Abstract
Study objectives:
Obesity is an important risk factor for obstructive sleep apnea syndrome (OSAS), and weight loss can reduce apnea severity or even lead to resolution in some patients. Effective CPAP therapy may lead to weight loss by any of several proposed mechanisms, including, but not limited to, increased physical activity and increased responsiveness to leptin. This retrospective study sought to determine whether subjects who adhered to prescribed CPAP treatment for OSAS would lose weight, or gain less weight than control subjects who were either untreated or did not adhere to prescribed CPAP treatment.
Methods:
BMI was determined at the time of diagnosis and at follow-up approximately 1 year (10–14 months) later. Subjects who used CPAP ≥ 4 h per night and ≥ 70% of nights were considered treatment subjects. Control subjects used no treatment for OSAS or used CPAP < 4 hours per night or < 70% of nights for 1 year.
Results:
BMI of treatment and control subjects did not significantly differ (p = 0.3157). BMI increased with 1 year of CPAP use in women but not men (p = 0.0228) and in non-obese subjects (p = 0.0443). BMI did not significantly decrease in any group treated with CPAP.
Conclusions:
CPAP was associated with weight gain in some; none lost weight. CPAP may affect weight in ways not measured here. Physicians should stress an active weight loss plan and not assume CPAP alone will lead to weight loss. A larger, prospective study may help clarify these findings.
Citation:
Redenius R; Murphy C; O'Neill E; Hamwi MA; Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med 2008;4(3):205-209.
Keywords: OSAS, OSA, sleep disordered breathing, BMI, CPAP, weight loss, obesity
Obstructive sleep apnea syndrome (OSAS) affects approximately 2% of women and 4% of men aged 30 to 60 years.1 Typical symptoms include loud snoring, restless sleep, and daytime sleepiness or fatigue.2 Anatomical features such as micrognathia or retrognathia, macroglossia, long soft palate, or large uvula can lead to airway obstruction; however, the most common cause of OSAS in adults is obesity.1 An estimated 70% of individuals with OSAS are obese.4,5 Conversely, 40% of obese men and women in the population have OSAS.5
Obesity may narrow the upper airway, making it more easily collapsible and resulting in the block of airflow.5 Factors such as BMI, neck circumference, and the size of the retroglossal space are considered to be the primary determinants of OSAS in obese individuals. Another key factor in obesity is the distribution of fat. An increased waist circumference may lead to OSAS even in non-obese individuals.5 Central obesity is often indicated as the biggest determinant of OSAS. Central obesity results from the buildup of brown adipose tissue, which is an important source of leptin, a satiety hormone that regulates energy intake and expenditure. Obese individuals have high serum leptin levels, which suggest a resistance to this hormone.5 Individuals with OSAS have been found to have even higher serum leptin levels than non-apneic individuals with similar BMIs.5 Leptin has also been shown to improve respiratory control during sleep.6 A resistance to this hormone may increase the likelihood of OSAS in obese individuals.
While obesity is a cause of OSAS, it may also be a result of the disorder. For example, a person who experiences daytime sleepiness may be less active and therefore at greater risk for weight gain. In addition, leptin resistance may make weight management difficult for these individuals.7–9
The most common and effective treatment for OSAS is CPAP, which provides a continuous flow of pressurized air to keep the upper airway from collapsing during sleep. CPAP is effective in 80% to 90% of sleep apneics.1 Despite its effectiveness, 20% to 40% of patients do not use CPAP, and others use it only part of the time.3 Some patients do not tolerate CPAP, and some perceive insufficient benefit to justify its use.
CPAP can improve daytime sleepiness due to sleep apnea, as evidenced by a decrease in mean Epworth Sleepiness Scale scores (ESS) among regular users.10
Since obesity is a common cause of OSAS, weight loss can be an effective treatment. Weight loss may be difficult to achieve in the face of untreated apnea, however, for several reasons. Daytime sleepiness and fatigue may decrease motivation to lose weight. As previously mentioned, leptin resistance in OSAS patients may lead to increased caloric intake.
Effective treatment of apnea may facilitate weight loss in some patients. We hypothesized (1) that subjects with OSAS would lose weight after regular CPAP use for approximately 1 year and (2) that subjects who used CPAP regularly for 1 year would lose more weight or gain less weight than control subjects with OSAS who were not treated or nonadherent with CPAP treatment.
METHODS
Protocol was approved by the Institutional Review Board at the University of Illinois College of Medicine at Peoria. This retrospective chart review identified adult subjects (≥ 18 years of age) diagnosed with OSAS from the patient database at the Illinois Neurological Institute Sleep Center at OSF Saint Francis Medical Center in Peoria, Illinois, from April 2001 to June 2006. OSAS diagnosis was defined as an apnea-hypopnea index (AHI) ≥ 5, as documented by a polysomnogram, plus clinical diagnosis by a sleep specialist. The following inclusion and exclusion criteria were used:
- Inclusion:
- Age ≥ 18 years at time of diagnosis
- Follow-up visit at the Illinois Neurological Institute Sleep Center at approximately 1 year (10–14 months)
- Exclusion:
- Women who were pregnant at the time of diagnosis or before the 1 year follow-up.
- Subjects reporting use of weight loss medications such as orlistat (Xenical), sibutramine (Meridia), phentermine (Adipex-P, Ionamin), rimonabant, phenylpropanolamine (Dexatrim, Acutrim), and ephedrine (Metabolife, Metabolite, MetaboMax).
- Subjects who had undergone bariatric surgery at any time before the 1 year follow-up.
Subjects who used CPAP ≥ 4 hours a night for ≥ 70% of nights (determined using the downloadable data from the patient's CPAP machine) at the time of follow-up 10–14 months after diagnosis were considered treatment subjects. Control subjects had no treatment (CPAP, oral appliances, or surgery) for OSAS or used CPAP < 4 hours a night or < 70% of nights.
Paired, 2-tailed t-tests were used to compare subjects' BMI at the time of diagnosis and at follow-up 1 year later. Unpaired, 2-tailed t-tests were used to compare the change in BMI of treatment and controls subjects 1 year after diagnosis. Data were considered significant at p ≤ 0.05. Data are expressed as means ± SD.
RESULTS
A total of 309 subjects were identified, 228 (64% men) of whom met inclusion criteria. Eighty-one subjects were excluded because they did not have a follow-up in the 10–14 month range, had used weight loss medications, or had used CPAP prior to their initial visit. There was no significant difference in age or initial BMI between treatment (n = 183) and control subjects (n = 45). The average age of subjects was 55.4 ± 12 years. Average initial BMI of treatment subjects was 35.9 ± 10 kg/m2 and control subjects was 33.5 ± 7.8 kg/m2. In the treatment group, 53.0% (97) of subjects gained or lost ≥ 1 kg/m2. In the control group, 55.6% (25) of subjects gained or lost ≥ 1 kg/m2.
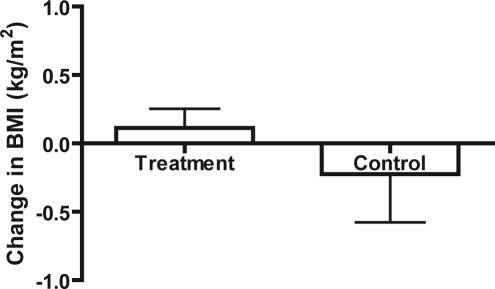
The change in BMI between treatment and control subjects did not significantly differ after 1 year (Figure 1, p = 0.3157).
Figure 1.
Change in BMI of treatment vs. control subjects
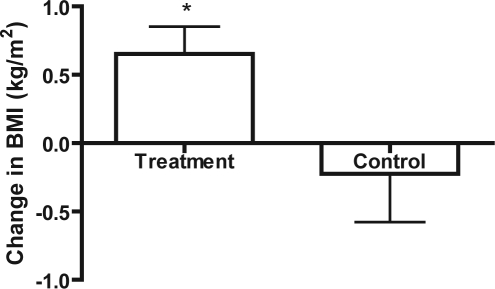
When CPAP compliance criteria were increased to ≥ 7 h/night for ≥ 90% of nights, treatment subjects (n = 63) had a significant increase in BMI compared to controls (n = 45; Figure 2, p = 0.0236).
Figure 2.
Change in BMI of treatment (with high compliance criteria) vs. controls
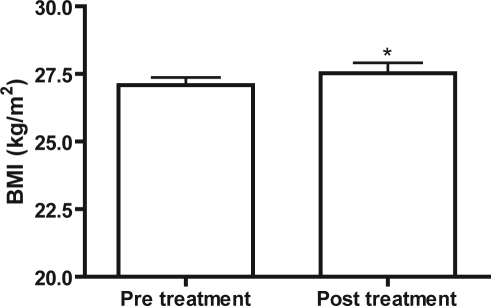
BMI significantly increased in non-obese (BMI < 30) treatment subjects following 1 year of CPAP use (Figure 3, p = 0.0443, n = 46). BMI did not significantly change in obese (BMI ≥ 30) treatment subjects (p = 0.9721, n = 137), obese controls (p = 0.4471, n = 28), or non-obese controls (p = 0.9124, n = 17) after 1 year. When separated by sex, neither non-obese men (p = 0.2240) nor non-obese women (p = 0.0647) in the treatment group lost or gained significant weight (Table 1).
Figure 3.
Pre vs. Post BMI of non-obese treatment subjects
Table 1.
Initial BMI, Final BMI, and Change in BMI for Obese and Non-Obese Subjects
| Group | n | Initial BMI | Final BMI | Change in BMI | p value when initial and final BMI are compared |
|---|---|---|---|---|---|
| Non-Obese treatment | 46 | 27.08 ± 0.3 | 27.53 ± 0.4 | 0.44 ± 0.2 | 0.0443 |
| Non-Obese control | 17 | 26.72 ± 0.5 | 26.77 ± 0.6 | 0.05 ± 0.4 | 0.9124 |
| Obese treatment | 137 | 39.69 ± 0.7 | 39.69 ± 0.6 | 0.00015 ± 0.2 | 0.9721 |
| Obese control | 28 | 38.79 ± 1.4 | 38.40 ± 1.4 | −0.39 ± 0.5 | 0.4471 |
| Non-Obese treatment men | 34 | 27.15 ± 0.3 | 27.39 ± 0.4 | 0.24 ± 0.2 | 0.2240 |
| Non-Obese treatment women | 14 | 26.51 ± 0.7 | 27.47 ± 0.9 | 0.96 ± 0.5 | 0.0647 |
When the change in BMI of non-obese treatment subjects was compared to that of non-obese control subjects, there was no significant difference between the two (p = 0.3790). Similarly, there was no significant difference between obese treatment subjects and obese controls (p = 0.3820). There was no significant difference in the change in BMI when non-obese men were compared to non-obese women in the treatment group (p = 0.0734).
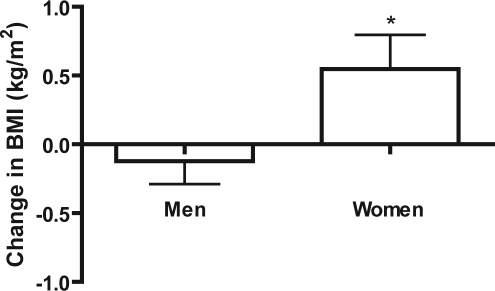
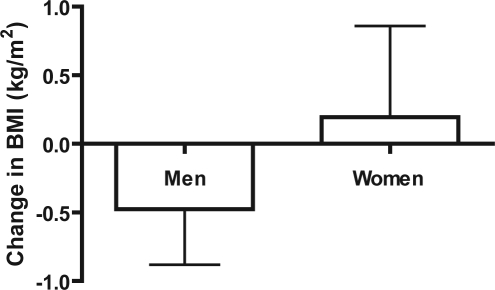
In the treatment group, there was a significant difference in the change in BMI when women were compared to men after 1 year of CPAP use (Figure 4, p = 0.0228). In the control group, the change in BMI of men compared to women did not significantly differ after 1 year (Figure 5, p = 0.3657). No significant difference in the change in BMI was found when men in the treatment group were compared to men in the control group (p = 0.3710). No significant difference was found when women in the treatment group were compared to women in the control group (p = 0.5520). Women in the treatment group gained significant weight with respect to their own initial BMIs (p = 0.0319). No difference was found between initial and final BMI in control women (p = 0.7751), treatment men (p = 0.4645), or control men (p = 0.2491; Table 2).
Figure 4.
Change in BMI of men vs. women (treatment)
Figure 5.
Change in BMI of men vs. women (control)
Table 2.
Initial BMI, Final BMI, and Change in BMI for Subjects Separated by Sex
| Group | n | Initial BMI | Final BMI | Change in BMI | p value when initial and final BMI are compared |
|---|---|---|---|---|---|
| Treatment men | 119 | 34.86 ± 0.7 | 34.73 ± 0.7 | −0.12 ± 0.2 | 0.4645 |
| Treatment women | 64 | 39.63 ± 1.2 | 40.18 ± 1.2 | 0.55 ± 0.3 | 0.0319 |
| Control men | 28 | 33.50 ± 1.5 | 33.03 ± 1.5 | −0.48 ± 0.4 | 0.2491 |
| Control women | 17 | 35.43 ± 2.2 | 35.62 ± 2.0 | 0.19 ± 0.7 | 0.7751 |
DISCUSSION
This study examined the link between regular CPAP usage and weight change. We believed, as do many physicians and patients, that effective use of CPAP may promote weight loss. Contrary to our hypothesis, however, the CPAP treatment group did not lose weight. Anecdotally, some subjects initially lost weight after starting CPAP but subsequently gained it back by follow-up 1 year later. Loube et al. found CPAP facilitated short-term weight loss in 35 obese OSAS patients.11 Weight was measured at an initial visit and at a follow-up visit approximately 6 months later. A study that examines weight changes both at shorter intervals and for longer duration may clarify this discrepancy.
BMI significantly increased in non-obese CPAP users. The increase was not seen in obese treatment subjects, obese controls, or non-obese controls. Although we did not measure leptin levels in this study, previous studies have shown CPAP treatment reduces leptin levels in OSAS patients.12 Harsch et al. found a greater decrease in leptin levels in subjects with a BMI < 30 than in subjects with a BMI ≥ 30 after 8 weeks of CPAP treatment.13 Because low levels of leptin stimulate increased caloric intake, lower leptin levels may have been responsible, at least in part, for the significant weight gain in our non-obese treatment subjects. Further study of CPAP use, weight changes, and leptin levels may advance our understanding of the pathophysiology of weight changes in sleep apnea.
Interestingly, when subjects with very good adherence to CPAP were compared to controls, a significant increase in BMI was seen in those treatment subjects. Perhaps greater CPAP adherence leads to a greater decrease in leptin and resultant increased caloric intake.
BMI increased with CPAP use in women but not men. There was no significant difference between men and women in the control group. In the general population, the incidence of weight gain is twice as high in women as men.14,17 Overweight women are even more likely to gain weight than women of normal weight. Since approximately 98% of women in the treatment group were overweight or obese at baseline, their baseline weight may have made further weight gain more likely.
Obesity results when energy intake exceeds energy expenditure (EE). In normal individuals, EE typically decreases during sleep.15 In individuals with sleep apnea, particularly severe sleep apnea, EE in sleep increases during apneic sleep and decreases with CPAP treatment.18 Treated apneics who do not concomitantly increase their EE while awake may therefore be more prone to a net weight gain while using CPAP. Kajaste et al. implemented a 2-year weight reduction program for 31 obese male sleep apneics.16 Subjects were randomized into 2 groups, CPAP and non-CPAP, to see if CPAP treatment during the first 6 months of the weight reduction program facilitated weight loss. No significant differences between the two groups were observed. Although each group lost significant weight with respect to baseline, this effect was independent of CPAP use. The Kajaste et al. study relied on self-reports of CPAP use, and the authors believed poor adherence to CPAP treatment may have been responsible for the lack of difference between the two groups. Our study obtained objective compliance data, but adherence to CPAP was not associated with weight loss. As noted above, our study found a larger increase in BMI in subjects with very good CPAP adherence. It may be that our group with very high CPAP compliance gained weight related due to reduced energy expenditure during sleep.
Limitations to this study include the small sample size and retrospective study design. The much smaller number of control subjects than treatment subjects requires a greater difference in BMI of controls to be significant. This difference in sample size reduces the power of the statistical findings; however, since the findings of our study concur with other findings, we believe our conclusions to be valid. It is possible the excluded subjects may have lost weight, which biases the results to the null. The retrospective study design limits the availability of data and the control of confounding factors. A larger, prospective study, which includes the measurement of leptin levels and energy expenditure, seems justified.
CPAP may impact weight in ways not accounted for here. Physicians should stress the importance of an active weight loss plan, as many patients may believe that treatment of OSAS with CPAP alone will lead to weight loss. Although CPAP can improve the sleepiness of OSAS, patients may not increase activity levels and caloric expenditure beyond energy intake. Since the majority of OSAS patients are obese when diagnosed, weight management may be difficult for this group in general. Weight change, naturally, involves more factors than leptin levels and improved alertness. Patients must take an active role in weight loss plans and use improved daytime alertness to their advantage to shift the balance of energy intake and energy expenditure. CPAP may facilitate weight loss in some patients but is probably not sufficient on its own for most.
ACKNOWLEDGMENTS
Institution where work was performed: Illinois Neurological Institute Sleep Center.
Financial Support: OSF Saint Francis Foundation.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Aldrich MS. Sleep medicine. Oxford: Oxford University Press; 1999. [Google Scholar]
- 3.Novak M, Mendelssohn D, Shapiro CM, Mucsi I. Diagnosis and management of sleep apnea syndrome and restless legs syndrome in dialysis patients. Semin Dial. 2006;19:210–6. doi: 10.1111/j.1525-139X.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 4.Palmer LJ, Buxbaum SG, Larkin E, et al. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet. 2003;72:340–50. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gami AS, Caples SM, Somers VK. Obesity and obstructive sleep apnea. Endocrinol Metab Clin North Am. 2003;32:869–94. doi: 10.1016/s0889-8529(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell CP, Tankersley CG, Polotsky VP, Schwartz AR, Smith PL. Leptin, obesity, and respiratory function. Respir Physiol. 2000;119:173–80. doi: 10.1016/s0034-5687(99)00111-5. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick M. Leptin and the obesity hypoventilation syndrome: a leap of faith? Thorax. 2002;57:1–2. doi: 10.1136/thorax.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel K, Leproult R, L'Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 10.Hui DSC, Choy DKL, Li TST, et al. Determinants of continuous positive airway pressure compliance in a group of chinese patients with obstructive sleep apnea. Chest. 2001;120:170–6. doi: 10.1378/chest.120.1.170. [DOI] [PubMed] [Google Scholar]
- 11.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight loss in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–7. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]
- 12.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–12. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 13.Harsch IA, Konturek PC, Koebnick C, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–7. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ. Prevalence of overweight and weight gain in the United States. Am J Clin Nutr. 1992;55:495S–502S. doi: 10.1093/ajcn/55.2.495s. [DOI] [PubMed] [Google Scholar]
- 15.Grunstein R. Endocrine disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. 4th ed. Philedephia: Elsevier Saunders; 2005. pp. 1242–3. [Google Scholar]
- 16.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med. 2004;5:125–31. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Hensrud DD, Klein S. Extreme obesity: a new medical crisis in the United States. Mayo Clin Proc. 2006;81:S5–10. doi: 10.1016/s0025-6196(11)61175-0. [DOI] [PubMed] [Google Scholar]
- 18.Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271:E1036–43. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]