Abstract
Study Objectives:
To evaluate the polysomnographic efficacy and the safety of a range of doses of eszopiclone relative to placebo in patients with primary insomnia. Zolpidem 10 mg was included as an active control.
Methods:
This multicenter, randomized, crossover study enrolled patients aged 21–64 years meeting the DSM-IV criteria for primary insomnia (n = 65). Patients received 2 nights treatment each with placebo, eszopiclone 1 mg, 2 mg, 2.5 mg, or 3 mg, and zolpidem 10 mg after randomization to one of 6 treatment sequences. Visits were separated by a 3–7 day washout. Objective efficacy was assessed by polysomnography (PSG). The primary endpoint was latency to persistent sleep (LPS); key secondary endpoints were sleep efficiency (SE) and wake time after sleep onset (WASO); other endpoints included wake time during sleep (WTDS) and number of awakenings (NAW), as well as patient-reported variables.
Results:
LPS and SE were significantly different than placebo for all active treatments (p < 0.05 for all). Significant differences from placebo were noted in the 3 objective sleep maintenance measures (WASO, WTDS, and NAW) for eszopiclone 3 mg (p < 0.05), which was not the case for zolpidem 10 mg or the other eszopiclone doses. The incidence of central nervous system adverse events was 23.4% for zolpidem 10 mg, 6.2% to 12.5% for the eszopiclone doses, and 7.9% for placebo.
Conclusions:
Relative to placebo, all active treatments were effective in reducing LPS and increasing SE. Eszopiclone 3 mg was significantly different from placebo on the 3 PSG measures of sleep maintenance (WASO, WTDS, and NAW). Significant differences between zolpidem 10 mg and eszopiclone (2 mg or 3 mg) were not observed for PSG-measured outcomes, although the study was not powered to detect differences between the active drug conditions.
Citation:
Erman MK; Zammit G; Rubens R; Schaefer K; Wessel T; Amato D; Caron J; Walsh JK. A polysomnographic placebo-controlled evaluation of the efficacy and safety of eszopiclone relative to placebo and zolpidem in the treatment of primary insomnia. J Clin Sleep Med 2008;4(3):229–234.
Keywords: Insomnia, eszopiclone, zolpidem, polysomnography, sleep maintenance
Insomnia is defined as a complaint of difficulty with sleep initiation, sleep maintenance, and/or sleep quality in the presence of adequate opportunity. Up to one third of adults report one or more insomnia symptoms, and approximately 10% of the adult population have chronic insomnia, or insomnia lasting more than one month.1 Insomnia's impact on quality of life,2,3 health care costs,4 and absenteeism5 make it a significant public health issue. In addition, insomnia can affect perception of daytime functioning such as impaired concentration, faulty memory, reduction in the ability to perform daily tasks, and loss of enjoyment of personal relationships.6
Many patients with insomnia not only experience difficulty with sleep onset, but also report problems with sleep maintenance and sleep quality at some point in time.7–11 Eszopiclone is a single-isomer, benzodiazepine receptor antagonist indicated for the treatment of insomnia that has been shown to improve sleep onset and sleep maintenance in non-elderly and elderly adults. Eszopiclone 3 mg significantly improved patient-reported measures of sleep onset, maintenance, duration, and quality, as well as daytime insomnia symptoms throughout two 6-month investigations of nightly treatment in adults with primary insomnia.12,13
This manuscript describes the findings of a study conducted to identify the dose(s) of eszopiclone most suited for development. We used polysomnography to investigate the efficacy of 4 doses of eszopiclone versus placebo in the treatment of adults with primary chronic insomnia. Zolpidem 10 mg was included as an active control to allow qualitative comparisons of eszopiclone. Although this study was not powered to test hypotheses of drug-drug differences, no other placebo-controlled, PSG study including zolpidem and eszopiclone has been published to our knowledge.
METHODS
This was a randomized, 6-way crossover study performed in 7 sleep laboratories in the United States between October 2000 and April 2001. Institutional review boards of participating institutions approved the protocol, and all patients provided written, informed consent before screening. The study was conducted in accordance with the standards of good clinical practice and followed guidelines and regulations established by the Declaration of Helsinki (1989).
Eligible patients were aged 21 to 64 years, met DSM-IV criteria for primary insomnia, and reported a sleep duration ≤6.5 h and time to fall asleep >30 min each night for at least 1 month. The clinical screening procedures included medical history, physical examination, a 12-lead electrocardiogram (ECG) and clinical laboratory evaluations. Exclusion criteria included any clinically significant and/or unstable medical condition or chronic disease; DSM-IV Axis I or Axis II psychiatric illness or personality disorder; sleep apnea or restless legs syndrome/periodic leg movements disorder; history of substance abuse/dependence; use of any psychotropic, hypnotic, or other medications (including herbal supplements or melatonin) known to affect sleep; or use of other prescription or over-the-counter medications (including those containing caffeine, diphenhydramine, or ephedrine) known to affect sleep or to be contraindicated for use with hypnotics.
Screening consisted of up to 3 nights in the sleep laboratory. On the first PSG night, respiratory and leg electromyography recordings allowed the exclusion of patients with an apnea-hypopnea index ≥10 or a periodic leg movement arousal index ≥10. Eligible patients also had to have a 2-night mean latency to persistent sleep (LPS) of ≥20 min with no night <15 min, plus either a 2-night mean total sleep time (TST) ≤420 min (7 h) or a WASO of ≥20 min for at least 2 nights with no nights <15 min. Consequently, patients were asked to return for a third night only if they continued to meet PSG inclusion criteria after 2 nights.
Patients received, in random order, placebo, eszopiclone 1 mg, 2 mg, 2.5 mg, and 3 mg, and zolpidem 10 mg. An unblinded third-party observer received 2 sets of 6 single-dose, opaque bottles (one set for each night of dosing) labeled in the order in which they were to be administered to the patient. Within each set, 5 bottles were prepackaged and contained the eszopiclone and/or matching placebo. Doses of eszopiclone were packaged utilizing combinations of 1 mg and 1.5 mg eszopiclone tablets and/or matching placebo, so that each bottle contained 2 tablets. For example, the bottle for the eszopiclone 1-mg dose contained one 1-mg eszopiclone tablet and 1 matching placebo tablet; the bottle for the eszopiclone 2 mg dose contained two 1-mg eszopiclone tablets, etc. The sixth empty bottle was filled by the third-party observer with zolpidem 10 mg and placebo that had been supplied to the site in bulk. The 6 bottles were given to blinded site personnel who administered them to patients in the clinic. The site personnel and patients were instructed not to examine the contents of any bottle, and all medications were administered to the patient directly from the bottle under the supervision of the blinded site personnel. Despite these requirements, the zolpidem treatment arm cannot technically be considered blinded.
Sleep studies were conducted on 2 consecutive nights for each treatment, and treatments were separated by 3 to 7 days. Patients were required to arrive at the sleep laboratory at least 2 h before their median bedtime, and study drug was administered 30 min before the beginning of PSG. PSG duration was held constant at 8 h, and all PSGs were scored in a blinded manner by trained individuals at a central scoring site.14 Patient-reported sleep variables and ratings of daytime alertness and ability to function were collected with morning and evening questionnaires. Morning questionnaires were administered within 30 min of awakening; evening questionnaires were administered each night (except on the first night) prior to dosing. Clinical laboratory measures, physical and neurological examinations, ECG recordings, and vital signs were obtained at screening and at an end-of-study visit (5 to 7 days after the last dose of study medication). Patients recorded adverse events throughout the trial and were questioned about adverse events upon awakening each morning in the sleep laboratory.
Objectives and Endpoints
The primary objective of the study was to evaluate the hypnotic efficacy of 4 doses of eszopiclone relative to placebo in adult subjects with primary insomnia, as measured by PSG. Other objectives were to: (1) assess the hypnotic efficacy of zolpidem 10 mg relative to placebo, (2) evaluate the safety of eszopiclone compared with placebo and zolpidem 10 mg, (3) assess the effect of treatment on the daytime symptoms of insomnia, and (4) make qualitative comparisons among the 5 drug conditions.
The primary efficacy endpoint was LPS (defined as the time from the onset of the PSG recording to the start of 10 continuous min of sleep) and there were 2 key secondary endpoints: sleep efficiency (SE; defined as the number of non-wake epochs from the beginning of recording to the end of recording or total sleep time in min, divided by total recording time in min x 100), and WASO (defined as the number of min of wakefulness after the onset of persistent sleep to the end of the PSG recording). Other secondary endpoints included 2 additional PSG measures of sleep maintenance: objective wake time during sleep (WTDS) and number of awakenings (NAW). WTDS represents the sum of all wake time after LPS until the final awakening of the night (i.e., WASO minus awake time after the last epoch of sleep).
Patient-reported efficacy measures were determined from the morning questionnaire and included sleep latency (sSL), sTST, sWASO, and sNAW, sleep quality, and depth of sleep. Sleep quality and depth of sleep were assessed using a 0-100 point Visual Analog Scale (VAS). Daytime function measures were determined from the evening (ability to function and daytime alertness) and morning questionnaires (morning sleepiness) using a 0–100 point VAS.
Statistical Analysis
The intent-to-treat (ITT) population consisted of all randomized participants who received at least one dose of study medication. All statistical tests were 2-sided and were carried out at the 0.05 level of significance using SAS software (Version 6.12 or higher). For each efficacy variable, the mean of the 2 consecutive nights on each treatment was used for the analyses. A sample size of 60 was chosen to confer power of at least 80% to detect differences between the individual eszopiclone dose groups and placebo for the primary endpoint (LPS: 15-min treatment difference) and one of the key secondary endpoints (SE: difference of 6.25%) simultaneously. This estimate assumed standard deviations of 30 min for LPS and 13% for SE on each condition.
For the primary efficacy analysis, comparisons of the eszopiclone 2 mg, 2.5 mg, and 3 mg dose groups combined versus placebo were based on an ANOVA model with treatment, visit, and sequence as fixed effects, and subject nested within sequence as a random effect. Data were rank-transformed prior to analysis. A contrast test between the 3 eszopiclone groups combined and the placebo group was used to assess the overall treatment effect of eszopiclone. Each of the 3 eszopiclone dose groups were also compared with the placebo group using pairwise contrasts from the model. A Fisher's protected approach was used to adjust for multiple comparisons. Statistical inferences based on the planned pairwise comparisons of each of the 3 eszopiclone groups with the placebo group was made only if the overall test was significant at the two-sided 5% level. The key secondary efficacy variables (SE and WASO) were analyzed using same method.
For all other secondary variables, rank-transformed data were also analyzed using the primary analysis model, but no overall test of the highest 3 eszopiclone dose groups versus placebo was conducted. Eszopiclone doses of 2 and 3 mg, and zolpidem 10 mg were compared with placebo and each other using the ANOVA model with pairwise contrasts. This approach limited the total number of unprotected paired comparisons (i.e., 66, of which 26 were significant) to those active drug conditions relevant to the treatment of insomnia in the adult population.
Pairwise comparisons among treatments were pre-planned for the PSG endpoints and patient-reported sleep, and post hoc on daytime function endpoints.
RESULTS
Of the 65 patients randomized, all received at least 1 dose of study medication (ITT population) and 63 completed the study. One patient withdrew voluntarily, and one was withdrawn due to use of an excluded medication. The mean age of the patients was 40.6 years, with a range of 22 to 63 years, and females outnumbered males approximately 3:1.
The overall tests of no difference between placebo and the 3 highest eszopiclone doses combined were statistically significant for the primary (LPS) and key secondary (SE and WASO) sleep efficacy variables.
All active treatments significantly reduced median LPS relative to placebo (p < 0.05) by 42% to 55%. The median LPS was 13.1 min for both eszopiclone 3 mg and zolpidem 10 mg. The median LPS was 29.0, 16.8, 15.5, and 13.8 min for the placebo, eszopiclone 1 mg, 2 mg, and 2.5 mg dose groups, respectively. The 2 highest doses of eszopiclone (2.5 mg and 3 mg) and zolpidem demonstrated significantly lower LPS when compared with eszopiclone 1 mg (p < 0.05).
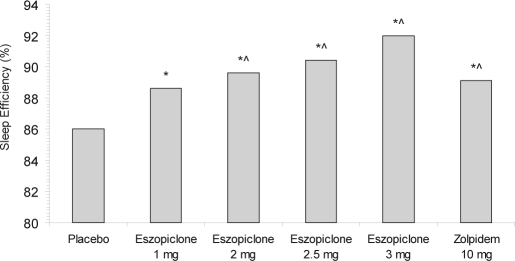
Significant differences were also noted between all active treatments in SE relative to placebo (p < 0.05). Eszopiclone 2 mg, 2.5 mg, and 3 mg, and zolpidem 10 mg demonstrated significantly higher SE when compared with eszopiclone 1 mg (p < 0.05). (Figure 1)
Figure 1.
Median objective sleep efficiency (SE), as determined by PSG. *p < 0.05 vs. placebo; ∧p < 0.05 vs. eszopiclone 1.0 mg.
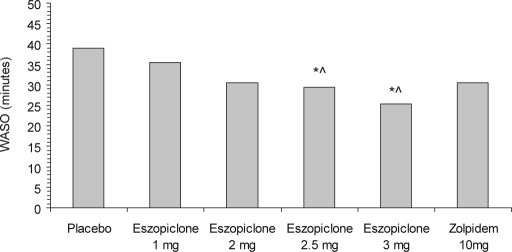
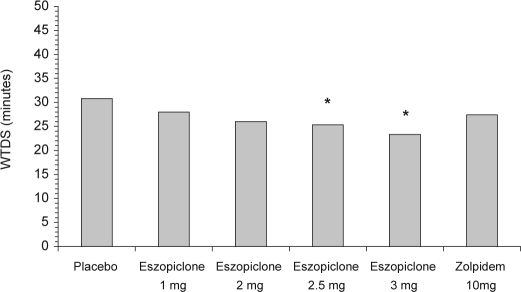
Treatment with eszopiclone 3 mg resulted in significant differences compared with placebo for PSG WASO (Figure 2), WTDS (Figure 3), and NAW. Eszopiclone 2.5 mg also demonstrated significant differences versus placebo for WASO and WTDS. Neither of the lower doses of eszopiclone nor zolpidem 10 mg were statistically different from placebo for WASO or WTDS. Comparisons of eszopiclone 3 mg and zolpidem 10 mg were not significantly different for WASO (p = 0.12), for WTDS (p = 0.07), or for NAW (p = 0.10).
Figure 2.
Median wake time after sleep onset (WASO), as determined by PSG. *p <0.02 vs. placebo; ∧p < 0.02 vs. eszopiclone 1.0 mg.
Figure 3.
Median wake time during sleep (WTDS), as determined by PSG. *p < 0.05 vs. placebo. Paired comparisons for WTDS were not performed for the eszopiclone 1 mg and 2.5 mg treatment arms. Paired comparisons for WTDS were performed only among the placebo, eszopiclone 2 mg, eszopiclone 3 mg, and zolpidem arms.
In general, treatment with eszopiclone 2 mg and 3 mg and zolpidem 10 mg showed improvements in patient-reported measures of sleep relative to placebo (Table 1). Both doses of eszopiclone and zolpidem 10 mg significantly improved sSL, sTST, quality of sleep, and depth of sleep relative to placebo (p < 0.05). Eszopiclone 2 mg and 3 mg and zolpidem 10 mg were significantly different from placebo for sNAW and sWASO (p < 0.05). Morning sleepiness was significantly less with eszopiclone 3 mg compared with placebo (p < 0.05). Evening ratings of daytime alertness were significantly increased with eszopiclone 2 mg and with zolpidem 10 mg compared with placebo (p < 0.05), and daytime ability to function was significantly improved for eszopiclone 2 mg and 3 mg and zolpidem 10 mg compared with placebo (p < 0.05). (Table 1)
Table 1.
Summary of Patient-reported and Next-day Insomnia Measures
| Placebo (n=63) |
Eszopiclone |
Zolpidem 10 mg (n=64) |
||||
|---|---|---|---|---|---|---|
| 1 mg# (n=63) | 2 mg (n=63) | 2.5 mg# (n=65) | 3 mg (n=64) | |||
| Patient-reported measures | ||||||
| sSL (min) | ||||||
| Median | 47.5 | 27.5 | 25.0* | 25.0 | 25.0* | 25.0* |
| Mean (SD) | 54.4 (39.3) | 37.5 (29.2) | 31.6 (25.7) | 42.2 (112.0) | 32.9 (34.0) | 30.8 (27.8) |
| sTST (min) | ||||||
| Median | 375.0 | 382.5 | 412.5* | 420.0 | 420.0* | 411.3* |
| Mean (SD) | 362.0 (63.8) | 432.5 (487.5) | 427. 5 (246.6) | 450.3 (439.1) | 453.1 (462.6) | 438.7 (406.4) |
| sNAW | ||||||
| Median | 3.5 | 3.0 | 3.0* | 3.0 | 2.5* | 2.5* |
| Mean (SD) | 3.7 (2.1) | 3.6 (1.9) | 3.0 (1.4) | 3.1 (1.5) | 3.0 (2.2) | 3.1 (1.8) |
| sWASO (min) | ||||||
| Median | 42.5 | 35.0 | 27.5* | 25.0 | 28.8* | 30.0* |
| Mean (SD) | 56.6 (48.3) | 83.7 (228.3) | 69.0 (229.8) | 46.2 (49.3) | 50.3 (124.0) | 74.7 (226.6) |
| Sleep Quality (VAS) | ||||||
| Median | 43.5 | 47.0 | 58.0* | 55.0 | 62.0* | 56.0* |
| Mean (SD) | 44.4 (19.9) | 49.5 (23.3) | 57.2 (20.9) | 54.4 (21.7) | 59.9 (22.8) | 55.6 (21.1) |
| Sleep Depth (VAS) | ||||||
| Median | 40.3 | 46.0 | 56.5* | 53.0 | 59.5* | 56.5* |
| Mean (SD) | 44.0 (19.3) | 48.9 (22.9) | 55.7 (22.5) | 54.4 (22.0) | 58.7 (23.2) | 55.1 (22.0) |
| Next-day insomnia measures | ||||||
| Morning sleepiness (VAS) | ||||||
| Median | 36.8 | 42.3 | 42.0 | 45.3 | 44.5* | 43.3 |
| Mean (SD) | 39.8 (20.1) | 43.8 (22.0) | 44.6 (21.3) | 44.7 (19.9) | 45.4 (22.8) | 43.5 (20.4) |
| Daytime alertness (VAS) | ||||||
| Median | 40.0 | 57.0 | 56.5* | 50.0 | 56.0 | 62.5* |
| Mean (SD) | 47.0 (24.1) | 52.5 (24.6) | 55.2 (24.3) | 50.7 (25.6) | 52.0 (27.5) | 55.8 (27.7) |
| Daily ability to function (VAS) | ||||||
| Median | 50.0 | 58.0 | 59.0* | 51.0 | 60.0* | 53.0* |
| Mean (SD) | 52.2 (22.9) | 58.7 (21.9) | 59.5 (22.4) | 54.1 (23.8) | 56.6 (26.2) | 56.2 (26.4) |
sSL, sTST, sNAW, and sWASO = patient-reported subjective measures of sleep latency, total sleep time, number of awakenings, and wake time after sleep onset, respectively; VAS = Visual Analog Scale of 0 to 100 mm (higher values are more positive, except for sleepiness)
* p <0.05 vs. placebo; # Paired comparisons between placebo and the 1 mg treatment arm, and between placebo and the 2.5 mg treatment arm were not performed.
The most common adverse events were headache, unpleasant taste, somnolence, dizziness, and nausea (Table 2). The overall p-values indicated statistically significant differences among the treatments for CNS adverse events, hallucinations, and dizziness (Table 2). The overall rate of CNS events was 7.9% for placebo, 6.2% to 12.5% for the eszopiclone groups, and 23.4% for zolpidem 10 mg. Hallucinations occurred only with zolpidem (4.7% of patients). The rate of dizziness was 4.8% in the placebo group, 4.7% for the eszopiclone 3 mg group, and 10.9% for the zolpidem group. There were no clinically relevant changes in other safety measures, including blood profiles, ECGs, physical findings, or vital signs.
Table 2.
Summary of Adverse Events
| Adverse event (%) | Placebo (n = 63) |
Eszopiclone |
Zolpidem 10 mg (n = 64) |
Overall p-value | |||
|---|---|---|---|---|---|---|---|
| 1 mg (n = 63) | 2 mg (n = 63) | 2.5 mg (n = 65) | 3 mg (n = 64) | ||||
| All adverse events | 25.4 | 19.0 | 25.4 | 35.4 | 32.8 | 32.8 | 0.300 |
| All CNS adverse events | 7.9 | 7.9 | 6.3 | 6.2 | 12.5 | 23.4 | 0.012 |
| Dizziness | 4.8 | 3.2 | 0 | 0 | 4.7 | 10.9 | 0.017 |
| Headache | 9.5 | 4.8 | 6.3 | 3.1 | 9.4 | 9.4 | 0.590 |
| Somnolence | 3.2 | 4.8 | 3.2 | 3.1 | 4.7 | 9.4 | 0.530 |
| Hallucinations | 0 | 0 | 0 | 0 | 0 | 4.7 | 0.010 |
| Unpleasant taste | 1.6 | 4.8 | 4.8 | 9.2 | 7.8 | 0 | 0.120 |
| Nausea | 3.2 | 3.2 | 1.6 | 3.1 | 3.1 | 6.3 | 0.810 |
Includes adverse events that were reported by ≥ 4% of patients in any treatment group.
DISCUSSION
This study was an eszopiclone dose-response study with zolpidem 10 mg as an active comparator. Based upon the results of this and other studies, eszopiclone 2 mg and 3 mg were investigated further for the treatment of insomnia in adults. A dose-response was noted for eszopiclone, supported by the finding that eszopiclone 2.5 mg and 3 mg were significantly different than the lowest dose of eszopiclone studied (1 mg) for LPS and SE, while among active treatments only eszopiclone 3 mg was significantly different than both placebo and eszopiclone 1 mg for WASO, NAW, and WTDS. The finding that higher doses of eszopiclone were more effective for sleep maintenance insomnia is consistent with the results of other PSG studies.15,16
The results for zolpidem reported in the current trial are consistent with previously published studies in which zolpidem 10 mg treatment resulted in significant improvements in objective LPS and SE relative to placebo without differences in objective measures of sleep maintenance, including WASO and WTDS.16,17 There were also significant differences relative to placebo of zolpidem 10 mg on next-day function measures (increased daytime alertness and daily ability to function). Consistent with previously reported findings in patients with primary insomnia12,13,16 and insomnia comorbid with other diseases,19,20,21 eszopiclone 3 mg also improved measures of sleep onset, sleep maintenance, and next-day function in the current trial.
While the pairwise tests of zolpidem relative to placebo, and the 2 mg and 3 mg doses of eszopiclone relative to zolpidem were not adjusted for multiple comparisons, the current investigation has value as these two commonly used treatments for insomnia were evaluated in a controlled design and well-defined study population. Significant differences were not noted when zolpidem 10 mg was compared with eszopiclone 2 mg and 3 mg on all but one sleep efficacy measure, although the study was not powered to detect differences between eszopiclone and zolpidem. The acute treatment schedule of the present study did not allow for assessments of tolerance or an evaluation of discontinuation effects. The doses of eszopiclone used acutely in this study were generally well tolerated. Unpleasant taste was the most common adverse event observed with eszopiclone,16 and this event was seen in 4.8% to 9.2% of eszopiclone-treated patients in this study and in none of the zolpidem-treated patients. For all benzodiazepine receptor agonist hypnotics, CNS adverse events are most relevant to clinicians and tend to have a dose-related incidence. In this study, approximately twice as many patients reported CNS adverse events with zolpidem 10 mg as compared with eszopiclone 3 mg, with dizziness, somnolence, nausea, and hallucinations comprising most of the difference. There were no reports of hallucinations on any eszopiclone dose. The differences between eszopiclone and zolpidem in the occurrence of CNS adverse effects may be due in part to differences in relative peak plasma concentrations, or to differential allosteric modulation of GABA activity associated with different binding profiles at various benzodiazepine receptors.22
In this study of adults with chronic insomnia, all active treatments produced statistical improvements in primary and key secondary objective PSG measures of sleep induction and sleep duration relative to placebo. Eszopiclone 3 mg also yielded improvements in the objective measures of sleep maintenance. Patient-reported insomnia variables were improved following treatment with the 2 mg and 3 mg doses of eszopiclone and zolpidem 10 mg. The treatments were well-tolerated in this study, and the efficacy and safety results are consistent with previously published randomized clinical trials with eszopiclone.
ACKNOWLEDGMENT
Support for this study provided by Sepracor Inc.
Footnotes
Disclosure Statement
Support for this study provided by Sepracor Inc. Dr. Erman has received research support from Sanofi, Takeda, Pfizer, Pharmacia, Merck, Schwarz, Organon, GlaxoSmithKline, Eli Lilly, Wyeth, and Neurocrine Biosciences; has consulted for Sanofi, Mallinckrodt, Cephalon, Takeda, and Neurocrine Biosciences; is on the advisory board of Sanofi, Cephalon, Takeda, and Neurocrine Biosciences; is on the speaker's bureau of Sanofi and Takeda; and has financial interests in Cephalon, Forest, Neurocrine Biosciences, Pfizer, Sepracor, Sanofi, and Somaxon. Dr. Zammit has received research support from Ancile Pharmaceuticals, Arena, Aventis, Cephalon, Elan, Epix, Evotec, Forest, GlaxoSmithKline, H. Lundbeck A/S, King, Merck, Neurim, Neurocrine Biosciences, Neurogen, Organon, Orphan Medical, Pfizer, Respironics, Sanofi-Aventis, Schering-Plough, Sepracor, Somaxon, Takeda, Transcept, UCB Pharma, Predix, Vanda, and Wyeth-Ayerst; has consulted for Aventis, Boehringer Ingelheim, Cephalon, Elan, GlaxoSmithKline, Jazz, King, Merck, Neurocrine Biosciences, Organon, Pfizer, Renovis, Sanofi-Aventis, Select Comfort, Sepracor, Shire, and Takada; has received honoraria from Neurocrine Biosciences, Kink, McNeil, Sanofi-Aventis, Sanofi-Synthelabo, Sepracor, Shire, and Takeda; and has ownership/Directorship in Clinilabs, Inc., Clinilabs IPA, Inc., and Clinilabs Physician Services, PC. Dr. Walsh has consulted for Pfizer, Sanofi-Aventis, Cephalon, Organon, Neurocrine Biosciences, Takeda, Actelion, Sepracor, Elan, Guilford, Respironics, Merck KgaA-Darmstadt, King, TransOral, Neurogen, GlaxoSmithKline, SleepTech, Somaxon, Eli Lilly, Evotec, Neurosciences, and Merck. Drs. Rubens, Wessel, Amato, Caron, and Ms. Schaefer are employees of Sepracor.
REFERENCES
- 1.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22:S354–8. [PubMed] [Google Scholar]
- 2.Hatoum HT, Kon SD, Kania CM, Wong JM, Mendelson WB. Insomnia, health-related quality of life and healthcare resource consumption: a study of managed-care organization enrollees. Pharmacoeconomics. 1998;14:629–37. doi: 10.2165/00019053-199814060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 4.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30:260–70. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 5.Godet-Cayre V, Pelletier-Fleury N, Le VM, Dinet J, Massuel MA, Leger D. Insomnia and absenteeism at work. Who pays the cost? Sleep. 2006;29:179–84. doi: 10.1093/sleep/29.2.179. [DOI] [PubMed] [Google Scholar]
- 6.Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65:13–9. [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22:S347–53. [PubMed] [Google Scholar]
- 8.Leger D, Poursain B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. 2005;21:1785–1792. doi: 10.1185/030079905X65637. [DOI] [PubMed] [Google Scholar]
- 9.Bliwise DL, King AC, Harris RB, Haskell WL. Prevalence of self-reported poor sleep in a healthy population aged 50–65. Soc Sci Med. 1992;34:49–55. doi: 10.1016/0277-9536(92)90066-y. [DOI] [PubMed] [Google Scholar]
- 10.Shochat T, Umphress J, Israel AG, Ancoli-Israel S. Insomnia in primary care patients. Sleep. 1999;22:S359–65. [PubMed] [Google Scholar]
- 11.Hohagen F, Kappler C, Schramm E, Riemann D, Weyerer S, Berger M. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening--temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994;17:551–4. [PubMed] [Google Scholar]
- 12.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 13.Walsh J, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep states of human subjects. NIH. 1968. Publication No. 204. [DOI] [PubMed]
- 15.Rosenberg R, Caron J, Roth T, Amato D. An assessment of the efficacy and safety of eszopiclone in the treatment of transient insomnia in healthy adults. Sleep Med. 2005;6:15–22. doi: 10.1016/j.sleep.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Zammit G, McNabb L, Caron J, Amato D, Roth T. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20:1979–91. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]
- 17.Scharf MB, Roth T, Vogel GW, Walsh JK. A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192–9. [PubMed] [Google Scholar]
- 18.Ware JC, Walsh JK, Scharf MB, Roehrs T, Roth T, Vogel GW. Minimal rebound insomnia after treatment with 10-mg zolpidem. Clin Neuropharmacol. 1997;20:116–25. doi: 10.1097/00002826-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Soares CN, Joffe H, Rubens R, Caron J, Roth T, Cohen L. Eszopiclone in patients with insomnia during perimenopause and early postmenopause. Obstet Gynecol. 2006;108:1402–10. doi: 10.1097/01.AOG.0000245449.97365.97. [DOI] [PubMed] [Google Scholar]
- 21.Pollack M, Kinrys G, Krystal A, et al. Eszopiclone co-administered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:551–62. doi: 10.1001/archpsyc.65.5.551. [DOI] [PubMed] [Google Scholar]
- 22.Smith AJ, Alder L, Silk J, et al. Effect of α subunit on allosteric modulation of ion channel function in stably expressed human recombinant λ-aminobutyric acid(A) receptors determined using (36)Cl ion flux. Mol Pharm. 2001;59:1108–18. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]