Abstract
Study Objectives:
We investigated the prevalence of silent cerebrovascular lesions in patients with obstructive sleep apnea (OSA) and the correlation between OSA severity and prevalence of silent cerebrovascular lesions in Japanese patients.
Methods:
Study subjects were 192 polysomnography (PSG)-confirmed patients who visited the sleep disorders clinic in our university hospital. None had a history of cerebrovascular disease (CVD). We performed a cross-sectional study on OSA severity and the prevalence of silent cerebrovascular lesions detected by brain MRI analysis.
Results:
The control (AHI < 5/h) group included 19 subjects with a mean AHI of 1.7 ± 1.6/h, the mild OSAS (AHI 5 to < 15/h) group included 25 patients with a mean AHI of 9.5 ± 3.7/h, the moderate OSAS (AHI 15 to < 30/h) group included 35 patients with a mean AHI of 22.0 ± 7.0/h while the severe OSAS (AHI ≥ 30/h) group included 113 patients with a mean AHI of 59.9 ± 20.5/h. A larger percentage of patients with severe OSAS had a higher BMI and hyperglycemia than those with mild or moderate OSAS and control subjects (p < 0.05). Silent lacunar infarction was identified in 4 (21.1%) control subjects, 3 (12.0%) patients with mild OSA, 17 (48.6%) with moderate OSA and 61 (54.0%) with severe OSA. Among control subjects and the mild, moderate, and severe OSA groups, 4 (21.1%), 5 (20.0%), 19 (54.3%) and 61(54.0%), respectively, had periventricular hyperintensity (PVH); most PVH was mild to moderate.
Conclusion:
Results indicate that patients with moderate to severe (AHI ≥ 15/h) OSA have a higher prevalence of silent cerebrovascular lesion than those with less severe OSA.
Citation:
Nishibayashi M; Miyamoto M; Miyamoto T; Suzuki K; Hirata K. Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesions. J Clin Sleep Med 2008;4(3):242–247.
Keywords: obstructive sleep apnea (OSA), silent lacunar infarction, periventricular hyperintensity (PVH), silent cerebrovascular lesion, polysomnography (PSG)
The prevalence of sleep disordered breathing among middle-aged adults was reported to be 4% in men and 2% in women in the cross-sectional survey of the Wisconsin Sleep Cohort Study.1,2 Prevalence estimates from studies with probability samples range for obstructive sleep apnea syndrome (OSAS) of at least mild severity as defined by apnea-hypopnea index (AHI) ≥ 5/h, from 3% to 28%; for OSAS of at least moderate severity defined by AHI ≥ 15/h, estimates range from 1% to 14%.3 Tishler et al. reported a 5-year follow-up survey on the population in the Cleveland Family Study. Among subjects with an AHI < 5/h, moderate sleep disordered breathing was found in 7.5% while mildly to moderate sleep disordered breathing was found in 16%.4 In Asian countries, on the other hand, a survey of Korean middle-aged or senior residents showed that sleep disordered breathing in those with an AHI ≥ 5/h was prevalent in 27% of males and 16% of females. When the coexistence of excessive daytime sleepiness was defined as OSAS, OSAS was prevalent in 4.5% of males and 3.2% of females.5 Asians have higher morbidity from severe OSAS than Caucasians.6 In addition, concomitance of obesity with OSAS is less frequent in Asians,7 and in this population OSAS becomes severe despite mild obesity.8 As causal factors, the craniomandibular factors7 have been suggested. All Japanese people may be at risk for OSAS.
Sleep fragmentation associated with nocturnal respiratory events and hypoxia adversely affects various physiological functions such as those of the respiratory, cardiovascular, and endocrine systems as well as mental function. Obstructive sleep apnea (OSA) might also increase morbidity and mortality related to ischemic heart failure, arrhythmia, and cerebrovascular disease (CVD).9–11 The Sleep Heart Health Study, conducted in 2001 in the USA, involved performing polysomnography (PSG) on 6,424 healthy subjects aged over 40 years. The results revealed that subjects with an AHI of more than 11.0/h had a significantly higher prevalence of CVD, coronary heart disease, and heart failure than those with an AHI of 0 to 1.3/h.9 In fact, OSA was identified as a risk factor for CVD independent of other risk factors, such as hypertension.12 Furthermore, accumulating evidence suggests that OSA severity correlates with the development of CVD.9 Also, it has been reported that the more severe the OSA, the higher the risk of CVD development.5,10,12 On the other hand, it has been suggested that the presence of silent cerebrovascular lesions is the preconditioning phase of CVD from the viewpoint of preventive medicine. In the Rotterdam Scan Study,13 which included follow-up for 4.2 years, brain magnetic resonance imaging (MRI) analysis was performed on 1,077 healthy adults aged 60 to 90 years without dementia; the results showed that the prevalence of symptomatic CVD was 5 times as high in people with silent cerebrovascular lesions as in those not so affected. SpO2 monitoring by pulse oximetry revealed that of all patients at high risk for CVD, a high prevalence of silent lacunar infarction was observed in those complicated with nocturnal hypoxia.14 The use of diagnostic imaging for clinical analysis of silent CVD has been the topic of several reports.1,2,9–12 However, investigations regarding symptomatic CVD have traditionally involved the use of self-assessment questionnaires to gain clinical information. The use of MRI to analyze in detail the relationship between silent cerebrovascular lesions and sleep disordered breathing was previously reported in the Sleep Heart Health Study.15 This study showed that central sleep apnea might contribute to the progression of white matter disease.15 But a correlation between severity of OSA and the prevalence of silent cerebrovascular lesions in patients with PSG-confirmed OSA has not been clarified. In this study, we examined the correlation between the prevalence of silent cerebrovascular lesions determined by brain MRI and the severity of PSG-confirmed OSA in Japanese patients.
METHODS
The study comprised 192 consecutive Japanese patients with no past history of CVD, who had undergone both an all-night PSG and brain MRI, over a period of 27 months, from March 2005 to June 2007. There were 170 men and 22 women (mean age, 50.6 years, range 22–76), with a mean body mass index (BMI) of 26.7kg/m2 (range 17.9–41.0) and a mean AHI of 40.7/h (range 0–118.4). These subjects had undergone all-night PSG at the Center of Sleep Medicine, Dokkyo Medical University Hospital, Tochigi Prefecture, Japan. Tochigi Prefecture is situated in the north of the Kanto region and is one of Tokyo's metropolitan regions based on its proximity. All patients were requested to fill out a questionnaire regarding their medical history, frequently used medications, and lifestyle-related habits such as smoking and alcohol consumption. Then they underwent an all-night PSG and brain MRI. The interval between PSG and brain MRI was 63.8 ± 86.6 days. Smokers were divided into two groups: current and ex-smokers. Patients were defined as heavy drinkers if their alcohol consumption levels were more than 60 g ethanol/day after converting the amount of alcohol consumed into grams of ethanol, as described previously16 (100 mL of Japanese beer contains 4 g of ethanol; 180 mL of rice wine 22 g of ethanol; 100 mL of distilled spirit 20 g of ethanol; and 100 mL of wine contains 10 g of ethanol). The other risk factors for arteriosclerosis analyzed included hypertension, hyperglycemia such as impaired fasting glycemia (IFG), impaired glucose tolerance (IGT), diabetes mellitus and hyperlipidemia. Hypertensive patients were defined as those under treatment with antihypertensive drugs or those with either a systolic blood pressure over 140 mm Hg or a diastolic blood pressure over 90 mm Hg when measured in the outpatient clinic, based on the criteria of the Joint National Committee 7 (JNC-7).17 The presence of IFG/IGT was defined according to a fasting plasma glucose level of > 100 mg/dL according to 2003 ADA recommendations or > 140 mg/dL at 2 h after meals or following an oral glucose tolerance test (OGTT), which was performed for all patients except those already under treatment with insulin or oral antidiabetic agents for diabetes mellitus.18 Hyperlipidemia was considered present if the blood total cholesterol level was > 220 mg/dL, the triglyceride level was >150 mg/dL or if treatment for hyperlipidemia was being given, in accordance to the Guidelines for the Diagnosis and Treatment of Atherosclerotic Cardiovascular Diseases issued by the Japan Atherosclerosis Society.19 The PSG monitoring included electroencephalography (C3, C4, O1, and O2), electrooculography, chin muscle electromyography, electrocardiography, detection of airflow by thermistor, plethysmography for ribcage and abdominal wall motion, oximetry for measurement of arterial oxyhemoglobin saturation, detection of changes in sleeping position, and bilateral electromyography of the tibialis anterior muscles. All signals were recorded using the Alice 5 system (Respironics, Inc., Murrysville, PA, USA). The PSGs were performed for at least 8 hours. Sleep stages were manually scored according to the criteria of Rechtschaffen and Kales.20 Apnea was defined as the absence of breathing for > 10 s. Hypopnea was defined as a reduction of more than 50% in breathing; in cases wherein the reduction in breathing was less than 50%, a condition of more than 3% oxygen desaturation or arousal for more than 10 s was defined as hypopnea. AHI was calculated as the average of the total number of apnea and hypopnea episodes experienced per hour of sleep. Oxygen desaturation index (ODI) was calculated according to the number of oxygen desaturation values greater than 3% per hour of sleep. In this study, patients were categorized into four groups according to the AHI: AHI < 5/h (control subjects), AHI 5 to < 15/h (mild OSA group), AHI 15 to < 30/h (moderate OSA group), and AHI ≥ 30/h (severe OSA group).
The presence of a silent cerebrovascular lesion was evaluated by MRI of the whole brain. The imaging protocol consisted of T1-weighted images (TR, 579 ms; TE, 15 ms), T2-weighted images (TR, 3800 ms; TE, 99 ms), and fluid-attenuated inversion recovery (FLAIR) images (TR, 9000 ms; TE, 105 ms) on 6-mm slices using a 1.5-T scanner (Siemens KK, Tokyo, Japan) for silent lacunar infarction and periventricular hyperintensity (PVH). Lacunar infarction represented lesions less than 15 mm in size in the region of the penetrating arteries on high intensity T2-weighted and FLAIR images.21 The severity of silent lacunar infarction was classified according to the number of lacunar infarctions. The degree of PVH was classified into 5 categories based on FLAIR images: PVH 0, no PVH detected; PVH 1, PVH detected in the apex of the frontal or posterior horn; PVH 2, mild PVH detected along the lateral ventricle; PVH 3, PVH strongly detected along the entire lateral ventricle; and PVH 4, diffuse PVH detected in the deep white matter.22 We used IDX Imagecast iPACS Viewer Version: 5.2.5.141841 (IDX Systems Corporation, Burlington, VT, USA) to measure silent cerebrovascular lesions. A single trained neurologist who was blinded to the patients' clinical details evaluated the existence, location, and size of lacunar infarcts and degree of PVH on MR imaging.
The study protocol was approved by the Human Ethics Review Committee of Dokkyo Medical University, and informed consent was obtained from each patient.
All statistical analyses in this study were performed using BASE /SAS(r), SAS STAT(r) (Ver.8.02) (SAS Institute, Inc., Cary, NC, USA). The obtained results were expressed as the mean ± standard deviation or as proportions. The χ2 test and Kruskal-Wallis test were used to compare proportions and continuous variables, respectively. The Cochran-Armitage and Jonckheere-Terpstra tests were used to evaluate whether AHI severity was correlated with the prevalence and severity of silent lacunar infarction and degree of PVH, respectively.
To assess the association of prevalence of silent cerebrovascular lesions or AHI severity with possible contributing risk factors, we used a stepwise multiple regression analysis of patients with OSA and control subjects. A p value < 0.05 was considered to be statistically significant.
RESULTS
Of the 192 patients included in the study, 70 were current smokers (36.5%) and 31 were heavy drinkers (16.1%). With regard to analyzed risk factors for arteriosclerosis, 109 (56.8%) patients were considered hypertensive, 94 (49.0%) had hyperglycemia, and 118 (61.5%) had hyperlipidemia. A wide range of AHI severity was observed; 19 patients were in the AHI < 5/h range, 25 were in the 5 to < 15/h range, 35 were in the 15 to < 30/h range, and 113 were in the AHI ≥ 30/h range. The backgrounds of the 19 control subjects were: OSAS suspected but PSG with AHI < 5/h, 11 cases; REM sleep behavior disorder, 4; restless leg syndrome, 2; and narcolepsy, 2. Table 1 shows characteristics of subjects with mild, moderate and severe OSA as well as control subjects. The 4 groups did not differ with regard to gender ratio, proportion of current or ex-smokers and heavy drinkers, the prevalence of hypertension, and the prevalence of hyperlipdemia. However, a significantly higher proportion of patients with severe OSA had a higher BMI (p < 0.0001) and had hyperglycemia compared with control subjects and the mild OSA and moderate OSA groups (p = 0.0173). Values for parameters related to sleep disordered breathing were significantly higher (p < 0.0001) in the severe OSA group than in the control subjects, and mild and moderate OSA groups.
Table 1.
Demographic Characteristics in Control Subjects and Patients with Mild, Moderate and Severe OSA
| AHI < 5 (n=19) |
AHI 5 to < 15 (n=25) |
AHI 15 to < 30 (n=35) |
AHI ≥ 30 (n=113) |
p value | |||
|---|---|---|---|---|---|---|---|
| gender (men/women) | 13 / 6 | 19 / 6 | 31 / 4 | 107 / 6 | 0.0013 | χ2 test | |
| Age, years | 45.6 ± 16.3 | 50.0 ± 15.4 | 52.7 ± 9.4 | 50.9 ± 11.2 | N.S. | Kruskal-Wallis test | |
| BMI, kg/m2 | 23.8 ± 4.1 | 26.1 ± 3.9 | 25.0 ± 2.8 | 27.9 ± 3.9 | <0.0001 | Kruskal-Wallis test | |
| ESS | 9.6 ± 6.2 | 8.5 ± 5.2 | 8.1 ± 4.4 | 9.6 ± 4.8 | N.S. | Kruskal-Wallis test | |
| Habitual smoking, n (%) | 5 (26.3) | 13 (52.0) | 9 (25.7) | 43 (38.1) | N.S. | χ2 test | |
| Heavy drinking, n (%) | 3 (15.8) | 7 (28.0) | 3 (8.6) | 18 (15.9) | N.S. | χ2 test | |
| Hypertension, n (%) | 7 (36.8) | 12 (48.0) | 21 (60.0) | 69 (61.1) | N.S. | χ2 test | |
| Hyperglycemia, n (%) | 6 (31.6) | 7 (28.0) | 16 (45.7) | 65 (57.5) | 0.0173 | χ2 test | |
| Hyperlipidemia, n (%) | 6 (31.6) | 13 (52.0) | 23 (65.7) | 76 (67.3) | N.S. | χ2 test | |
| AHI, /h | 1.7 ± 1.6 | 9.5 ± 3.7 | 22.0 ± 7.0 | 59.9 ± 22.5 | <0.0001 | Kruskal-Wallis test | |
| Arousal index, /h | 25.0 ± 7.8 | 22.2 ± 8.0 | 30.2 ± 13.1 | 58.5 ± 22.4 | <0.0001 | Kruskal-Wallis test | |
| ODI, /h | 0.8 ± 0.7 | 6.4 ± 3.7 | 16.6 ± 7.0 | 50.3 ± 22.5 | <0.0001 | Kruskal-Wallis test | |
| mean SpO2, % | 97.7 ± 0.8 | 96.6 ± 1.1 | 94.7 ± 10.8 | 94.6 ± 2.8 | <0.0001 | Kruskal-Wallis test |
Silent lacunar infarction was identified in 4 (21.1%) normal subjects, 3 (12.0%) mild OSA patients, 17 (48.6%) moderate OSA patients, and 61 (54.0%) severe OSA patients (p < 0.0001). Prevalence of silent lacunar infarction among subjects with moderate and severe OSA (AHI ≥15/h) was higher than among the control subjects and the patients with mild OSA (p < 0.0001).
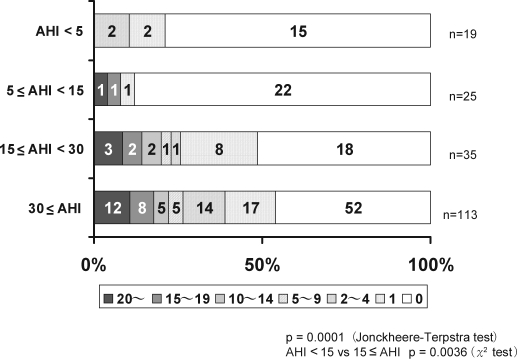
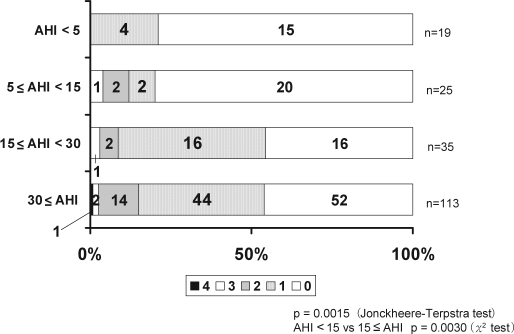
There were 0.4 ± 0.8, 1.4 ± 4.9, 3.7 ± 6.5 and 4.4 ± 6.9 silent lacunar infarctions per person among the control subjects and mild, moderate and severe OSA groups, respectively (p < 0.0001). Among the patients with moderate and severe OSA (AHI ≥ 15/h), the prevalence and number of lacunar lesions were higher than among control subjects and those with mild OSA (AHI < 15/h) (Figure 1). PVH was identified in 4 (21.1%) of the control subjects and in 5 (20.0%), 19 (54.3%), and 61 (54.0%) patients in the mild, moderate, and severe OSA groups, respectively (p = 0.0004). The prevalence of PVH was significantly higher in the moderate and severe OSA (AHI ≥ 15/h) groups (p < 0.0001). With regard to the degree of PVH, 66 patients (34.4%) had PVH 1; 18 (9.4%) had PVH 2; 4 (2.1%) had PVH 3; and 1 (0.5%) had PVH 4. Furthermore, the degree of PVH according to OSA severity was evaluated. Among the control subjects, 4 (21.1%) had PVH 1, and none had PVH 2, 3, or 4. Two (8%) of the patients in the mild OSA group had PVH 1, while 2 (8.0%) and 1 (4.0%) had PVH 2 and 3, respectively; none had PVH 4. In the moderate OSA group, 16 (45.7%) had PVH 1, 2 (5.7%) had PVH 2, 1 (2.9%) had PVH 3 and none had PVH 4. Finally, in the severe OSA group, 44 (38.9%) had PVH 1, 14 (12.4%) had PVH 2, 2 (1.8%) had PVH 3, and 1 (0.9%) had PVH 4 (Figure 2).
Figure 1.
Number of lacunar infarctions in patients with moderate and severe OSA (AHI ≥ 15/h) was significantly higher than in those with mild OSA (AHI < 15/h) patients.
Figure 2.
In patients with moderate and severe OSA (AHI ≥ 15/h), the degree of PVH was significantly higher than in those with mild OSA (AHI < 15/h).
In this study, we used the prevalence of silent lacunar infarction or PVH as a dependent variable and evaluated the order of inclusion in the model of the following independent variables: age, gender, BMI, smoking, alcohol consumption, hypertension, hyperglycemia, hyperlipidemia, AHI, and ODI. Among the 10 independent variables, age (p < 0.001 or p < 0.001) and AHI (p = 0.001 or p = 0.006) were predictors of the prevalence of silent lacunar infarction or PVH in patients with OSA and control subjects. On the other hand, we used AHI severity as a dependent variable and evaluated the order of inclusion in the model of the following independent variables: age, gender, BMI, smoking, alcohol consumption, hypertension, hyperglycemia, hyperlipidemia, the prevalence of lacunar infarction and the prevalence of PVH. Among the 10 independent variables, gender (p < 0.001), BMI (p < 0.001), hyperlipidemia (p = 0.020), the prevalence of lacunar infarction (p = 0.021) and the prevalence of PVH (p = 0.013) were predictors of AHI severity in patients with OSA and control subjects.
DISCUSSION
Our results indicate that moderate and severe OSA (AHI ≥ 15/h) are associated with a higher prevalence of silent lacunar infarctions and PVH than is mild OSA and that severe OSA is a possible risk factor for silent cerebrovascular lesions.
The prevalence of silent cerebrovascular infarction has been the topic of several epidemiological investigations. Kobayashi et al.23 in analyzing asymptomatic cerebral infarction to evaluate the prevalence of silent lacunar infarction examined 505 patients (282 men and 223 women; age, 33 to 81 years) who had no history of CVD. Of these patients, 13.3% were diagnosed with silent lacunar infarction (16.3% and 6.7% of the men and women, respectively). The prevalence of silent lacunar infarction increased with age, with no patient less than 50 years of age being affected. Takagi et al24 performed brain MRI on 1,258 patients (882 men and 376 women; mean age, 51 ± 10 and 53 ± 10 years, respectively) who had no history of CVD and found silent lacunar infarction in 9.5% of the male and in 5.9% of the female patients. Here, we investigated OSA patients with no history of CVD, and the results revealed a distinctly high prevalence of silent lacunar infarction among those with OSA. Approximately 50% of the study patients with moderate and severe OSA were found to have silent lacunar infarction. With regard to the severity of PVH, another study25 examined 946 patients to determine the prevalence of asymptomatic cerebral infarction. They found that the prevalence of PVH increased with age; 21.1% of those with PVH were from 50 to 60 years of age. In our study, approximately 50 years was the average age of the subjects with moderate and severe OSA, and there was a high prevalence of PVH in these groups (54.3% and 54.0%, respectively). We also found that the prevalence of PVH in OSA patients is higher than that detected previously during health screening of the brain.25 It has been generally accepted that age and hypertension are risk factors for silent lacunar infarction,26,27 and that hypertension is also a risk factor for PVH.28,29 The present results showed that moderate and severe OSA are associated with a high prevalence of silent lacunar infarction and PVH; however, it was not clarified whether OSA severity is an independent risk factor for the development of silent lacunar infarction or PVH.
The association between silent lacunar infarction and OSA severity is considered to be due to various biological reactions occurring during hypoxia, hypercapnia, and arousal associated with respiratory events.30 The first biological reactions probably include an inflammatory response, oxidative stress, and relevant atherosclerotic changes due to abnormal hemostasis. Recently, blood levels of C-reactive protein (CRP) were reported to be high in atherosclerosis-based diseases such as cardiac and cerebral infarction.31 Another study of the association between oxidative stress and CVD found that plaque instability was induced by increased oxidative stress followed by lipid oxidation.32 Lavie et al33 observed significantly high levels of oxidative stress in OSA patients and that OSA severity was associated with the levels of thiobarbituric-acid reactive substances and peroxides, which are markers of oxidative stress. Minoguchi et al34 reported that the presence of silent brain infarction was increased in patients with OSA and was associated with an elevation in markers of platelet activation, such as soluble CD40L and soluble P-selectin, and systemic inflammation (CRP). These reports reveal that due to various changes associated with OSA, OSA tends to result in atherosclerotic changes. The second biological reaction in OSA is the hemodynamic change associated with respiratory events. Abnormal cerebrovascular hemodynamics have been reported to be associated with increased intracranial pressure followed by decreased cerebral perfusion pressure related to apnea.35 The cerebrovascular system is generally controlled by an autoregulation mechanism that can secure blood flow to a certain extent if the cerebral perfusion pressure decreases. However, hemodynamic changes may still occur since this autoregulation mechanism is inadequate to protect the brain from the rapid fluctuations in pressure that are associated with apnea.36 Hypercapnia associated with apnea and the accompanying changes in cerebral blood flow velocity and vessel wall tension that are associated with both these conditions were reported to result in long-term damage of cerebral blood vessels.37 Further, atherosclerosis and hemodynamic changes by OSA are considered to be responsible for OSA-associated silent cerebrovascular lesions, and the association between silent cerebrovascular lesions and OSA severity can be predicted based on these factors.
On the other hand, CVD is a form of organopathy occurring due to hypertension, and it is considered to be related to the development of silent cerebrovascular lesions. OSA-associated hypertension might also be related to silent cerebrovascular lesions. Morning hypertension was found to be an independent risk factor for CVD based on the finding that symptoms of CVD commonly develop within 4 h after rising.38 Shimada et al 39 reported that the prevalence of CVD is significantly higher in patients with non-dipping and extreme-dipping circadian blood pressure patterns than in those with normal dipping patterns. Moreover, it has been reported38 that a large number of people with the non-dipping pattern have OSA. Davies et al40 conducted a case-control study involving 2 groups (healthy subjects and OSA patients) and observed that the blood pressure values recorded, including overall diastolic pressure, daytime diastolic pressure, nighttime systolic pressure, and nighttime diastolic pressure, were significantly higher for OSA patients than for the healthy subjects. A possible explanation for the development of CVD in OSA patients may be that recurrent respiratory events occurring during the night cause frequent arousal accompanied by hypertension due to sympathetic hyperactivity, and that this hypertension continues until morning. In this study, we did not identify an association between systemic hypertension and the prevalence or severity of silent cerebrovascular lesions in OSA patients. This may be because our protocol involved measuring casual blood pressure in the outpatient department. It will be necessary to measure blood pressure during both the day and night rather than obtaining a single daytime measurement.
Munoz et al11 reported that severe OSA with an AHI of 30/h is a risk factor for CVD in elderly people aged over 70 years. We found that subjects with moderate and severe OSA (AHI ≥15/h) had a high prevalence of silent cerebrovascular lesion.
Since gender and BMI were not matched in the present study, multivariate analysis was carried out considering latent factors that might affect the prevalence of silent cerebrovascular lesions other than a sleep related breathing disorder. As a result, age and AHI severity were found to be significant risk factors for the prevalence of silent lacunar infarction or PVH. Regarding AHI severity, gender, BMI, hyperlipidemia, prevalence of lacunar infarction, and prevalence of PVH were found to be significant risk factors. The result that males accounted for a majority of the study subjects and that the BMI was not matched among the 4 groups seemed to explain why gender and BMI were found to be significant factors. Interestingly, the result that the presence of lacunar infarction or PVH significantly affected AHI severity suggested the possibility that the presence of silent cerebrovascular lesions was involved in the process of deterioration of apnea. In elderly subjects (mean age, 77 ± 4.3 years), the potential importance of central sleep apnea was identified as a possible pathogenetic factor in the development of white matter disease rather than as a consequence of white matter disease in the Sleep Heart Health Study.15 Robbins et al. noted that recurrent changes in cerebral blood flow with central sleep apnea may contribute causally to the observed deterioration in white matter grade.15
In other words, patients with silent cerebrovascular lesions can be considered to be at high risk for symptomatic CVD. However, the long-term course of silent cerebrovascular infarction in patients with moderate and severe OSA and its outcome remains unclear; follow-up studies regarding silent cerebrovascular infarction in OSA patients have been conducted only on a small scale41 rather than on a sufficiently large scale to clarify this issue. However, based on this association as revealed at present, treatment of OSA is essential and serves as surrogate prophylactic therapy for CVD.
In conclusion, results presented here suggest that the presence of silent cerebrovascular lesions in OSA patients is an important part of a preconditioning phase of symptomatic CVD. Therefore, from the viewpoint of stroke prevention, further studies are required to investigate the relationships between silent cerebrovascular lesions and the development of symptomatic CVD in patients with moderate and severe OSA.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population. JAMA. 2003;289:2230–7. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 6.Ong KC, Clerk AA. Comparison of the severity of sleep-disordered breathing in Asian and Caucasian patients seen at a sleep disorders center. Respir Med. 1998;92:843–8. doi: 10.1016/s0954-6111(98)90386-9. [DOI] [PubMed] [Google Scholar]
- 7.Li KK, Powell NB, Kushida C, et al. A comparison of Asian and White patients with obstructive sleep apnea syndrome. Laryngoscope. 1999;109:1937–40. doi: 10.1097/00005537-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Ip SM, Tsang WTK, Lam WK, Lam B. Obstructive sleep apnea syndrome: an experience in Chinese adults in Hong Kong. Chin Med J. 1998;111:257–60. [PubMed] [Google Scholar]
- 9.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;63:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vincente E, Aguisti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1043–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 11.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 12.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnoea as a risk factor for stroke and death. N Engl J Med. 2006;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 13.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–9. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 14.Eguchi K, Kario K, Hoshide S, Ishikawa J, Morinari M, Shimada K. Nocturnal hypoxia is associated with silent cerebrovascular disease in a high-risk Japanese community-dwelling population. Am J Hypertens. 2005;18:1489–95. doi: 10.1016/j.amjhyper.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Robbins J, Redline S, Ervin A, Walsleben JA, Ding J, Nieto FJ. Associations of sleep-disordered breathing and cerebral changes on MRI. J Clin Sleep Med. 2005;1:159–65. [PubMed] [Google Scholar]
- 16.Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289:579–88. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Japan Atherosclerosis Society (JAS) Tokyo: 2002. Guidelines for diagnosis and treatment of atherosclerotic cardiovascular diseases. [Google Scholar]
- 20.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service / Brain Research Institute, UCLA; 1968. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. [Google Scholar]
- 21.Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases. Stroke. 1990;21:637–76. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda H, Kobayashi S, Okada K, Tsunematsu T. Frontal white matter lesions and dementia in lacunar infarction. Stroke. 1990;21:1143–9. doi: 10.1161/01.str.21.8.1143. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S. Asymptomatic cerebral infarction. Nippon Naika Gakkai Zasshi. 1997;86:1800–5. doi: 10.2169/naika.86.1800. [DOI] [PubMed] [Google Scholar]
- 24.Takagi S, Ide M, Yasuda S, Shohtsu A. Risk factors for asymptomatic lacunar infarction in subjects without symptomatic cerebrovascular disease. Jpn J Stroke. 1996;18:85–92. [Google Scholar]
- 25.Masana Y, Iwamoto F, Yamada M, Motozaki T, Yoshimine T. Prevalence and risk factors for silent lacunar infarct in white matter lesion–brain multiphasic screening. No To Shinkei. 2003;55:1027–32. [PubMed] [Google Scholar]
- 26.Shintani S, Shiigai T, Arinami T. Silent lacunar infarction on magnetic resonance imaging (MRI): risk factors. J Neurol Sci. 1998;160:82–6. doi: 10.1016/s0022-510x(98)00182-8. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi S, Okada K, Yamashita K. Prevalence of silent lacunar lesion in normal adults and its relation to cerebral blood flow and risk factors. Stroke. 1991;22:1379–83. doi: 10.1161/01.str.22.11.1379. [DOI] [PubMed] [Google Scholar]
- 28.Chamorro A, Pujol J, Saiz A, et al. Periventricular white matter lucencies in patients with lacunar stroke. A marker of too high or too low blood pressure? Arch Neurol. 1997;54:1284–8. doi: 10.1001/archneur.1997.00550220082018. [DOI] [PubMed] [Google Scholar]
- 29.Sierra C, de la Sierra A, Mercader J, Gomez-Angelats E, Urbano-Marquez A, Coca A. Silent cerebral white matter lesions in middle-aged essential hypertensive patients. J Hypertens. 2002;20:519–24. doi: 10.1097/00004872-200203000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implication for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 31.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105:2107–11. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 33.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–8. [PubMed] [Google Scholar]
- 34.Minoguchi K, Yokoe T, Tazaki T, et al. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:612–7. doi: 10.1164/rccm.200608-1141OC. [DOI] [PubMed] [Google Scholar]
- 35.Jennum P, Borgesen SE. Intracranial pressure and obstructive sleep apnea. Chest. 1989;95:279–83. doi: 10.1378/chest.95.2.279. [DOI] [PubMed] [Google Scholar]
- 36.Mohsenin V, Culebras A. Sleep-related breathing disorders and risk of stroke editorial comment: balancing sleep and breathing. Stroke. 2001;32:1271–8. doi: 10.1161/01.str.32.6.1271. [DOI] [PubMed] [Google Scholar]
- 37.Klingelhofer J, Hajak G, Sander D, Schulz-Varszegi M, Ruther E, Conrad B. Assessment of intracranial hemodynamics in sleep apnea syndrome. Stroke. 1992;23:1427–33. doi: 10.1161/01.str.23.10.1427. [DOI] [PubMed] [Google Scholar]
- 38.Kario K. Time for focus on morning hypertension: pitfall of current antihypertensive medication. Am J Hypertens. 2005;18:149–51. doi: 10.1016/j.amjhyper.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Shimada K, Kawamoto A, Matsubayashi K, Nishinaga M, Kimura S, Ozawa T. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–8. [PubMed] [Google Scholar]
- 40.Davies CW, Crosby JH, Mullins RL, et al. Case control study of cerebrovascular damage defined by magnetic resonance imaging in patients with OSA and normal matched control subjects. Sleep. 2001;24:715–20. doi: 10.1093/sleep/24.6.715. [DOI] [PubMed] [Google Scholar]
- 41.Hentschel F, Schredl M, Dressing H. Sleep apnea syndrome and cerebral lesions–a prospective MRI study. Fortschr Neurol Psychiatr. 1997;65:421–4. doi: 10.1055/s-2007-996347. [DOI] [PubMed] [Google Scholar]