Abstract
Study Objectives:
We investigated autonomic balance and resting metabolic rate to explore their possible involvement in obesity in hypocretin/orexin-deficient narcoleptic subjects.
Methods:
Resting metabolic rate (using indirect calorimetry) and variability in heart rate and blood pressure were determined in the fasted resting state. Subjects included 15 untreated, hypocretin-deficient male narcoleptics and 15 male controls matched for age and body mass index.
Results:
Spectral power analysis revealed greater heart rate and blood pressure variability in hypocretin-deficient male narcoleptic patients (heart rate: p = 0.01; systolic blood pressure: p = 0.02; diastolic: p < 0.01). The low to high frequency ratio of heart rate power did not differ between groups (p = 0.48), nor did resting metabolic rate (controls: 1767 ± 226 kcal/24 h; patients: 1766 ± 227 kcal/24h; p = 0.99).
Conclusions:
Resting metabolic rate was not reduced in hypocretin-deficient narcoleptic men and therefore does not explain obesity in this group. Whether the increased heart rate and blood pressure variability—suggesting reduced sympathetic tone—is involved in this regard remains to be elucidated.
Citation:
Fronczek R; Overeem S; Reijntjes R; Lammers GJ; van Dijk JG; Pijl H. Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J Clin Sleep Med 2008;4(3):248–254.
Keywords: narcolepsy, hypocretin, orexin, obesity, metabolism, heart rate variability, autonomic balance
Narcolepsy is a sleep disorder that affects 25–50 out of 100,000 people in Western countries.1 It is classically characterized by the tetrad of excessive daytime sleepiness, cataplexy, sleep paralysis, and hypnagogic hallucinations.2 Obesity in narcolepsy was first described as early as the 1930s3,4 and has since been described repeatedly.5–12 The discovery of hypocretin/orexin deficiency in human narcolepsy with cataplexy and the fact that hypocretin peptides may be involved in metabolic control sparked renewed interest in the pathophysiology of obesity in narcolepsy. Indeed, various observations suggest that hypocretin deficiency is involved in the pathogenesis of weight gain in narcoleptic patients. Increased bodyweight has been reported in human narcolepsy as well as in the ataxin-3 hypocretin-deficient animal model of the disease.13,14 Moreover, patients with idiopathic hypersomnia, similarly marked by excessive daytime sleepiness but exhibiting normal cerebrospinal hypocretin levels, are not obese.12
There is evidence that hypocretin peptides stimulate feeding behavior:15 Injection of hypocretins into the lateral cerebral ventricle promotes food intake in rats,16 whereas ablation of hypocretin neurons leads to hypophagia in mice.14 Accordingly, narcoleptic humans and mice eat less than age and sex matched controls,13,18 which clearly indicates that other components of energy balance must drive weight gain in this disease. The effects of hypocretin peptides on wakefulness and the sympathetic nervous system may be involved. Injection of hypocretins into the lateral ventricle stimulates arousal and sympathetic activity in rats, elevating arterial blood pressure (BP), heart rate (HR), oxygen consumption, body temperature and plasma catecholamine levels.19–22 Thus, hypocretin deficiency could promote obesity through the concerted effects of several behavioral and neuroendocrine corollaries. It may reduce sympathetic tone and thereby resting metabolic rate. Moreover, since adipose tissue is innervated by both sympathetic and parasympathetic nerves, where sympathetic inputs stimulate lipolysis and parasympathetic signals are anabolic, dominance of parasympathetic tone could promote fat storage through direct effects on adipocytes.23,24 Finally, excessive daytime sleepiness may limit physical activity, which obviously would reduce daily energy expenditure. Thus, hypocretin deficiency may dampen sympathetic tone in narcoleptic patients, and thereby reduce resting metabolic rate and promote adipocyte lipogenesis to induce weight gain in the face of diminished food intake.
We studied resting metabolic rate and HR and BP variability, as a proximate measure of autonomic balance, in hypocretin-deficient narcoleptic patients and healthy controls matched for gender, body weight, and age. Since (lean) body mass is an important determinant of resting metabolic rate, matching for body weight was necessary to preclude confounding the data on metabolic rate by anthropometric features of our volunteers. We hypothesized that sympathetic tone and resting metabolic rate per unit of (lean) body mass would be reduced in narcoleptic subjects.
MATERIALS AND METHODS
Subjects
The study was approved by the local medical ethical committee. All narcoleptic patients were male and fulfilled the ICSD-2 criteria of narcolepsy with cataplexy.27 Patient characteristics are shown in Table 1. Known reasons for obesity other than narcolepsy formed an exclusion criterion. No patient used any medication at the time of the study. Four patients had received narcolepsy medication in the past, but at least 2 years previous to the study. One patient had used methylphenidate until 2 weeks before participating. Hypocretin-1 was not detectable in the cerebrospinal fluid of any patient, using a radioimmunoassay (Phoenix Pharmaceuticals, Inc., Belmont, CA) in duplicate 100 μL aliquots. All samples were run in a single assay with a detection limit of 50 pg/mL and an intra-assay variability less than 5%. We used a validated reference sample to adjust levels to previously reported values.28 Healthy male controls were recruited using an advertisement in a local newspaper.
Table 1.
Narcolepsy Patient Characteristics
| ID | Sex | Hypocretin (CSF) |
HLA (DQB1*0602) |
Age of Onset (yr) |
Disease Duration (yr) |
Current Medication |
Past Medication (> 2 yr ago) |
|---|---|---|---|---|---|---|---|
| 1 | M | N.D. | + | 17 | 54 | - | - |
| 2 | M | N.D. | + | 24 | 9 | - | Methylphenidate, modafinil, selegiline, anafranil, sodium oxybate |
| 3 | M | N.D. | + | 7 | 17 | - | - |
| 4 | M | N.D. | + | 33 | 3 | Methylphenidate | - |
| 5 | M | N.D. | ? | 15 | 11 | - | - |
| 6 | M | N.D. | + | 20 | 2 | - | Modafinil, methylphenidate |
| 7 | M | N.D. | + | 12 | 46 | - | Methylphenidate, dexamphetamine |
| 8 | M | N.D. | + | 10 | 17 | - | - |
| 9 | M | N.D. | + | 16 | 14 | - | - |
| 10 | M | N.D. | + | 8 | 14 | - | - |
| 11 | M | N.D. | + | 21 | 7 | - | - |
| 12 | M | N.D. | + | 31 | 11 | - | - |
| 13 | M | N.D. | + | 19 | 17 | - | - |
| 14 | M | N.D. | + | 10 | 42 | - | Methylphenidate |
| 15 | M | N.D. | + | 27 | 3 | - | Methylphenidate, modafinil, tofranil |
N.D. Not Detectable; Current medication was stopped at least 2 weeks before participating in the current study. ? unknown
Metabolic Measurements
Metabolic studies were conducted in 15 patients and 15 controls. Subjects were instructed to fast and drink only water from 22:00 the night before until after the metabolic measurement had been performed. Studies started at 09:00, when subjects were asked to lie down in supine position for 30 minutes. Care was taken to keep subjects awake during this period by talking to them. Resting metabolic rate (RMR) was measured by indirect calorimetry29 using a computerized open-circuit ventilated hood system (Oxycon B; Jaeger, Breda, The Netherlands).30 Measurements from the first 10 min of recording were not used in the analysis as this period reflects the subjects coming to rest and getting acquainted with the experimental conditions.
Oxygen consumption (VO2, L/min) and carbon dioxide production (VCO2, L/min) were used to calculate the respiratory index (VCO2/VO2). RMR and carbohydrate (C) and fat (F) combustion were calculated using the Weir formula and were expressed as kilocalories per 24 h (per kilogram body weight) and grams per minute (per kilogram body weight) respectively.31,32 The following formulas were used:
Autonomic Measurements
Autonomic measurements were recorded simultaneously with the metabolic measurements. These measurements were added to the original protocol and were obtained in 9 patients and 9 controls, i.e., a subpopulation of the subjects included for the metabolic measurements. HR was calculated from the ECG. Beat-to-beat arterial BP was noninvasively measured (Finometer, TNO-Biomedical Instruments, The Netherlands). The hand used for these finger BP measurements was held in a constant position at heart level. Analysis of autonomic data was restricted to the last 20 min of the recording time, as was done for metabolic measurements.
HR and BP calculations were performed using software written in MatLab (MatLab v7.0, Mathworks, MA, USA). HR variability (HRV) was estimated by calculating the mean and SD of consecutive R-R intervals and with spectral analysis performed by interpolating the series of RR intervals by cubic splines, resampling the signal at 3 Hz and performing a fast Fourier transformation using a Hamming window.33 Power was calculated for the very low frequency (VLF, 0–0.04 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.15–0.4 Hz) bands. Total power was calculated by adding the VLF, LF, and HF power values. The LF/HF ratio was also calculated.
Of note, the LF band is usually considered to represent sympathetically mediated baroreflex activity, while the HF band, largely derived from respiratory influences, mostly concerns parasympathetic activity. Increases in total power can be caused by a reduction in sympathetic tone.34,35 Although the LF/HF ratio is generally used as a measure of the autonomic parasympathetic-sympathetic balance,36 some authors regard it as an indication of sympathetic activity.37
Mean systolic (SBP) and diastolic (DBP) BPs were calculated, and the total power in their frequency spectrum (calculated in a similar fashion as the HRV spectrum) was taken as an estimate of variability. Note that finger BP measurement using the aforementioned Finometer has a tendency to underestimate BP. While this can be corrected using additional conventional measurements,38 we chose not to do so because analyses involved groupwise comparisons.
Statistics
Differences between groups were calculated using Student's t-test for unpaired samples. Pearson correlation coefficient was used to evaluate potential correlations. P-values below 0.05 were considered significant.
RESULTS
Resting Metabolic Rate
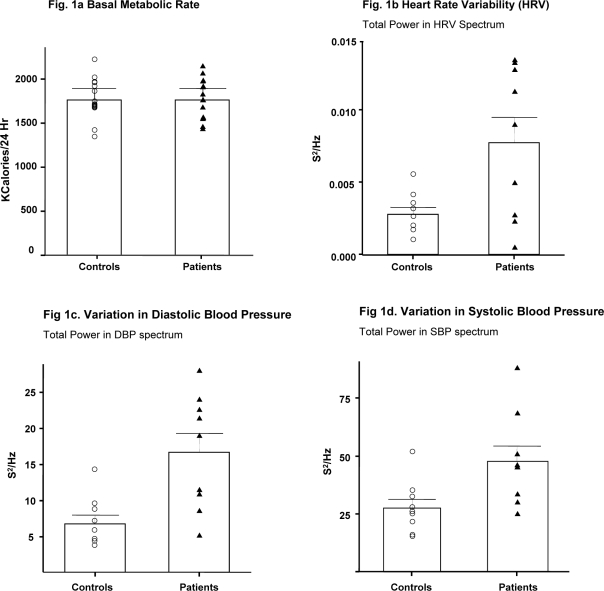
Summarized data are shown in Table 2. Age and BMI did not differ between groups (Figure 1a). There were no significant differences in RMR, VO2, VCO2, RI and carbohydrate or fat substrate combustion between narcoleptics and controls. RMR, carbohydrate and fat combustion expressed per kg of body weight were not significantly different between groups either (Table 2).
Table 2.
Metabolic Measures
| Controls (N = 15) | Narcolepsy (N = 15) | P-value | |
|---|---|---|---|
| male/female | 15/0 | 15/0 | |
| Age (years) | 36.3 ± 13.8 | 35.6 ± 13.8 | 0.89 |
| BMI (kg/m2) | 26.0 ± 2.8 | 26.8 ± 2.3 | 0.42 |
| VO2 (mL/min) | 250.9 ± 34.2 | 252.4 ± 33.4 | 0.91 |
| VCO2 (mL/min) | 225.9 ± 24.6 | 220.3 ± 31.0 | 0.58 |
| RI | 0.88 ± 0.06 | 0.86 ± 0.07 | 0.45 |
| RMR (kcal/24 h) | 1767.1 ± 226.5 | 1766.5 ± 226.5 | 0.99 |
| RMR/kg | 19.9 ± 2.0 | 20.1 ± 2.2 | 0.77 |
| C (Carbohydrate) (g/min) | 222.5 ± 56.9 | 191.9 ± 95.0 | 0.29 |
| C/kg | 2.5 ± 0.6 | 2.1 ± 1.0 | 0.34 |
| C in % of RMR | 72.5 ± 18.5 | 62.6 ± 31.0 | 0.29 |
| F (Fat) (g/min) | 41.7 ± 29.5 | 53.7 ± 38.9 | 0.35 |
| F/kg | 0.5 ± 0.3 | 0.6 ± 0.5 | 0.35 |
| F in % of RMR | 30.6 ± 21.6 | 39.4 ± 28.5 | 0.35 |
Values in the table are means ± standard deviation. T-tests were used to assess group differences; no significant differences were found.
BMI, body mass index; kcal, kilocalories; 24 h, per 24 hours; RMR, resting metabolic rate; kg, kilograms; RI, respiratory index; VO2, oxygen consumption; VCO2, carbon dioxide consumption.
Figure 1.
Resting metabolic rate (a), heart rate variation (b), variation in systolic blood pressure (c) and variation in diastolic blood pressure (d) estimated by total power in the SBP and DBP frequency spectrum in narcoleptic patients and controls. Bars represent means, error bars indicate standard deviation, triangles represent patients, and circles represent controls.
Correlations between patient age, age of disease onset, disease duration on the one hand and RMR on the other hand were not significant (all r < 0.22, p > 0.43). Furthermore, there was no significant difference in RMR according to whether or not patients had used medication in the past (p = 0.94). There was, however, a significant correlation between disease duration and BMI (r = 0.55, p = 0.04).
Autonomic Balance
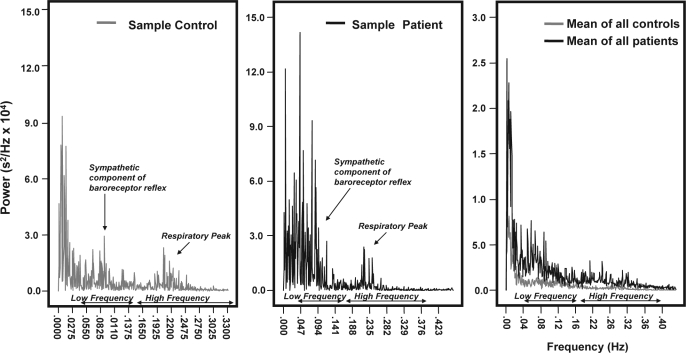
Data are shown in Table 3. Age, BMI, mean HR, mean SBP, and mean DBP did not differ significantly between groups. However, HR and BP variability did differ: the total power in the spectrum of both SBP and DBP was significantly higher in patients than in controls (SBP: p < 0.02, DBP: p < 0.001; Figure 1c and 1d). A HF peak could be identified in the HR spectra of all subjects (Figure 2). Total power (p < 0.01), VLF power (p < 0.03), and LF power (p < 0.02) were all significantly higher in patients than in controls. HF power also tended to be higher in patients (p = 0.05, Figure 2). In contrast, the LF/HF ratio did not differ between groups (p = 0.48).
Table 3.
Autonomic Measures
| Controls (N = 9) | Narcolepsy (N = 9) | P-value | |
|---|---|---|---|
| male/ female | 9/0 | 9/0 | |
| Age (years) | 29.2 ± 4.1) | 32.6 ± 16.2 | 0.55 |
| BMI (kg/m2) | 24.9 ± 2.6) | 26.2 ± 2.1 | 0.31 |
| Total Power in HRV spectrum × 104s2/Hz | 27.8 ± 14.1 | 77.4 ± 52.0 | 0.01* |
| - VLF (0–0.04 Hz) × 104s2/Hz | 12.6 ± 8.2 | 30.7 ± 22.0 | 0.03* |
| - LF (0.04–0.15 Hz) × 104s2/Hz | 9.7 ± 5.5 | 30.6 ± 24.2 | 0.02* |
| - HF (0.15–0.4 Hz) × 104s2/Hz | 5.6 ± 2.9 | 16.2 ± 14.9 | 0.05 |
| LF/HF Ratio | 1.9 ± 1.1 | 2.3 ± 1.2 | 0.48 |
| Mean heart rate (BPM) | 59.6 ± 8.8 | 56.7 ± 5.4 | 0.42 |
| Mean diastolic blood pressure (DBP, mm Hg) | 55.8 ± 9.0 | 52.8 ± 6.9 | 0.44 |
| Mean systolic blood pressure (SBP, mm Hg) | 104.0 ± 13.7 | 99.5 ± 17.6 | 0.55 |
| Total power in SBP spectrum × 104mm Hg2/Hz | 27.5 ± 11.1 | 47.7 ± 19.7 | 0.02* |
| Total power in DBP spectrum × 104mm Hg2/Hz | 6.8 ± 3.5 | 16.7 ± 7.9 | <0.00* |
Values in the table are means ± standard deviation. T-tests were used to assess group differences.
BMI, body mass index; HRV, heart rate variability; s, seconds; Hz, hertz, VLF, very low frequency; LF, low frequency; HF, high frequency; BMP, beats per minute; SBP, systolic blood pressure; DBP; diastolic blood pressure.
Figure 2.
Heart Rate Variability (HRV) in the frequency domain. Example of a typical power spectrum including respiratory peak, sympathetic component of the baroreceptor reflex and LF/HF regions for a control (left) and a narcoleptic patient (middle), and mean spectrum for both groups (right).
Total power did not significantly correlate with age, age of disease onset or disease duration in patients (all r < 0.64, p > 0.09). HRV parameters did not differ between patients who had vs. those who had not received medication in the past (p = 0.43).
DISCUSSION
The pathogenesis of obesity in narcoleptic patients remains unexplained. Obviously, eating more or moving less are potential explanations. However, hypocretin neuron-ablated narcoleptic mice13 and human patients18 eat less than normal controls (total daily food intake, narcoleptic humans: 8,756 ± 2,312 kilojoules; controls: 10,640 ± 3,129 kJ; p < 0.001, data from Lammers et al.18), which is in accordance with the orexigenic qualities of hypocretin peptides. Actigraphy studies have shown that, although periods of activity and inactivity are more scattered in narcoleptic subjects versus controls, the total intensity of physical activity does not differ.39,40 Furthermore, narcoleptic subjects are more obese than equally active subjects suffering from idiopathic hypersomnia.12 Thus, hypocretin deficiency must have other metabolic consequences to explain why narcoleptic animals and humans are obese. Since hypocretin peptides were shown to activate the sympathetic nervous system and increase oxygen consumption in rats,19–22 we hypothesized that hypocretin deficiency would lead to a reduction of sympathetic tone and resting metabolic rate in patients with narcolepsy.
Spectral analysis of HR and BP variability revealed higher power in all frequency bands in narcoleptic patients, while the LF/HF ratio did not differ from that in healthy controls. These findings support our hypothesis that sympathetic tone is reduced in narcoleptic patients. However, at least one observation requires further clarification. The HF peak almost exclusively reflects vagal effects on HR. Thus, diminution of sympathetic tone cannot be the proximate cause of the increase of HF power we observed. However, studies employing selective antagonists of (para)sympathetic neurotransmitters revealed that reduction of sympathetic tone elevates both LF and HF power,34,35 perhaps because diminished sympathetic control causes larger than normal fluctuations in BP that in turn require considerable adaptive fluctuations of parasympathetic activity to control heart rate in response. Thus, reduced sympathetic tone can explain the high power in all frequency bands as well as the similarly elevated HF power in narcoleptic patients. Furthermore, sympathetic tone is already low in the supine position, so any further decreases are not likely to affect the ratio much under these circumstances,34,35 hampering a straightforward interpretation of this ratio.37 The only finding that is not readily compatible with decreased sympathetic tone is that mean HR was not lower in the narcolepsy group.34,35
Hypocretin deficiency may directly inhibit sympathetic activity. Various studies have shown that the hypocretin system is heavily involved in autonomic control and that hypocretins stimulate sympathetic activity.45 Indeed, obese hypocretin neuron-ablated mice have lower sympathetic vasoconstrictor outflow.46 Alternatively, narcoleptic subjects may not have been as awake as the control subjects, although special care was taken to keep patients alert during the measurements. Narcolepsy is commonly seen as a loss of state boundary control, which means that patients are unable to remain awake steadily.42 A tendency to drift into drowsiness could lead to a higher variability in autonomic parameters, as autonomic control differs in the various sleep stages. The transition between wakefulness and sleep affects the power in both the HF and the LF band.43 Frequent shifts from waking to drowsiness might therefore cause increased HRV. Drowsiness would not only affect autonomic parameters, but might also have lead to an underestimation of the RMR in narcoleptic subjects, since RMR is lower during sleep.44 However, this would mean that the actual RMR in narcoleptic subjects is higher, which obviously would not explain their obesity. We suggest that further studies should take drowsiness into account.
Surprisingly, although sympathetic activity drives resting energy expenditure, at least in rodents, RMR was similar in narcoleptic patients and controls. This may also hold for hypocretin-knockout mice, also having a normal RMR (C.M. Sinton, personal communication). Some caution is necessary, inasmuch as large numbers of subjects are required to detect small differences in energy expenditure with indirect calorimetry. Nonetheless, small differences in RMR can be relevant, as they may lead to weight gain in the long term.41 There is direct autonomic innervation of adipose tissue,23 implying that a low sympathetic tone can directly promote fat accrual.24
Other studies on autonomic nervous function in narcoleptic patients reported no abnormalities during provocations25 and no primary disturbances between 18:00 and 20:00 or during sleep.26 An increased LF/HF ratio compared with controls was found just before sleep onset, but this was thought to be related to the impairment of the sleep-wake cycle in narcolepsy and not to a primary disturbance.26 The sample of 9 patients used for autonomic measurements in our study was relatively small, increasing the chance of type II errors. We thus advocate replicating and extending the studies on autonomic activity in narcoleptic patients.
In a recent study,47 RMR was determined in 7 typical narcolepsy patients with cataplexy who were assumed to be hypocretin-deficient. The control group consisted of 9 healthy controls. RMR tended to be lower in narcoleptic patients (p = 0.07). Note that the controls were not matched for BMI in that study. In our larger patient and BMI-matched control sample, metabolic parameters were so similar between the two groups that increasing the number of subjects is not likely to result in a significant difference in RMR. To obtain a definite answer, a large, collaborative study in subjects with a broad range of BMIs should be conducted. It would also be of interest to include leptin measurements in such a study. Given the controversy in the literature, multiple measurement points over the day should then be performed.
In conclusion, resting metabolic rate appears to be normal in narcoleptic humans as determined by indirect calorimetry. However, there are signs of reduced sympathetic activity, which may lead to fat accrual through direct effects on adipocytes.
ACKNOWLEDGMENTS
We thank P.J. van Someren, S. van Nues, B. Ladan, and P. Kok for their help in acquiring the data and R. D. Thijs for his help in interpreting the data.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, Van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Overeem S, Mignot E, van Dijk JG, Lammers GJ. Narcolepsy: clinical features, new pathophysiologic insights, and future perspectives. J Clin Neurophysiol. 2001;18:78–105. doi: 10.1097/00004691-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Cave H. Narcolepsy. Arch Neurol Psychiatr. 1931:50–101. [Google Scholar]
- 4.Daniels L. Narcolepsy. Medicine. 1934;13:1–122. [Google Scholar]
- 5.Kotagal S, Hartse KM, Walsh JK. Characteristics of narcolepsy in preteenaged children. Pediatrics. 1990;85:205–9. [PubMed] [Google Scholar]
- 6.Allsopp MR, Zaiwalla Z. Narcolepsy. Arch Dis Child. 1992;67:302–6. doi: 10.1136/adc.67.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahl RE, Holttum J, Trubnick L. A clinical picture of child and adolescent narcolepsy. J Am Acad Child Adolesc Psychiatry. 1994;33:834–41. doi: 10.1097/00004583-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Roth B. Narkolepsie und Hypersomnie. VEB Verlag Volk und Gesundheit. 1962 [Google Scholar]
- 9.Bell IR. Diet histories in narcolepsy. In: Guilleminault C, Dement WC, Passouant P, editors. Narcolepsy. 1976. pp. 221–8. [Google Scholar]
- 10.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 11.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251:85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- 12.Kok SW, Overeem S, Visscher TL, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 13.Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–42. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 15.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–58. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 17.Taylor MM, Samson WK. The other side of the orexins: endocrine and metabolic actions. Am J Physiol Endocrinol Metab. 2003;284:E13–7. doi: 10.1152/ajpendo.00359.2002. [DOI] [PubMed] [Google Scholar]
- 18.Lammers GJ, Pijl H, Iestra J, Langius JA, Buunk G, Meinders AE. Spontaneous food choice in narcolepsy. Sleep. 1996;19:75–6. doi: 10.1093/sleep/19.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept. 2002;104:91–5. doi: 10.1016/s0167-0115(01)00352-4. [DOI] [PubMed] [Google Scholar]
- 20.van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–82. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Osaka T, Inoue S. Orexin-A-sensitive site for energy expenditure localized in the arcuate nucleus of the hypothalamus. Brain Res. 2003;971:128–34. doi: 10.1016/s0006-8993(03)02437-5. [DOI] [PubMed] [Google Scholar]
- 22.Monda M, Viggiano A, Fuccio F, De Luca V. Injection of orexin A into the diagonal band of Broca induces sympathetic and hyperthermic reactions. Brain Res. 2004;1018:265–71. doi: 10.1016/j.brainres.2004.05.084. [DOI] [PubMed] [Google Scholar]
- 23.Kreier F, Fliers E, Voshol PJ, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest. 2002;110:1243–50. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eikelis N, Esler M. The neurobiology of human obesity. Exp Physiol. 2005;90:673–82. doi: 10.1113/expphysiol.2005.031385. [DOI] [PubMed] [Google Scholar]
- 25.Hublin C, Matikainen E, Partinen M. Autonomic nervous system function in narcolepsy. J Sleep Res. 1994;3:131–137. doi: 10.1111/j.1365-2869.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferini-Strambi L, Spera A, Oldani A, et al. Autonomic function in narcolepsy: power spectrum analysis of heart rate variability. J Neurol. 1997;244:252–5. doi: 10.1007/s004150050080. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 28.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 29.Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol. 1990;258:399–412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- 30.Carter J, Jeukendrup AE. Validity and reliability of three commercially available breath-by-breath respiratory systems. Eur J Appl Physiol. 2002;86:435–41. doi: 10.1007/s00421-001-0572-2. [DOI] [PubMed] [Google Scholar]
- 31.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–34. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 32.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. Nutrition. 1990;6:213–21. [PubMed] [Google Scholar]
- 33.Furlan R, Malliani AF. Assessment of the autonomic control of the cardiovascular system by frequency domain approach. In: Robertson D, editor. Primer on the autonomic nervous system. Amsterdam; Boston: Elsevier Academic Press; 2004. pp. 228–30. [Google Scholar]
- 34.Hojgaard MV, Holstein-Rathlou NH, Agner E, Kanters JK. Dynamics of spectral components of heart rate variability during changes in autonomic balance. Am J Physiol. 1998;275:H213–9. doi: 10.1152/ajpheart.1998.275.1.H213. [DOI] [PubMed] [Google Scholar]
- 35.Jokkel G, Bonyhay I, Kollai M. Heart rate variability after complete autonomic blockade in man. J Auton Nerv Syst. 1995;51:85–9. doi: 10.1016/0165-1838(95)80010-8. [DOI] [PubMed] [Google Scholar]
- 36.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 37.Matsumoto T, Miyawaki C, Ue H, Kanda T, Yoshitake Y, Moritani T. Comparison of thermogenic sympathetic response to food intake between obese and non-obese young women. Obes Res. 2001;9:78–85. doi: 10.1038/oby.2001.10. [DOI] [PubMed] [Google Scholar]
- 38.Guelen I, Westerhof BE, Van Der Sar GL, et al. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit. 2003;8:27–30. doi: 10.1097/00126097-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Middelkoop HA, Lammers GJ, Van Hilten BJ, Ruwhof C, Pijl H, Kamphuisen HA. Circadian distribution of motor activity and immobility in narcolepsy: assessment with continuous motor activity monitoring. Psychophysiology. 1995;32:286–91. doi: 10.1111/j.1469-8986.1995.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 40.Durrer M, Hess K, Dursteler M. [Narcolepsy and activity monitor] Schweiz Arch Neurol Psychiatr. 1991;142:313–8. [PubMed] [Google Scholar]
- 41.Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int J Obes (Lond) 2005;29:287–91. doi: 10.1038/sj.ijo.0802888. [DOI] [PubMed] [Google Scholar]
- 42.Broughton R, Valley V, Aguirre M, Roberts J, Suwalski W, Dunham W. Excessive daytime sleepiness and the pathophysiology of narcolepsy-cataplexy: a laboratory perspective. Sleep. 1986;9:205–15. doi: 10.1093/sleep/9.1.205. [DOI] [PubMed] [Google Scholar]
- 43.Busek P, Vankova J, Opavsky J, Salinger J, Nevsimalova S. Spectral analysis of the heart rate variability in sleep. Physiol Res. 2005;54:369–76. [PubMed] [Google Scholar]
- 44.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson AV, Samson WK. The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front Neuroendocrinol. 2003;24:141–50. doi: 10.1016/s0091-3022(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Sakurai T, Fukuda Y, Kuwaki T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00704.2005. [DOI] [PubMed] [Google Scholar]
- 47.Chabas D, Foulon C, Gonzalez J, et al. Eating disorder and metabolism in narcoleptic patients. Sleep. 2007;30:1267–73. doi: 10.1093/sleep/30.10.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]