symp. 2007;284:142–163. doi: 10.1002/9780470319390.ch10. [DOI] [PubMed] [Google Scholar]
- 8.Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, et al. An integrated gene regulatory network controls stem cell proliferation in teeth. Public Library Sci Biol. 2007;5(6):e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashiro T, Zheng L, Shitaku Y, Saito M, Tsubakimoto T, Takada K, et al. Wnt10a regulates dentin sialophospho-protein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation. 2007;75(5):452–462. doi: 10.1111/j.1432-0436.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Samudrala R. Automated functional classification of experimental and predicted protein structures. Biomed Central Bioinformatics. 2006;7:278. doi: 10.1186/1471-2105-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Samudrala R. The effect of experimental resolution on the performance of knowledge-based discriminatory functions for protein structure selection. Protein Eng Des Sel. 2006;19(9):431–437. doi: 10.1093/protein/gzl027. [DOI] [PubMed] [Google Scholar]
- 12.Hung LH, Samudrala R. An automated assignment-free Bayesian approach for accurately identifying proton contacts from NOESY data. J Biomol NMR. 2006;36(3):189–198. doi: 10.1007/s10858-006-9082-1. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan HL, Berry JE, Foster BL, Gibson CW, Li Y, Kulkarni AB, et al. Amelogenin: a potential regulator of cementum-associated genes. J Periodontol. 2003;74(10):1423–1431. doi: 10.1902/jop.2003.74.10.1423. [DOI] [PubMed] [Google Scholar]
- 14.Foster BL, Popowics TE, Fong HK, Somerman MJ. Advances in defining regulators of cementum development and periodontal regeneration. Curr Top Dev Biol. 2007;78:47–126. doi: 10.1016/S0070-2153(06)78003-6. [DOI] [PubMed] [Google Scholar]
- 15.Stupp SI, LeBonheur VV, Walker K, Li LS, Huggins KE, Keser M, Amstutz A. Supramolecular materials: self-organized nanostructures. Science. 1997;276(5311):384–389. doi: 10.1126/science.276.5311.384. [DOI] [PubMed] [Google Scholar]
- 16.Palmer LC, Velichko YS, de la Cruz MO, Stupp SI. Supramolecular self-assembly codes for functional structures. Philos Trans R Soc Lond A: Math Phys Eng Sci. 2007;365(1855):1417–1433. doi: 10.1098/rsta.2007.2024. [DOI] [PubMed] [Google Scholar]
- 17.Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102(33):11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 20.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Sargeant T, Hulvat JF, Mata A, Bringas JP, Koh CY, et al. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J Bone Miner Res. doi: 10.1359/JBMR.080705. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajangam K, Behanna HA, Hui MJ, Han X, Hulvat JF, Lomasney JW, Stupp SI. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6(9):2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 23.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. Public Library Sci ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12(10):2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 25.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Lin MC, Yao H, Wang H, Zhang AQ, Yu J, et al. Lentivirus-mediated RNA interference targeting enhancer of zeste homolog 2 inhibits hepatocellular carcinoma growth through down-regulation of stathmin. Hepatology. 2007;46(1):200–208. doi: 10.1002/hep.21668. [DOI] [PubMed] [Google Scholar]
- 27.Oren EE, Tamerler C, Sahin D, Hnilova M, Seke UO, Sarikaya M, Samudrala R. A novel knowledge-based approach to design inorganic binding peptides. Bioinformatics. 2007;21:2816–2822. doi: 10.1093/bioinformatics/btm436. [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Zin MT, Zareie MH, Kang MS, Kang SH, Kim KS, et al. Assembly of nanomaterials through highly ordered self-assembled monolayers and peptide-organic hybrid conjugates as templates. J Nanosci Nanotechnol. 2007;7(8):2549–2566. doi: 10.1166/jnn.2007.615. [DOI] [PubMed] [Google Scholar]