Abstract
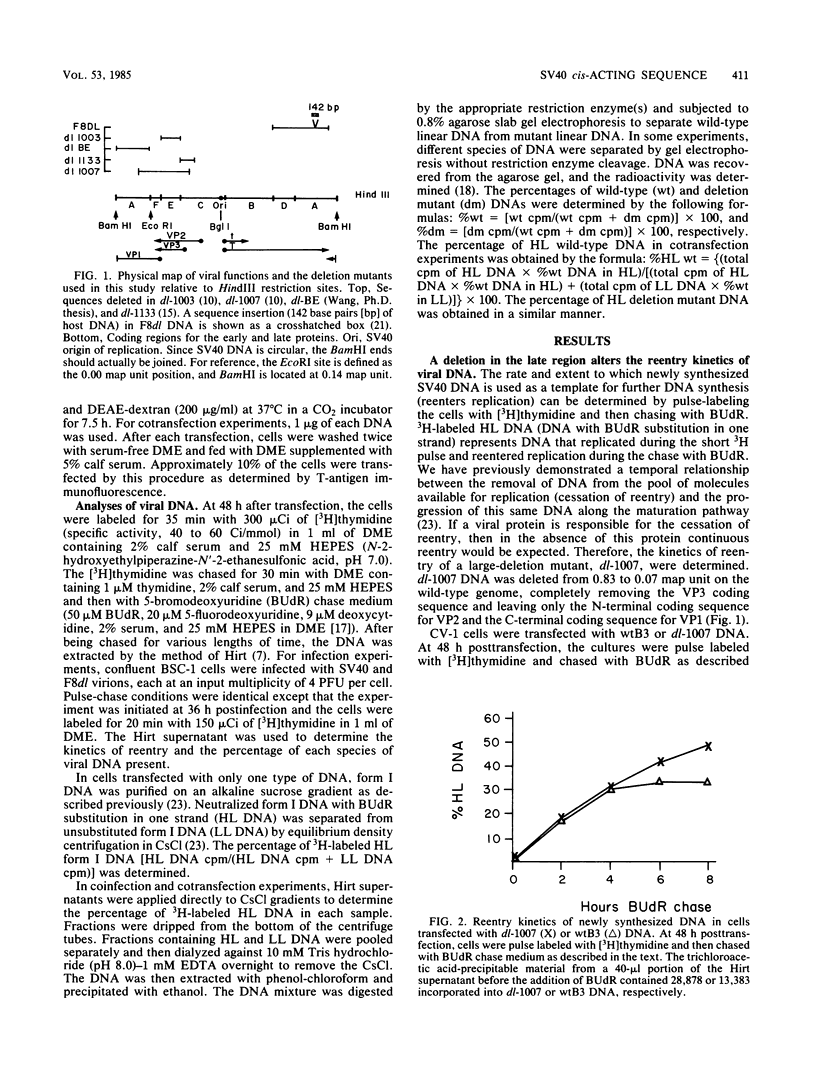
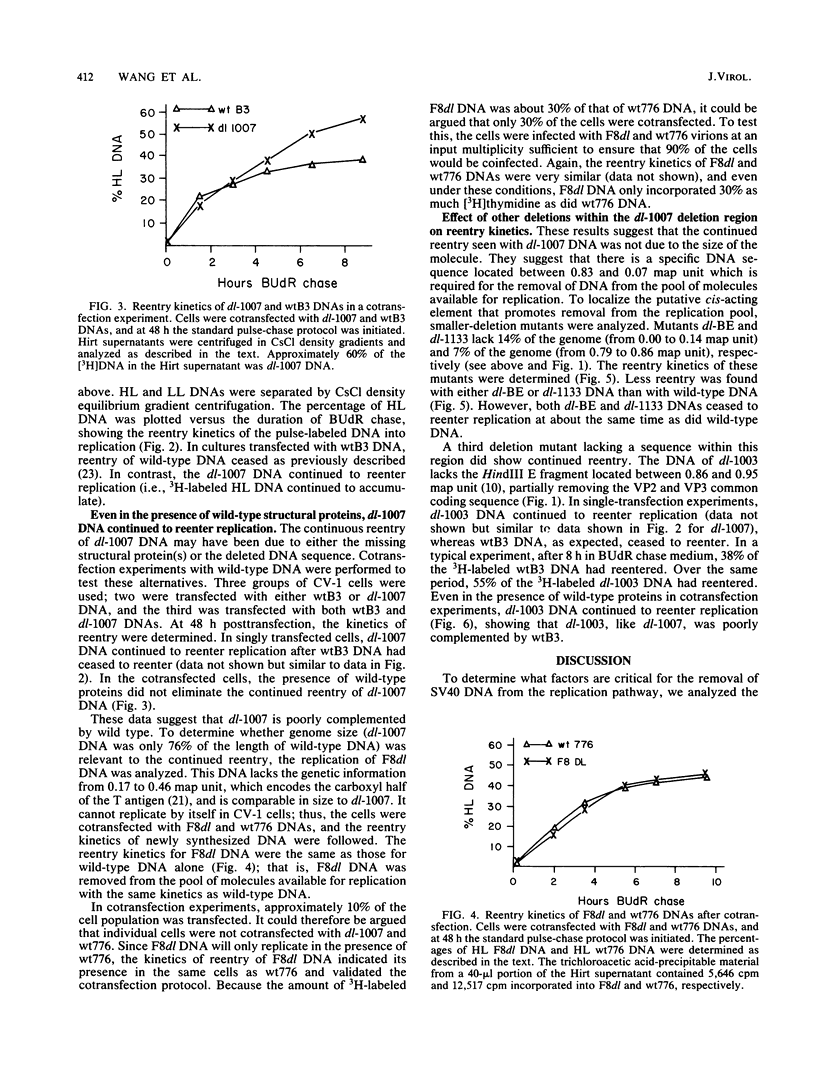
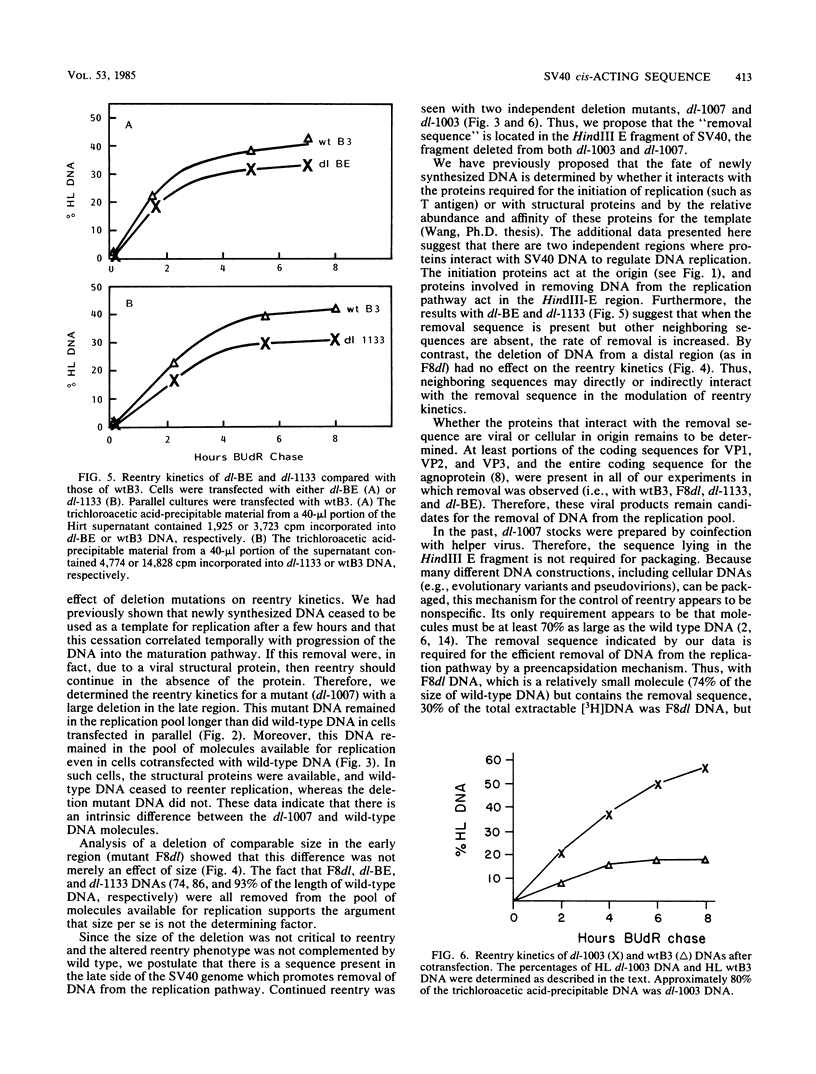
Pulse-labeled simian virus 40 (SV40) DNA is removed from the pool of molecules available for replication (i.e., it ceases to reenter replication) a few hours after synthesis. We studied this cessation of reentry with mutants containing different deletions in the structural genes of SV40. The DNAs of two independent deletion mutants, dl-1007 (24% deletion) and dl-1003 (8% deletion), were used as templates for further DNA synthesis (i.e., they reentered replication) to a greater extent than was wild-type DNA. The alteration in reentry kinetics was not because the DNAs were smaller; other deletion mutations that were from 76 to 85% of the length of wild-type DNA (dl-BE and dl-1133 with a deletion in the late region and F8dl with a deletion in the early region) did not reenter replication to a greater extent than the wild type did. Cotransfection experiments showed that the mutant phenotypes of dl-1007 and dl-1003 were poorly complemented, if at all, by the wild type. Thus, we propose that there is a cis-acting sequence located in the HindIII E fragment of SV40, not present in either of these mutants, that promotes the efficient removal of DNA from the replication pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockman W. W., Lee T. N., Nathans D. Characterization of cloned evolutionary variants of simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):119–127. doi: 10.1101/sqb.1974.039.01.017. [DOI] [PubMed] [Google Scholar]
- Ganem D., Nussbaum A. L., Davoli D., Fareed G. C. Isolation, propagation and characterization of replication requirements of reiteration mutants of Simian Virus 40. J Mol Biol. 1976 Feb 15;101(1):57–83. doi: 10.1016/0022-2836(76)90066-8. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. Intracellular SV40 nucleoprotein complexes: synthesis to encapsidation. Virology. 1980 Dec;107(2):389–401. doi: 10.1016/0042-6822(80)90306-2. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Green M. H., Brooks T. L. Recently replicated simian virus 40 DNA is a preferential template for transcription and replication. J Virol. 1978 May;26(2):325–334. doi: 10.1128/jvi.26.2.325-334.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Khoury G., Fareed G. C., Berry K., Martin M. A., Lee T. N., Nathans D. Characterization of a rearrangement in viral DNA: mapping of the circular simian virus 40-like DNA containing a triplication of a specific one-third of the viral genome. J Mol Biol. 1974 Aug 5;87(2):289–301. doi: 10.1016/0022-2836(74)90150-8. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. The B/C gene of simian virus 40. Virology. 1976 Dec;75(2):335–345. doi: 10.1016/0042-6822(76)90032-5. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Nathans D. Evolutionary variants of simian virus 40: replication and encapsidation of variant DNA. Virology. 1979 Jan 30;92(2):291–298. doi: 10.1016/0042-6822(79)90134-x. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Teresky A. K. Deoxyribonucleic acid replication in simian virus 40-infected cells. II. Detection and characterization of simian virus 40 pseudovirions. J Virol. 1970 Apr;5(4):451–457. doi: 10.1128/jvi.5.4.451-457.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N. Construction of an SV40-derived cloning vector. Gene. 1980 Oct;11(1-2):63–77. doi: 10.1016/0378-1119(80)90087-6. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Roman A., Dulbecco R. Fate of polyoma form IDNA during replication. J Virol. 1975 Jul;16(1):70–74. doi: 10.1128/jvi.16.1.70-74.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman A. Kinetics of reentry of polyoma progeny form I DNA into replication as a function of time postinfection. Virology. 1979 Jul 30;96(2):660–663. doi: 10.1016/0042-6822(79)90125-9. [DOI] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Regulatory mutants of simian virus 40: constructed mutants with base substitutions at the origin of DNA replication. J Mol Biol. 1979 Jul 15;131(4):801–817. doi: 10.1016/0022-2836(79)90202-x. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Isolation and characterization of simian virus 40 early region deletion mutants. J Virol. 1982 Jul;43(1):328–331. doi: 10.1128/jvi.43.1.328-331.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trilling D. M., Axelrod D. Encapsidation of free host DNA by simian virus 40: a simian virus 40 pseudovirus. Science. 1970 Apr 10;168(3928):268–271. doi: 10.1126/science.168.3928.268. [DOI] [PubMed] [Google Scholar]
- Wang H. T., Roman A. Cessation of reentry of simian virus 40 DNA into replication and its simultaneous appearance in nucleoprotein complexes of the maturation pathway. J Virol. 1981 Jul;39(1):255–262. doi: 10.1128/jvi.39.1.255-262.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. B., Aposhian H. V. Polyoma pseudovirions. I. Sequence of events in primary mouse embryo cells leading to pseudovirus production. J Virol. 1972 Sep;10(3):340–346. doi: 10.1128/jvi.10.3.340-346.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


