Abstract
Cell rolling is an important physiological and pathological process that is used to recruit specific cells in the bloodstream to a target tissue. This process may be exploited for biomedical applications to capture and separate specific cell types. One of the most commonly studied proteins that regulate cell rolling is P-selectin. By coating surfaces with this protein, biofunctional surfaces that induce cell rolling can be prepared. Although most immobilization methods have relied on physisorption, chemical immobilization has obvious advantages, including longer functional stability and better control over ligand density and orientation. Here we describe chemical methods to covalently immobilize P-selectin on glass substrates. The chemistries were categorized based on the functional groups on modified glass substrates: amine, aldehyde, and epoxy. The prepared surfaces were first tested in a flow chamber by flowing microspheres functionalized with a cell surface carbohydrate (Sialyl Lewis(x)) that binds to P-selectin. Adhesion bonds between P-selectin and Sialyl Lewis(x) dissociate readily under shear forces leading to cell rolling. P-selectin immobilized on the epoxy glass surfaces exhibited enhanced long term stability of the function and better homogeneity as compared to surfaces prepared by other methods and physisorbed controls. The microsphere rolling results were confirmed in vitro with isolated human neutrophils. This work is essential for future development of devices for isolating specific cell types based on cell rolling, which may be useful for hematologic cancers and certain metastatic cancer cells that are responsive to immobilized selectins.
Introduction
Cell rolling along vascular endothelium in viscous shear flow is of primary biological importance given its role in recruitment of leukocytes to sites of inflammation, homing of hematopoietic progenitor cells after intravenous injection, tumor cell metastasis and other inflammatory processes1-3. Rolling is a receptor-ligand mediated event that initiates an adhesion process to a target tissue through a reduction in cell velocity followed by activation, firm adhesion, and transmigration. Rolling response is primarily mediated by a family of transmembrane domain based glycoprotein receptors called selectins which are expressed on the surface of leukocytes and activated endothelial cells4. Selectins bind to carbohydrates via a lectin-like extracellular domain and the broad family of selectins are divided into L-(CD62L), E-(CD62E), and P-selectin (CD62P). L-selectin (74-100 kDa) is found on most leukocytes and can be rapidly shed from the cell surface5, E-selectin (100 kD) is transiently expressed on vascular endothelial cells in response to IL-1 beta and TNF-alpha, and P-selectin (140 kDa) is typically stored in the secretory granules of platelets and endothelial cells6-8.
Since the adhesion bonds between selectins and their complementary ligands dissociate readily under shear forces, these adhesions do not firmly immobilize the cells and thus are referred to as ‘transient adhesions’. Although rolling experiments are useful for uncovering fundamental biology and for potentially building devices for cell separation, most studies to date have employed random placement of selectins onto a 2-D substrate utilizing protein physisorption9, 10. Furthermore, the stability of physisorbed selectins is weak as adsorbed proteins tend to rapidly desorb from the surfaces. Commonly used selectin immobilization methods do not afford a high degree of control over the presentation of selectins which may hinder the ability to mimic relevant complexities of the in situ rolling response and to design efficient and effective separation tools.
The rolling velocity of each cell type is distinctive as a function of local shear rate, the distribution of receptors on cell membranes, and the total number of receptors present on the cell which may differ from one cell type to another1, 11-13. This suggests a number of parameters to improve specificity. Through exhibiting greater control over the presentation14 and stability of selectins, one may be able to model and interrogate more complex phenomena leading to enhanced biological understanding. Covalent immobilization of proteins offers great potential for enhanced control over presentation and stability of biomolecules from surfaces15. Specifically, covalent immobilization of selectins has advantages over conventional complexation and physisorption since it would facilitate optimization of cell-material interactions via control over density in the surface coating, spatial patterning, active site orientation, stability and shelf life, and topology as facilitated by linkers to achieve specific rolling characteristics. Although covalent immobilization procedures for peptides and enzymes have been extensively studied for decades, covalent immobilization of large molecular weight biomolecules such as selectins present significant challenges due to the increase of binding to non-specific sites16 and due to the requirement for mild processing conditions to prevent protein inactivation.
Here we investigate three conjugation chemistries based on amine, aldehyde and epoxy functionalized glass substrates using a parallel plate flow chamber which mimics physiologic flow conditions. The prepared surfaces are characterized by x-ray photoelectron scattering (XPS) and contact angle measurements. To prescreen each chemistry before conducting cell-based studies we used 10 μm microspheres conjugated with Sialyl Lewis(x)9, 17, 18. We found that epoxy chemistry which is stable at neutral pH in aqueous environments allowed us to achieve the longest term storage and the most bond stability without protein aggregation among the three chemistries. This chemistry led to significant enhancement in the stability of microsphere rolling. These results were validated through in vitro cell rolling experiments as previously described11, 12.
Experimental Section
Materials
Recombinant Human P-selectin/Fc chimera (P-selectin) and mouse monoclonal antibody specific for human P-selectin (clone AK-4) were purchased from R&D systems (Minneapolis, MN). All the functionalized glass surfaces (plain, amine, aldehyde, and epoxy glass) were provided by TeleChem International, Inc (Sunnyvale, CA). Heterobifunctional poly(ethylene glycol) (NH2-PEG-COOH) was acquired from Nektar (San Carlos, CA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). All the materials employed in this study were used without further purification unless specified.
Preparation of Surfaces
A synthetic route for surface preparation is illustrated in Figure 1. Briefly, P-selectin immobilization was performed on four different glass substrates. Glass surface with physically adsorbed P-selectin was prepared on the plain glass. The plain glass substrate (SuperClean2®) was washed with PBS three times, 5 min for each washing. 600 μL of P-selectin at a 5 μg/mL concentration was placed on top of the glass and incubated on a plate shaker for 18 hrs. For covalent immobilization of P-selectin, the amine (SuperAmine2®), aldehyde (SuperAldehyde2®), and epoxy (SuperEpoxy2®) functionalized glass surfaces were employed. AFM analysis and other characterization results of all underlying glass substrates can be found at http://www.arrayit.com/Products/Substrates/.
Figure 1. Surface preparation via a synthetic route.
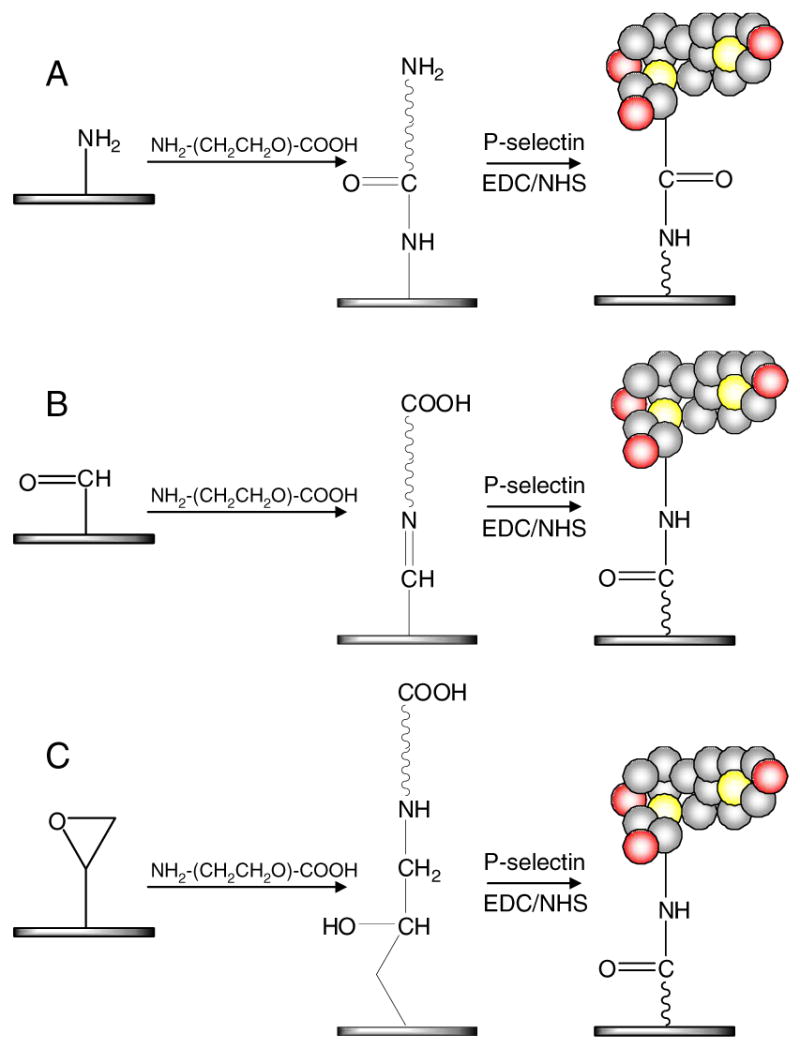
P-selectin was immobilized on A) amine, B) aldehyde, and C) epoxy functionalized glass surfaces through a PEG linker (NH2-PEG-COOH). On the amine glass, NH2-PEG-COOH and P-selectin were pre-activated by EDC and NHS in solution before they were placed on the surfaces. For covalent immobilization on aldehyde and epoxy surfaces, carboxylated groups on the PEGylated surfaces were pre-activated using EDC/NHS and P-selectin was conjugated on top of the PEGylated glass surfaces. For comparison of surface stability with physical adsorption of P-selectin, plain glass substrate as well as PEGylated aldehyde and epoxy surfaces were employed without pre-activation (i.e. without EDC/NHS).
To ensure effective surface modification, all reagents were used in excess quantities. According to the supplier, the SuperAmine2® contains 2×1013 reactive groups per mm2 whereas the SuperAldehyde2®and SuperEpoxy2® have 5×1012 reactive groups per mm2. Therefore a total surface area of 10 cm2 has approximately 2×1016 or 5×1015 reactive groups. Reagents including NH2-PEG-COOH were used with an excess molarity of 10-100X as described below.
Detailed protocols for each surface are as follows: On the amine glass, 500 μL of NH2-PEG-COOH at a 5 mg/mL concentration (∼1 mM) was pre-activated by 1:1 mixture (500 μL) of 50 mM (1.9 mg/mL) 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) and 50 mM (2.2 mg/mL) N-hydroxysuccinimide (NHS) in distilled water for 5 min, immediately followed by incubation on the glass at r. t. for 1 hr. One milliliter of P-selectin at a concentration of 5 μg/mL was also pre-activated in solution by EDC (19 μg) and NHS (22 μg) for 5 min, added on top of the PEGylated glass, and incubated at r. t. overnight. The glass surfaces were washed thoroughly with PBS at each step. On the aldehyde glass, 600 μL of NH2-PEG-COOH (5 mg/mL) was added onto the glass surface and incubated for 2 hrs. After washing with PBS three times, some of the surfaces were treated by 10X molar excess of sodium cyanoborohydride (5×10-6 mol) compared to the concentration of NH2-PEG-COOH. This was used to reduce the unstable Schiff's bases to stable secondary amines. EDC (160 μg) and NHS (180 μg) were added to 500 μL of PBS and used to activate the surface bound COOH groups for 30 min. This volume was removed from the surface and 600 μL of P-selectin (5 μg/mL) was immediately added and permitted to react at r.t. for 18 hrs. On the epoxy glass, NH2-PEG-COOH was immobilized, activated by EDC/NHS, and reacted with P-selectin under the same conditions for the aldehyde glass except the reduction reaction (in this reaction, stabilization by a reducing agent is not necessary).
For the stability tests, surfaces were immersed in PBS and placed on a plate shaker at room temperature. The aged surfaces were compared to freshly prepared surfaces in subsequent flow chamber experiments.
X-ray photoelectron spectroscopy (XPS) and contact angle measurement
Surfaces at each step were characterized by XPS and contact angle measurement (Table 1). XPS measurements were performed using an Axis Ultra X-ray Photoelectron spectrometer (Kratos Analytical, Manchester, UK) equipped with a monochromatic Al K-alpha source (1486.6 eV, 150 W) and a Hemispherical analyzer. The mass concentration % was obtained at a take-off angle of 20° with pass energy of 80 eV and step size of 0.2 eV.
Table 1.
Relative surface composition and contact angles of various surfaces
| Plain Glass Substrate | Aldehyde Glass Substrate | Epoxy Glass Substrate | ||||||
|---|---|---|---|---|---|---|---|---|
| Plain | P-selectin | Aldehyde | PEGylated | P-selectin | Epoxy | PEGylated | P-selectin | |
| Ca | 11% | 58% | 20% | 23% | 55% | 18% | 19% | 56% |
| Na | 1% | 12% | - | 0% | 9% | 1% | 0% | 8% |
| Oa | 47% | 23% | 43% | 43% | 34% | 43% | 44% | 33% |
| Sia | 37% | 7% | 30% | 33% | 2% | 32% | 37% | 3% |
| Contact Angle (°)b | 34±2 | 68±4 | 49±5 | 45±5 | 66±4 | 43±6 | 50±5 | 65±5 |
All standard deviations for XPS data (mass concentration %) were less than ±5% (pass energy 80 eV, step size 0.2 eV) at take-off angle 20° measured by XPS.
Contact angles of each surface were measured 4 times using double distilled water. Mean ± SD.
Contact angles of double distilled water on surfaces were measured using VCA2000 (AST Products, Inc., Billerica, MA). Drops, of 3 μL, were deposited onto the sample surface using a micro syringe attached to the system and data was analyzed using VCA optima XE software.
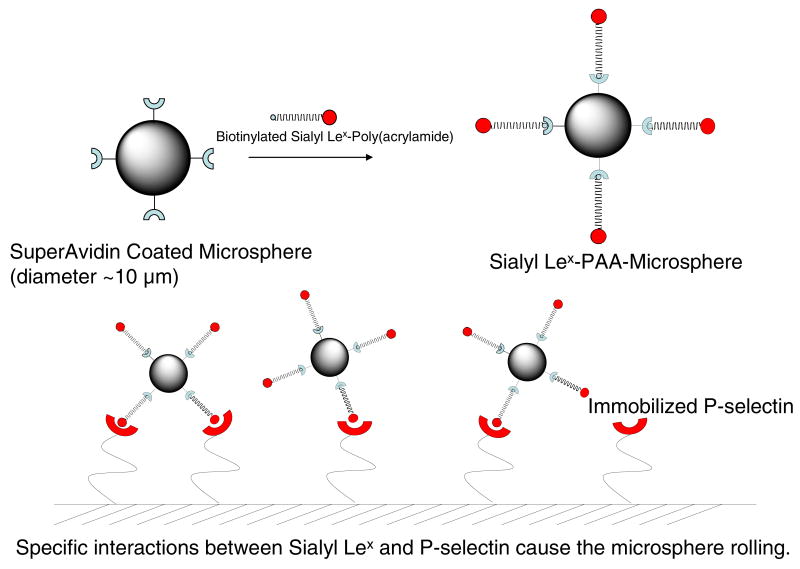
Preparation of microsphere conjugates
SuperAvidinTM-coated microspheres with a diameter of 9.95 μm (Bangs Laboratories, Fishers, IN) were conjugated with multivalent biotinylated Sialyl Lewis(x)-poly(acrylamide) (sLex-PAA-biotin, Glycotech, Gaithersburg, MD) to be used as a cell mimic for our pre-screening tests according to the previous report (Figure 2)9. Briefly, a 104.8 μL bead solution (containing 2×106 beads) was dissolved into 1 ml of PBS containing 1% BSA (BPBS). The mixture was washed with BPBS three times by centrifuge at 10,000 rpm for 2 min. Four microliters of 1 mg/ml sLex-PAA-biotin (4 μg sLex) was added into the mixture and incubated for 1 h at room temperature with occasional vortexing. The resulting solution was then washed again with BPBS three times by centrifuge at 10,000 rpm for 2 min. The final solution was resuspended in BPBS and diluted at a concentration of 1×105 beads/mL to be used in adhesion experiments. In control experiments, native SuperAvidinTM-coated microspheres (without sLex modification) at the same concentrations were also used to assess the velocity of non-interacting microspheres.
Figure 2. A schematic diagram of preparation of microsphere conjugates and their rolling on the P-selectin coated surface.
Flow chamber assay with microsphere-ligand conjugates
A rectangular parallel-plate flow chamber (Glycotech) with a gasket with thickness of 250 μm and length of 6 cm was placed on the glass surfaces with P-selectin. Flow rate – shear stress relationship was calculated based on the following equation19.
Where, (τs*)max is the maximum shear stress on the surfaces, μ is the viscosity of the fluid (water = 0.01 dyn s/cm2), Q2-D* is the flow rate per unit width in the system, and H is the height of the channel. All the flow chamber experiments using the microspheres were performed at a flow rate of 50 μL/min which is translated into a wall shear stress of 0.24 dyn/cm2 in this system. Note that different conditions were used for cell-based experimentation.
For the microsphere experiment, 5×105 mL-1 multivalent sLex-coated microspheres were prepared in PBS containing 1% BSA and perfused into a flow chamber at a shear stress of 0.24 dyn/cm2 using a syringe pump (New Era Pump Systems, Inc., Farmingdale, NY). During each microsphere experiment flow was interrupted for 1 min, followed by image recording for 2 min. The flow was stopped to promote microsphere-surface contact via sedimentation. Images were taken every 5 seconds on the Axiovert 200 Zeiss microscope (Carl Zeiss, Thornwood, NY) equipped with a camera controlled by Hamamatsu camera controller (Hamamatsu, Japan) and the velocities were calculated by measuring the displacement of each microsphere in subsequent images using Axioveision software version 3.1 (Carl Zeiss, Thornwood, NY). The average velocities were obtained by averaging velocities of at least 20 microspheres. For control experiments, instantaneous velocities were measured during the first 30s (without stopping the flow) using non-interacting microspheres without sLex (at the same shear stress and concentration). Other control surfaces were also employed such as BSA treated glass and P-selectin coated surfaces that were post-treated with P-selectin antibody (10 μg/mL) at r. t. for 2 hrs in order to block the specific interaction, Rolling dynamic data of microspheres was presented as mean ± SEM.
Flow chamber assay with neutrophils
Human blood was collected into a sterile tube containing sodium heparin (BD Biosciences, San Jose, CA) via venipuncture after informed consent was obtained. Neutrophils were then isolated by centrifugation (480 ×g at 23°C for 50 min) with 1-Step Polymorphs (Accurate Chemical & Scientific Co., Westbury, NY). After isolation, neutrophils were kept in sterile Hanks’ Balanced Salt Solution containing 0.5% human serum albumin, 2 mM Ca2+, 10 mM HEPES, at pH 7.4, until flow experiments. A rectangular parallel-plate flow chamber (Glycotech) with a gasket of thickness 127 μm and length 6 cm was placed on a P-selectin-immobilized glass surface. The assembled flow chamber was placed on an inverted microscope, Olympus IX81 (Olympus America Inc., Center Valley, PA) and the neutrophil solution, at a concentration of 2.5×105/mL, was perfused into the chamber using a syringe pump (New Era Pump Systems, Inc.) at different flow rates. The perfusion pump generated a laminar flow inside the flow chamber, allowing regulation of calculated wall shear stresses from 1 to 10 dyn/cm2.
Data acquisition and cell tracking
A microscope-linked CCD camera (Hitachi, Japan) was used for monitoring neutrophil rolling interactions with adhesive P-selectin substrates. Rolling of neutrophils was observed using phase contrast microscopy and recorded on high quality DVD+RW discs for cell tracking analyses. Cell rolling videos were re-digitized to 640×480 pixels at 29.97 fps with ffmpegX software. Rolling fluxes and velocities of neutrophils interacting with immobilized P-selectin were then acquired using a computer-tracking program coded in ImageJ 1.37m (NIH) and MATLAB 7.3.0.267 (R2006b) (Mathworks). A cell was classified as rolling if it rolled for > 10 seconds while remaining in the field of view (864×648 μm2 using a 10× objective (NA = 0.30; Type: Plan Fluorite; Olympus America Inc.)) and if it translated at an average velocity less than 50% of the calculated free stream velocity of a non-interacting cell (Note that this criteria was specific to the cell-based study). The free stream velocity was calculated using the theory of Goldman et al.20. Rolling dynamic data was presented as mean ± SEM of duplicate observations. Each observation was measured for 1 min under each shear stress tested. To confirm statistical significance among the data, p values were calculated using a paired student's t-test method.
Results and Discussion
An early example of cell rolling studies was performed by Tim Springer's laboratory in 1991 using selectins within lipid bilayers21. This model system was used to reproduce early leukocyte interactions with vascular endothelium and the subsequent interest in this area is evident from the nearly 4500 references ascribed to the original report4. In addition to using in vitro model systems to study leukocyte rolling, the rolling phenomena may be applied as a tool for cell separations22, 23. Specifically, selectin molecules coated on a solid surface can be used as an affinity chromatography mechanism to capture a number of cell types from blood, including hematopoietic stem cells, leukocytes, platelets, and some metastatic cancer cell lines1. To date most of these studies have employed physisorption of selectins to affect cell rolling. Since physisorbed proteins adhere mainly through weak intermolecular forces (i.e. van der Waals interactions) with an equilibrium established between the adsorbed and free protein, these surfaces have limited stability and are typically only useful for immediate or short term use. Here we explore the covalent immobilization of selectins using a variety of substrate chemistries to enhance stability and offer the potential of controlling spatial orientation.
Covalent Immobilization of P-selectin – amine substrate chemistry does not improve P-selectin presentation
As illustrated in Figure 1, surfaces for covalent immobilization of P-selectin were pre-coated with NH2-PEG-COOH. The heterobifunctional PEG was used to provide reactive sites for P-selectin and to produce non-fouling surfaces24. On top of the PEGylated surfaces, P-selectin was immobilized forming amide bonds between the carboxylate groups and primary amine groups.
The availability of primary amines and carboxylics on the surface of proteins makes amine coupling commonplace. Amine reactive groups on a solid substrate (silanized glass) bind to the carboxyl groups of the PEG linker and the amine groups of the linker react with carboxyl termini of proteins. This chemistry was initially thought to be useful for enhanced orientation of P-selectin since the active site of the protein is known to be near the amine termini (the opposite end of the carboxyl termini). However, given the relatively low reactivity of amine groups, carboxyl termini on P-selectin must be activated by EDC and NHS for the reaction to occur. We found that the EDC/NHS activation of P-selectin in solution led to aggregation of P-selectin which resulted in undesirable formation of micron sized particles as observed by fluorescence microscopy with an antibody-FITC conjugate (see supplementary Figure 1). Since we were unable to substantially curb this aggregation by changing the reaction conditions, we rationalized that this strategy was not suitable for enhancing control over P-selectin presentation.
We next investigated aldehyde chemistry given that this chemistry does not require activation of P-selectin or the PEG linker in solution as aldehyde groups on silanized glass possess a high reactivity towards amine groups. Aldehydes bind through Schiff base aldehyde-amine chemistry to amines on the PEG. After activation of the PEG with EDC/NHS the carboxylate terminus on the PEG reacts with amine groups within lysine residues of proteins, or via the primary amine terminus. For an additional strategy, P-selectin was immobilized on the PEGylated epoxy-coated glass substrate that have been widely used for protein conjugation particularly in microarrys25. Epoxy-coated slides are derivatized with epoxysilane where proteins are covalently attached through an epoxide ring-opening reaction primarily with surface amino groups on proteins. In comparison to the amine chemistry, the common advantage of epoxy-based and aldehyde-based chemistry is that EDC/NHS activation can be performed on the surfaces, which does not cause protein aggregation due to intramolecular loop formation and/or intermolecular interactions. In addition, the epoxy-based chemistry has the added advantage over aldehyde chemistry given that the reaction between epoxy and amine results in very stable bond formation. A stable bond can be also formed using the aldehyde-based chemistry if the bond is reduced by a reducing agent such as sodium cyanoborohydride26. However, this requires an additional step and in our experiments reduced the functionality of the immobilized P-selectin.
Surface modifications were confirmed by XPS and contact angle measurements as shown in Table 1. The aldehyde and epoxy functionality was evidenced from the increased carbon:oxygen ratios as compared to the plain glass substrate. P-selectin immobilization (both physisorbed and chemically bound) was evidenced from the increase in nitrogen composition, decreased visibility of silicon in the underlying glass substrate, and increased contact angle. All surfaces treated with P-selectin had a high degree of coverage as evidenced from the lack of visible underlying silicon. Furthermore, the microspheres and cells encountered similar substrate properties given the consistency in elemental composition and surface energy (contact angle values). Higher relative oxygen concentrations were observed on the chemically immobilized P-selectin surfaces likely due to the presence of underlying PEG in comparison to the physisorbed substrates.
Aldehyde-based Chemistry Exhibits a Lack of Long Term Stability
To test the adhesion properties of the prepared surfaces, microspheres conjugated with the ligand sLex-PAA-biotin were employed prior to testing with human cells. Adhesion properties of the P-selectin immobilized surfaces were tested with the sLex microspheres using a flow chamber as previously described9. To confirm that the observed response was due to specific interaction between sLex and P-selectin, a series of control experiments were carried out. Microspheres without ligands (sLex) demonstrated no rolling behavior (average velocities of 32-40 μm/s on the P-selectin coated surfaces) and surfaces without P-selectin did not reduce velocities of flowing microsphere conjugates (Supplementary Fig. 2A). In addition, to block specific interaction between P-selectin and sLex on microspheres, P-selectin coated surfaces were post-treated using P-selectin antibody, followed by perfusion of microsphere conjugates into the flow chamber. After antibody treatment, the microsphere average velocities on the P-selectin coated surfaces were increased from 0.4 to 31.6 μm/s and 3.4 to 29.2 μm/s on P-selectin immobilized epoxy and aldehyde surfaces, respectively (Supplementary Fig. 2B). These results indicate that the observed velocity reduction on P-selectin coated surfaces is solely due to a P-selectin mediated interaction.
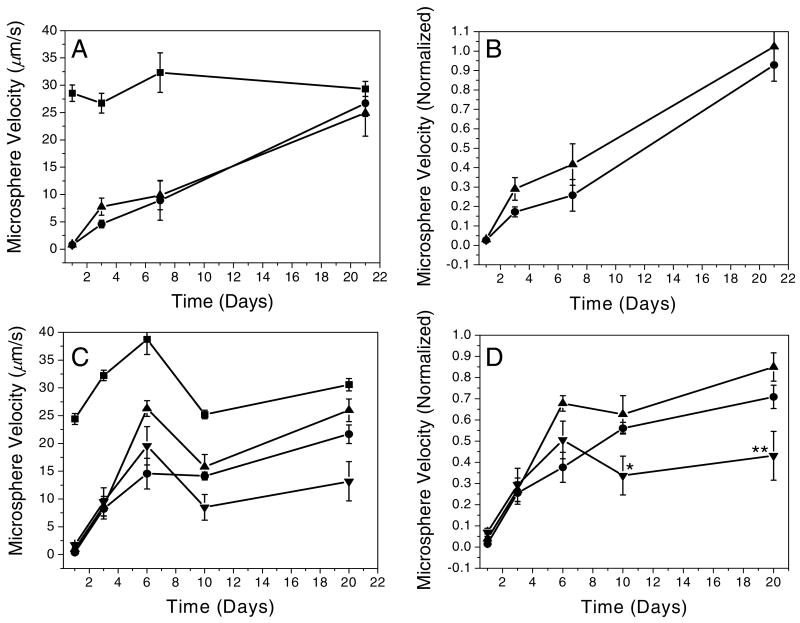
All the freshly made surfaces including P-selectin-adsorbed plain glass and P-selectin-immobilized aldehyde (Fig. 3A) and epoxy (Fig. 3B) glass substrates significantly reduced the microsphere velocities. The microsphere conjugates traveled on the PEGylated aldehyde and PEGylated epoxy surfaces without P-selectin at average velocities of 25-30 μm/s and 25-40 μm/s, respectively. Note that the calculated velocity of a microsphere with diameter of 9.95 μm is 57.5 μm/s at a wall shear stress of 0.24 dyn/cm2 according to the Goldman's calculation20. The velocities of sLex-bound microspheres on control surfaces without P-selectin were examined each day and used to standardize day-to-day variation in data as plotted in the Figure 3B and 3D.
Figure 3. Comparison of surface stability detected through microsphere rolling.
A solution of 1.0 × 105 microspheres/ml was perfused at 0.24 dyn/cm2 of shear stress. (A) Aldehyde surface microsphere velocities and (B) normalized velocities (with respective to PEGylated physisorbed surface controls). (C) Epoxy surface microsphere velocities and (D) normalized velocities. For (A) and (B), microsphere velocities on PEGylated aldehyde glass (■) and P-selectin immobilized on the PEGylated aldehyde surface without EDC/NHS pre-activation (●) and with EDC/NHS pre-activation (▲). For (C) and (D), microsphere velocities on PEGylated epoxy glass (■), P-selectin adsorbed on plain glass (●), and P-selectin immobilized on the PEGylated epoxy surface without EDC/NHS pre-activation (▲) and with EDC/NHS pre-activation (▼). Although surfaces prepared on aldehyde glass do not show any enhanced stability regardless of EDC/NHS pre-activation, P-selectin-immobilized surfaces prepared on the PEGlyated epoxy glass pre-activated using EDC/NHS exhibit significantly enhanced stability. All the rolling dynamic data is represented as mean ± SEM. In (D), *p<0.05 (both ▼ vs ● and ▼ vs ▲), **p<0.05 (▼ vs ●) and p<0.005 (▼ vs ▲)
After 20 days in PBS at r. t., P-selectin immobilized surfaces prepared using the aldehyde glass lost their adhesion property, leading to a loss in rolling behavior (Figure 3A and 3B). Moreover, there was no significant difference between pre-activated (EDC/NHS) and untreated surfaces in terms of sustained adhesive function. This result can be attributed to the unstable chemical bond (Schiff base) between aldehydes and the PEG leading to P-selectin detachment from the surface over time.
Epoxy-based Chemistry Exhibits Enhanced Stability of P-selectin observed via microsphere rolling
In comparison to the aldehyde chemistry, P-selectin covalently immobilized onto epoxy glass exhibited a significant enhancement in long term stability compared to both physisorbed P-selectin and unactivated surfaces (without NHS/EDC) as shown in Figure 3C and 3D. Specifically, after 20 days in PBS at r.t., the P-selectin immobilized surface (pre-activated) exhibited the highest reduction in the microsphere velocity (∼40% compared to the PEGylated epoxy surface without P-selectin) wheareas the P-selectin immobilized epoxy glass untreated with EDC/NHS (∼85%) and P-selectin-adsorbed plain glass (∼70%) allowed the conjugates to travel relatively faster. After 21 days, the average microsphere velocity was 13.1 μm/s on the P-selectin immobilized surfaces compared to 30.6 μm/s on the PEGylated surfaces without P-selectin. This result may be important for design of devices for separating or isolating cells based on rolling behavior where one would need specific functionality for extended periods of time.
Although covalently bound P-selectin on the epoxy surfaces appeared to be more stable than physisorbed P-selectin, it is curious why all the surfaces tested in this study exhibit an increase in microsphere velocity overtime, particularly during the first 3 days. This implies that P-selectin immobilization on the surfaces occurs through both covalent binding and physisorption, or P-selectin forms multi-layers on the surfaces. In other words, P-selectin molecules that are adsorbed on top of other P-selectin and/or directly on the surfaces can be readily desorbed from the surfaces for the first few days. This is not surprising because proteins can be spontaneously adsorbed on surfaces by enthalpic contributions such as van der Waals, electrical double layer, and hydrophobic interactions27, 28. After all of the additionally presented P-selectin is desorbed, the observed difference in terms of functional stability can then be attributed to the difference between covalent immobilization and physisorption. Therefore, actual stability should be compared after aging the surfaces for at least 3 days as the adhesive function of the covalently bound P-selectin was constant after that period of time. This issue can be resolved by employing a flow system for the P-selectin immobilization so that the additional P-selectin on the surfaces can be rapidly removed by shear force. A study in this regard is now being conducted and will be the subject of future publications.
Covalent immobilization enhances Neutrophil Cell Rolling
To confirm that the microsphere results are consistent with live human leukocytes, we investigated neutrophil rolling interaction with the immobilized P-selectin using a parallel-plate chamber under flow. First, control surfaces which did not have P-selectin (i.e., plain glass and a PEGylated epoxy glass slides) showed no cell adhesion (data not shown). From this in vitro cell rolling assay at four different wall shear stresses (1, 3, 5 and 10 dyn/cm2), the number of rolling cells was significantly greater on the P-selectin immobilized surface with having pre-activation of EDC/NHS than on the rest of the P-selectin-surfaces at 28 days after preparation (Figure 4). In contrast, the rolling fluxes dramatically decreased on the older P-selectin-adsorbed surfaces on plain glass and on a PEGylated epoxy glass slide without having EDC/NHS activation compared with those on a newer (3-day-old) surface under the same condition, as shown in Figure 5A. Specifically, at 3 dyn/cm2, rolling flux on the older P-selectin immobilized on epoxy surface (pre-activated with EDC/NHS) did not significantly decrease (80.6 ± 19.1% (mean ± SEM) of that on the new surface), but fluxes on the older P-selectin adsorbed glass and on the older P-selectin immobilized on epoxy surface without EDC/NHS pre-activation dropped to 30.1 ± 5.2% and 1.1 ± 1.1%, respectively (Figure 5B).
Figure 4. Representative phase contrast micrographs of neutrophil rolling adhesion on P-selectin-adsorbed substrates.
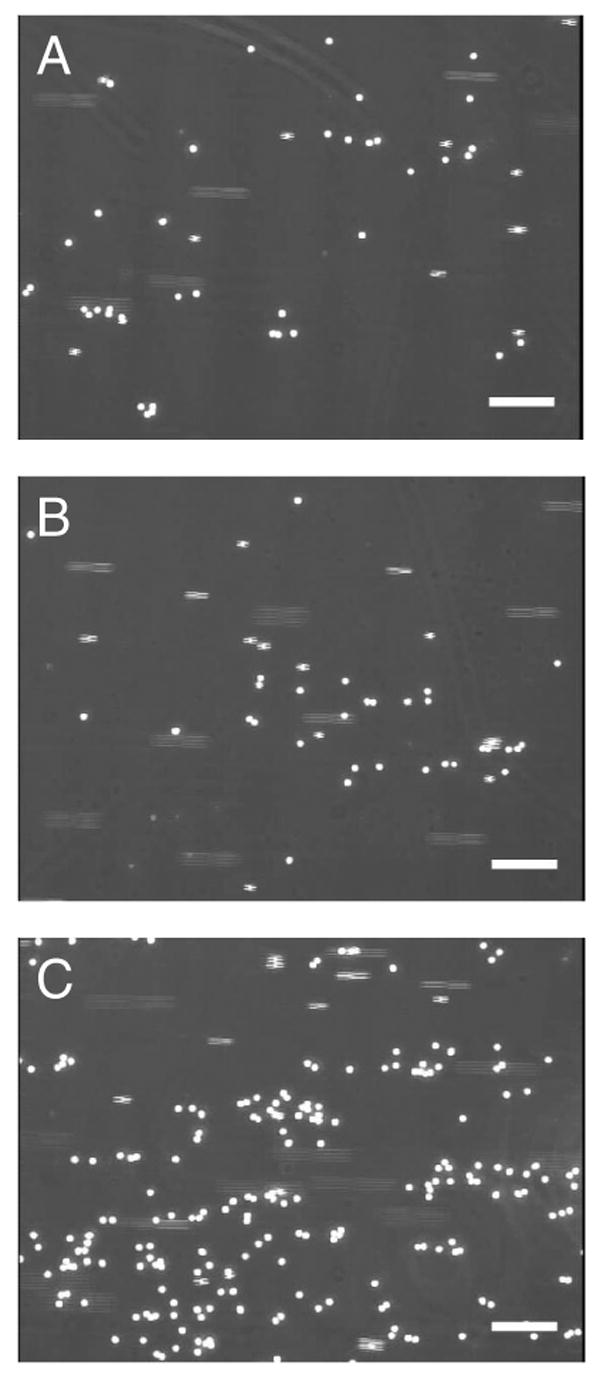
(A) A still image of rolling adhesion of neutrophils on a P-selectin-adsorbed surface on plain glass, a PEGylated epoxy glass slide without (B) or with (C) pre-activation using EDC/NHS. 2.5 × 105/ml of neutrophil solution was perfused on the 28-day-old P-selectin-surface under 1 dyn/cm2 of shear stress. A total magnification of 100× was applied and all scale bars indicate 100 μm.
Figure 5. Rolling dynamics of neutrophils on P-selectin-immobilized surfaces under shear flow.
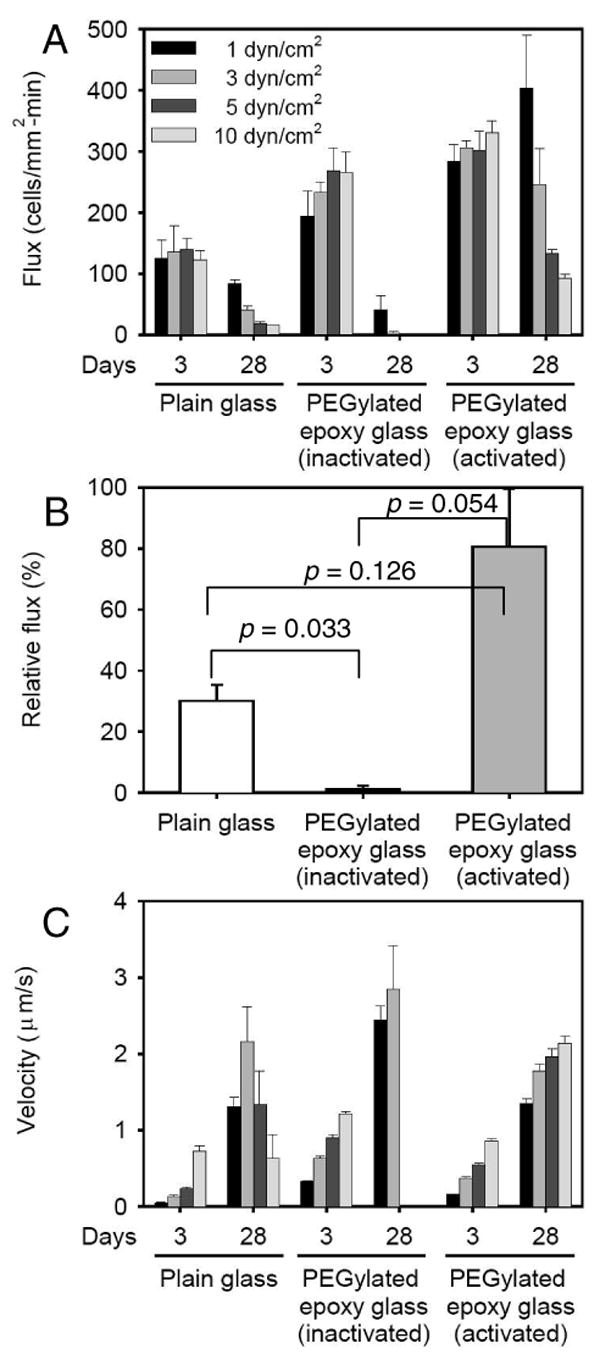
2.5×105/ml of neutrophil solution was perfused on a 3 or 28-day-old P-selectin-surface under wall shear stresses from 1 to 10 dyn/cm2. Rolling fluxes (A) and rolling velocities (C) of neutrophils were measured for each condition. In (B), relative rolling fluxes on the 28-day-old P-selectin-surfaces at 3 dyn/cm2 are plotted. The mean values of fluxes from the 3-day-old surface each are set to 100% and data from the 28-day-old surface are expressed as the mean ± SEM (%). All p values in (A) are listed in supplementary Table 1. All p values in (C) were < 0.0005 except for the plain glass slide at 10 dyn/cm2 which had p = 0.319 between the 3-day-old and 21-day-old surfaces.
Cell rolling velocity analysis indicates that a large number of neutrophils on the aged P-selectin immobilized epoxy surface sustain continuous rolling as the shear stress increased while most of cells on the other two surfaces detached and rejoined the free stream (Figure 5C). It is noteworthy that the observed rolling velocities of cells were significantly lower than those of microspheres, especially given that the shear stresses were higher for the cells by an order of magnitude. This is due to two main differences: (1) the microvilli on the neutrophil surface that extend to reconcile the dissociation force applied on the P-selectin-ligand bond, and (2) neutrophils possess the stronger-binding selectin ligand PSGL-1, whereas the microspheres were coated with the weaker-binding sLex group29, 30. Note that the glycoprotein PSGL-1 possesses not only the sLex carbohydrate, but also two sulfated tyrosines which strengthen the bond with P-selectin relative to sLex alone31. In addition, the contact area of a neutrophil with a ligand-bearing surface is known to flatten and increase during cell rolling, making additional receptors available for binding32. As shown in Figure 4 and 5, the average rolling velocities of neutrophils on all P-selectin-coated surfaces were lower than those of sLex-microspheres, although the microspheres traveled at a reduced wall shear stress of 0.24 dyn/cm2. While most previous studies of carbohydrate-coated microspheres and neutrophils rolling on P-selectin did not test wall shear stresses below 0.5 dyn/cm2, Yago et al. did test the entire range of shear stresses from 0.25 to 32.5 dyn/cm2 (both neutrophils and microspheres on P-selectin), and our results are in good qualitative agreement with that work33.
In addition, the small number of rolling cells which rolled slower on the older P-selectin-plain surface at 5 and 10 dyn/cm2 is likely to be from small patches of P-selectin retaining their adhesive activity. These data are consistent with data obtained using microspheres, indicating that our pre-screening tests are reliable to quickly test prepared surfaces in terms of adhesive function.
Here we have demonstrated that covalent immobilization of P-selectin enhances cell rolling interactions through improved long-term stability (Figure 3, 4, and 5) and homogeneity (Figure 5) compared to typical adsorption protocols. Given the difficulty in cost-effectively isolating large quantities of P-selectin, it is important to note that the immobilization conditions presented here utilized the same amounts of P-selectin that were used for the adsorbed controls, thus indicating the practical utility of these methods. The improved stability is a requirement for developing implantable devices that capture specific target cell types based on cell rolling. Through further optimization of presentation of active P-selectin binding sites, the efficiency of these surfaces may be significantly improved. Additionally, orientation and density control through chemical immobilization should be useful for performing controlled studies required to uncover the mechanisms of physiological and pathological cell rolling.
Conclusions
We have achieved stable immobilization of P-selectin using the epoxy based conjugation chemistry without compromising its biological adhesive function. The immobilized surfaces were demonstrated to be superior to the conventional physisorbed controls and the observed rolling response was dependent on a P-selectin mediated interaction. These results are directly applicable to the design therapeutic or diagnostic devices for capturing specific cells. Covalent immobilization techniques can be extended to enhance the control over the presentation of P-selectin including surface density and patterning. We are presently translating this result to animal studies to capture hematopoetic stem cells and circulating cancer cells from whole blood.
Supplementary Material
Acknowledgments
The authors thank Ms. Minhee Sung for her assistance in performing and analyzing microsphere flowing experiments. This work was supported by StemCapture, Inc. M.R. King serves on the scientific advisory board of StemCapture, a company in which he holds financial interest. The XPS measurements were performed using the Shared Experimental Facilities of Center for Materials Science and Engineering at MIT supported by the MRSEC Program of the National Science Foundation under award number DMR 02-13282.
Refereneces
- 1.Geng JG, Chen M, Chou KC. P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Curr Med Chem. 2004;11(16):2153–2160. doi: 10.2174/0929867043364720. [DOI] [PubMed] [Google Scholar]
- 2.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14(5):581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 3.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Engin. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. Traffic Signals for Lymphocyte Recirculation and Leukocyte Emigration - the Multistep Paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 5.Lee D, Schultz JB, Knauf PA, King MR. Mechanical shedding of L-selectin from the neutrophil surface during rolling on sialyl Lewis x under flow. J Biol Chem. 2007;282(7):4812–20. doi: 10.1074/jbc.M609994200. [DOI] [PubMed] [Google Scholar]
- 6.Kneuer C, Ehrhardt C, Radomski MW, Bakowsky U. Selectins - potential pharmacological targets? Drug Discovery Today. 2006;11(2122):1034–1040. doi: 10.1016/j.drudis.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr Opin Cell Biol. 2001;13(5):569–577. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- 8.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79(1):181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 9.King MR, Hammer DA. Multiparticle adhesive dynamics: Hydrodynamic recruitment of rolling leukocytes. Proc Natl Acad Sci USA. 2001;98(26):14919–14924. doi: 10.1073/pnas.261272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike J, Nagata K, Kudo S, Tsuji T, Irimura T. Density-dependent induction of TNF-alpha release from human monocytes by immobilized P-selectin. FEBS Lett. 2000;477(12):84–88. doi: 10.1016/s0014-5793(00)01765-8. [DOI] [PubMed] [Google Scholar]
- 11.King MR. Scale invariance in selectin-mediated leukocyte rolling. Fractals. 2004;12(2):235–241. [Google Scholar]
- 12.King MR, Sumagin R, Green CE, Simon SI. Rolling dynamics of a neutrophil with redistributed L-selectin. Math Biosc. 2005;194(1):71–79. doi: 10.1016/j.mbs.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia SK, Hammer DA. Influence of receptor and ligand density on the shear threshold effect for carbohydrate-coated particles on L-selectin. Langmuir. 2002;18(15):5881–5885. [Google Scholar]
- 14.Nalayanda DD, Kalukanimuttam M, Schmidtke DW. Micropatterned surfaces for controlling cell adhesion and rolling under flow. Biomed Microdevices. 2007;9(2):207–14. doi: 10.1007/s10544-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 15.Larsen K, Thygesen MB, Guillaumie F, Willats WGT, Jensen KJ. Solid-phase chemical tools for glycobiology. Carbohyd Res. 2006;341(10):1209–1234. doi: 10.1016/j.carres.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 16.Leckband D, Langer R. An Approach for the Stable Immobilization of Proteins. Biotechnol Bioengin. 1991;37(3):227–237. doi: 10.1002/bit.260370305. [DOI] [PubMed] [Google Scholar]
- 17.Kaila N, Thomas BE. Design and synthesis of sialyl Lewis(x) mimics as E- and P-selectin inhibitors. Med Res Rev. 2002;22(6):566–601. doi: 10.1002/med.10018. [DOI] [PubMed] [Google Scholar]
- 18.King MR, Rodgers SD, Hammer DA. Hydrodynamic collisions suppress fluctuations in the rolling velocity of adhesive blood cells. Langmuir. 2001;17(14):4139–4143. [Google Scholar]
- 19.Farokhzad OC, Khademhosseini A, Yon SY, Hermann A, Cheng JJ, Chin C, Kiselyuk A, Teply B, Eng G, Langer R. Microfluidic system for studying nanoparticles and microparticles the interaction of with cells. Anal Chem. 2005;77(17):5453–5459. doi: 10.1021/ac050312q. [DOI] [PubMed] [Google Scholar]
- 20.Goldman AJ, Cox RG, Brenner H. Slow viscous motion of a sphere parallel to a plane wall. II Couette flow. Chem Engin Sci. 1967;22:653–660. [Google Scholar]
- 21.Lawrence MB, Springer TA. Leukocytes Roll on a Selectin at Physiological Flow-Rates - Distinction from and Prerequisite for Adhesion through Integrins. Cell. 1991;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 22.Chang WC, Lee LP, Liepmann D. Biomimetic technique for adhesion-based collection and separation of cells in a microfluidic channel. Lab Chip. 2005;5(1):64–73. doi: 10.1039/b400455h. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg AW, Hammer DA. Cell separation mediated by differential rolling adhesion. Biotechnol Bioengin. 2001;73(2):111–124. doi: 10.1002/bit.1043. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka H, Nagasaki Y, Kataoka K. Self-assembly of poly(ethylene glycol)-based block copolymers for biomedical applications. Curr Opin Colloid Interface Sci. 2001;6(1):3–10. [Google Scholar]
- 25.Janzi M, Odling J, Pan-Hammarstrom Q, Sundberg M, Lundeberg J, Uhlen M, Hammarstrom L, Nilsson P. Serum microarrays for large scale screening of protein levels. Mol Cell Proteom. 2005;4(12):1942–1947. doi: 10.1074/mcp.M500213-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira L, Ramos MA, Dordick JS, Gil MH. Influence of different silica derivatives in the immobilization and stabilization of a Bacillus licheniformis protease (Subtilisin Carlsberg) J Mol Cat B. 2003;21(46):189–199. [Google Scholar]
- 27.Kambhampati D. Protein Microarray Technology. Wiley-VCH; Weinheim: 2004. [Google Scholar]
- 28.Pasche S, Voros J, Griesser HJ, Spencer ND, Textor M. Effects of ionic strength and surface charge on protein adsorption at PEGylated surfaces. J Phys Chem B. 2005;109(37):17545–17552. doi: 10.1021/jp050431+. [DOI] [PubMed] [Google Scholar]
- 29.Park EY, Smith MJ, Stropp ES, Snapp KR, DiVietro JA, Walker WF, Schmidtke DW, Diamond SL, Lawrence MB. Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters. Biophys J. 2002;82(4):1835–47. doi: 10.1016/S0006-3495(02)75534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodgers SD, Camphausen RT, Hammer DA. Sialyl Lewis(x)-mediated, PSGL-1-independent rolling adhesion on P-selectin. Biophys J. 2000;79(2):694–706. doi: 10.1016/S0006-3495(00)76328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodgers SD, Camphausen RT, Hammer DA. Tyrosine sulfation enhances but is not required for PSGL-1 rolling adhesion on P-selectin. Biophys J. 2001;81(4):2001–9. doi: 10.1016/S0006-3495(01)75850-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King MR, Heinrich V, Evans E, Hammer DA. Nano-to-micro scale dynamics of P-selectin detachment from leukocyte interfaces. III. Numerical simulation of tethering under flow. Biophys J. 2005;88(3):1676–83. doi: 10.1529/biophysj.104.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yago T, Leppanen A, Qiu H, Marcus WD, Nollert MU, Zhu C, Cummings RD, McEver RP. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J Cell Biol. 2002;158(4):787–99. doi: 10.1083/jcb.200204041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.