Abstract
The bacterial flagellum assembles in a strict order, with structural subunits delivered to the growing flagellum by a type III export pathway. Early rod-and-hook subunits are exported before completion of the hook, at which point a subunit-specificity switch allows export of late filament subunits. This implies that in bacteria with multiple flagella at different stages of assembly, each export pathway can discriminate and sort unchaperoned early and chaperoned late subunits. To establish whether subunit sorting is distinct from subunit transition from the cytosol to the membrane, in particular docking at the membrane-associated FliI ATPase, the pathway was manipulated in vivo. When ATP hydrolysis by the FliI ATPase was disabled and when the pathway was locked into an early export state, both unchaperoned early and chaperoned late subunits stalled and accumulated at the inner membrane. Furthermore, a chaperone that attenuates late subunit export by stalling when docked at the wild-type ATPase also stalled at the ATPase in an early-locked pathway and inhibited export of early subunits in both native and early-locked pathways. These data indicate that the pathways for early and late subunits converge at the FliI ATPase, independent of ATP hydrolysis, before a distinct, separable sorting step. To ascertain the likely signals for sorting, the export of recombinant subunits was assayed. Late filament subunits unable to bind their chaperones were still sorted accurately, but chaperoned late subunits were directed through an early-locked pathway when fused to early subunit N-terminal export signal regions. Furthermore, while an early subunit signal directed export of a heterologous type III export substrate through both native and early-locked pathways, a late subunit signal only directed export via native pathways. These data suggest that subunits are distinguished not by late chaperones but by N-terminal export signals of the subunits themselves.
Keywords: flagella assembly, type III export, sorting mechanism, export chaperone
Bacterial motility is commonly conferred by cell surface flagella, comprising a long helical filament that is connected by a flexible hook to a central rod in the cell envelope basal body that also houses the flagellar motor.1–3 Flagella substructures are assembled in strict sequence, with formation of the basal body and rod structures preceding polymerisation of the hook and, finally, the filament subunits.1–3 The order of assembly is achieved by sequential expression of the gene hierarchy4,5 and by a subunit-specificity switch in the flagellar type III export pathway. This ensures that, prior to hook completion, only ‘early’ rod-and-hook subunits are exported,6,7 while those forming the later distal substructures of the filament, filament cap and hook–filament junction are not. The outline of the subunit-specificity switching mechanism is evident. When the hook reaches its mature length, a signal is transmitted by the hook length control protein FliK to the integral membrane export component FlhB, triggering a switch in subunit specificity to allow export of late subunits.6–9 Nevertheless, peritrichously flagellated bacteria such as Escherichia coli and Salmonella have multiple flagella at different stages of assembly,10 so an individual export pathway potentially encounters both unchaperoned early and chaperoned late subunits from the cytosol. This implies that early and late subunits are discriminated and sorted by the pathway.
We have previously shown that late filament subunits are piloted by their chaperones to dock at the membrane-associated FliI ATPase.11 Here we manipulate the export pathway to determine whether subunit docking and sorting are separable and sequential events. We also assess the relative influence of subunit export signals and bound export chaperones in discriminating early and late subunits.
Stalling of early and late subunits at the membrane in an early-locked pathway attenuated in ATP hydrolysis
To examine the relationship between the proposed sorting step and subunit transition from the cytosol to the inner membrane, we aimed to generate stalled export intermediates of both early and late subunits. Our previous work had exploited export-defective chaperones to stall late cognate (hook–filament junction) subunits, which they piloted to and docked at the membrane-associated FliI ATPase.11 To similarly interrupt the movement of unchaperoned early subunits, we attenuated FliI ATP hydrolysis, which, as in other export systems,12,13 is envisaged to drive unfolding and export of substrates engaged at the membrane machinery,14 in this case prior to assembly into the growing flagellum. After creating single-amino-acid substitutions in the active site region, one variant was chosen for full study, variant FliIE211A, which is mutated immediately adjacent to the Walker A motif. ATP was still bound by FliIE211A [Km = 0.2 mM, compared to wild type (1 mM); triplicate assays ± 15%] but was poorly hydrolysed [Vmax = 0.22 μmol min− 1mg− 1 compared to wild type (2.30)] in a coupled assay in the presence of phospholipids.15
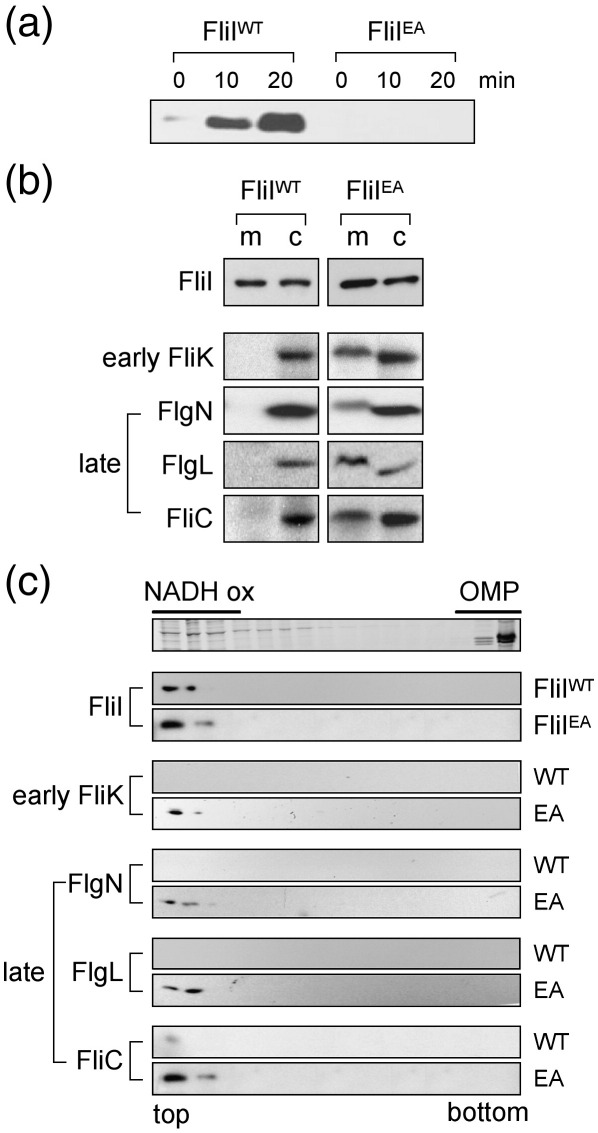
Export supported by FliIE211A was assayed in a fliIflgKflgM triple mutant (by our previously published method11). The resulting pathway is not subject to negative feedback (via the FlgM anti-sigma factor) arising from the disabling of the export apparatus (fliI ATPase), and late and early subunits are thus constitutively synthesized. When wild-type fliI is expressed in trans, exported late subunits such as filament subunit FliC accumulate in the culture supernatant as a result of the flgK hook–junction lesion that precludes filament polymerisation (Fig. 1a). Export of FliC was severely attenuated by substitution of FliI by FliIE211A. Like the wild-type ATPase, FliIE211A assembled into hexamers in vitro in the presence of phospholipids and the short-arm crosslinker disuccinimidylglutamate (Supplementary Data), and cell fractionation and sucrose gradient ultracentrifugation11,16 showed that, in vivo, it localised normally to the inner membrane (Fig. 1b and c).
Fig. 1.
Membrane accumulation of early and late subunits in the pathway attenuated by enzymatically impaired FliI ATPase. (a) FliC export, assayed by immunoblotting, filtered supernatants from midexponential Luria broth (LB) cultures (A600 = 1.0) of ΔfliIflgKflgM cells (made by P22 transduction combined with the method of Datsenko and Wanner27) expressing in trans either wild-type FliI (FliIWT) or variant FliIE211A (FliIEA) from pBAD33 (0.1% arabinose). A ΔfliIflgKflgM strain containing empty pBAD33 was shown to be nonmotile and attenuated in the export of early FliK subunit and late FliC subunit (data not shown). (b) Salmonella fliIflgKflgM cultures expressing wild-type FliIWT or variant FliIEA separated into membrane (m) and cytoplasmic (c) fractions.11,16 Immunoblotted for FliI ATPase, FlgN chaperone and subunits. (c) Separation of the membrane fractions into outer membrane (OMP; Coomassie stained) and inner membrane (NADH oxidase marker) by sucrose gradient ultracentrifugation (0.8–2.0 M11,16 top and bottom of the gradient indicated). Proteins immunoblotted using antisera described above.
The fliIflgKflgM pathway containing the nonhydrolysing FliIE211A is locked into an early export state.6,7,11 In vivo localisation of nonexported subunits in this pathway revealed (Fig. 1b and c) that the unchaperoned early subunit FliK17 accumulated as a membrane-associated intermediate in a FliI-dependent manner. This indicates that, like chaperoned late subunits, unchaperoned early subunits can be stalled at the membrane, putatively docked at the FliI ATPase. If late subunits are sorted before they dock at FliI, then late subunit–chaperone complexes should not accumulate at the membrane in the FliIE211A early-locked fliIflgKflgM pathway, but they should accumulate if sorting occurs after late subunit docking. The in vivo fractionation and sucrose gradients of fliIflgKflgM cells expressing FliIE211A (Fig. 1b and c) revealed that the late subunits FliC and FlgL and the FlgN chaperone18,19 accumulate, like early FliK, at the inner membrane.
The data indicate that FliI enzymatic activity is not required for in vivo docking of late subunits at the membrane ATPase (compatible with in vitro interaction of virulence chaperones with a catalytically inactive type III export ATPase14), and they indicate that this is also true for unchaperoned early subunits. Furthermore, they argue that sorting is separable from docking at FliI, occurring most likely afterwards, and that progression to sorting requires ATP hydrolysis by FliI.
Early and late subunits converge at the ATPase prior to sorting
We have described a late FlgN chaperone variant (now called FlgNrel, as it putatively fails to release from the ATPase) that attenuates export of cognate and noncognate late subunits when expressed in trans in wild-type pathways, with chaperoned subunits trapped after docking at the membrane FliI, accumulating chaperone–subunit–ATPase intermediates.11 We used this dominant-negative chaperone variant to extend indications that chaperoned late subunits engage the wild-type FliI ATPase before sorting, asking whether FlgNrel-stalled membrane intermediates accumulate in an early export-locked pathway and attenuate early subunit export.
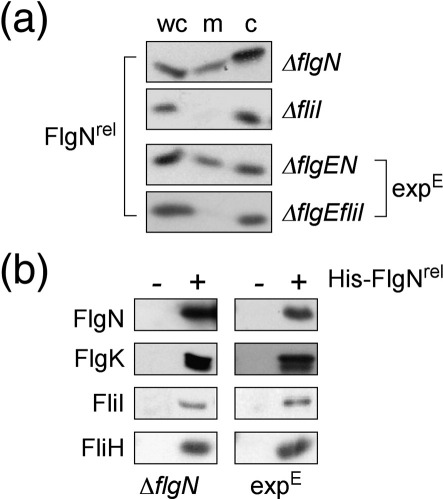
Cell fractionation was performed to establish FlgNrel localisation and putative interaction with the ATPase in the actively secreting early-locked pathway lacking the hook protein FlgE and the wild-type FlgN chaperone (expE; ΔflgEΔflgN), and also in native pathways of ΔflgN control (expE + L) that export early subunits and then late subunits after completion of the hook substructure. The results (Fig. 2a) show that FlgNrel localised to the membrane in a FliI-dependent manner in both pathways. When His-FlgNrel was used as bait in in vivo affinity chromatography (Fig. 2b), cognate subunit FlgK, FliI and its regulator FliH were all copurified, showing that ATPase–chaperone–subunit intermediate complexes were formed in early-locked pathways analogous to native pathways.
Fig. 2.
Membrane accumulation of early and late subunits in an early-locked pathway attenuated by stalling docked chaperone FlgNrel. (a) Localisation of FlgNrel (expressed in trans by 0.01% arabinose) in the whole cell (wc), membrane (m) and cytoplasm (c)11,15 of the ΔflgN and ΔfliI pathways, and in the isogenic (expE) early-locked pathways ΔflgEN and ΔflgEfliI. (b) Affinity copurification of stalled docking complexes by His–FlgNrel bait (+)11 from native ΔflgN and early-locked (expE; ΔflgEN) pathways [(−) vector-only controls]. Cell extracts were incubated with Ni–NTA resin [20 mM tris(hydroxymethyl)aminomethane–HCl pH 8.0, 300 mM NaCl and 5 mM imidazole] before washing (10 mM imidazole) and elution in SDS sample buffer. FlgN chaperone, FlgK cognate subunit and ATPase complex components FliI and FliH were detected by immunoblotting.
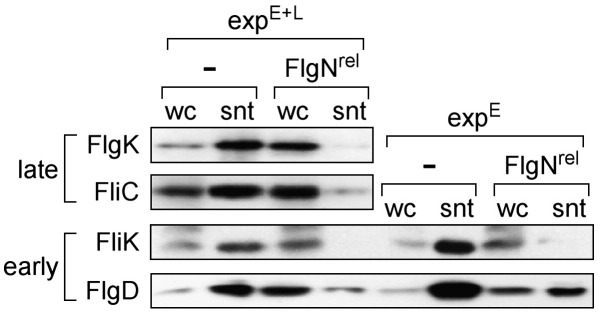
These findings support the earlier indication that both unchaperoned early and chaperoned late subunits engage the export ATPase before they are sorted for export or exclusion, and furthermore suggest that the export pathways for the two classes of subunit converge at the ATPase. To substantiate the idea of convergence, we again used the docked but stalled late FlgNrel to assess whether it could attenuate export of not only chaperoned late subunits but also early subunits in native export pathways of ΔflgN. The results (Fig. 3) confirm that export of cognate (FlgK) and noncognate (FliC) chaperoned late subunits is reduced by 5- to 10-fold and reveal a comparable attenuation in the export of unchaperoned early subunits FlgD and FliK. Significantly, they show that FlgNrel caused a comparable reduction of FlgD and FliK export in an early export-locked (ΔflgEΔflgN; expE) pathway (Fig. 3). These FlgNrel experiments strengthen the indication from those using FliIE211A, that is, that the pathways for unchaperoned early and chaperoned late subunits converge at the ATPase before progressing (dependent on ATP hydrolysis) to sorting.
Fig. 3.
Attenuation of early and late subunits export by stalling FlgNrel. Export of subunits by native ΔfliD (expE + L) and early-locked ΔflgE (expE) pathways containing FlgNrel [expressed using 0.01% arabinose; (−) vector-only controls], assayed following precipitation from supernatants (snt) of midexponential LB cultures (wc, whole culture) by SDS-PAGE and immunoblotting for early (FliK and FlgD) and late (FliC and FlgK) subunits.
Are subunits discriminated by their own signals or by late chaperones?
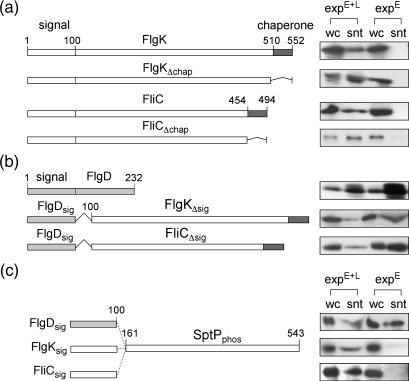
An obvious difference between early and late subunits is that only late subunits are bound by export chaperones, acting as cytosolic bodyguards and pilots for docking at the membrane export ATPase.11,20,21 Chaperones could act as flagellar sorting signals, labelling subunits for rejection during the early stages of flagella assembly. This is especially so as in a virulence type III secretion system, bound chaperones (e.g., Salmonella InvB chaperone of the SopE effector), are reported to form part of the secretion signal, preventing promiscuous export through the flagellar pathway.20,21 If this were true, late subunits from which C-terminal polymerisation and chaperone-binding domains are deleted might be exported as early subunits. To test this possibility, we assessed the export of recombinant FliC and FlgK late subunit variants lacking their chaperone-binding domains (FlgKΔchap, and FliCΔchap)6,18,22 and found (Fig. 4a) that, although these variants were exported in native pathways that export both early and late subunits (expE + L), neither variant was exported in an early-locked pathway (expE; i.e., the unchaperoned subunits were still faithfully sorted by the export pathway). We then assessed the export of recombinant subunits in which the N-terminal export signal residues 1–100 (FlgDsig) were fused to truncated late FliC (FliCΔsig) or FlgK (FlgKΔsig) lacking their N-terminal export signals23 but still able to bind their respective chaperones. Like wild-type FlgD, these hybrid subunits were exported by both early export-locked (expE) and native export (expE + L) pathways (Fig. 4b), substantiating the view that chaperones are not a sorting signal to preclude late subunit export before hook completion. The marginal (twofold) reduction in the export of early subunits (FlgD, FlgDsig–FliCΔsig, FlgDsig–FlgKΔsig) in native pathways (Fig. 4b; expE + L), compared to the early export-locked strain (expE), possibly indicates that once an export apparatus has switched specificity, it no longer accepts early subunits for export (a view compatible with observations in Yersinia T3SS indicating that once pathways have switched specificity to late effectors, they do not export early substrates24).
Fig. 4.
Influence of subunit domains on sorting. (a) Export of chaperoned late subunits (FlgK and FliC) and their variants that cannot bind chaperone (FlgKΔchap and FliCΔchap) in export pathways that are either early locked (expE; ΔflgEKL27 ΔflgE, SJW1353 acquired from Ohnishi et al.28) or native (expE + L; ΔflgKL or ΔfliC). Proteins from whole cells (wc) and supernatants (snt) were immunoblotted with FlgK or FliC antisera. (b) Export of FlgD and recombinant late subunits FlgKΔsig and FliCΔsig lacking amino acids 1–100 fused to residues 1–100 of early FlgD in export pathways (described above) that are either early locked (expE) or native (expE + L). Proteins from whole cells (wc) and supernatants (snt) were immunoblotted with FlgD, FlgK or FliC antisera. (c) Export of recombinant fusion proteins comprising putative early or late subunit N-terminal signal regions (FlgDsig, FlgKsig and FliCsig; amino acids 1–100) fused to the signal-less SptP tyrosine phosphatase domain (SptPphos; residues 161–543) in early locked (expE; ΔflgE) and native (expE + L; ΔfliC) pathways. Proteins were immunoblotted with SptP antisera (V. Koronakis, University of Cambridge). Genes encoding variant wild-type and variant FliC, FlgK, FlgD and SptP were amplified by overlap extension PCR using Salmonella chromosomal DNA as template. PCR products were inserted into XbaI–HindIII restriction sites of the pBAD18 expression vector. Recombinant genes were expressed (LB, 0.01% arabinose) and proteins were assayed as in Fig. 2b. Control experiments performed in isogenic ΔfliI and ΔflgEfliI strains (created by P22 transduction of ΔfliI allele into ΔflgE) showed that none of the recombinant proteins was exported (data not shown).
These assays also suggest that the subunit N-terminal 100 residues contain both export and sorting signals. To confirm this, we constructed recombinant subunits comprising the N-terminal 100 residues of the late subunit FliC (FliCsig) or FlgK (FlgKsig), or the early subunit FlgD (FlgDsig) fused to the catalytic phosphatase domain of the Salmonella SPI-1 SptP effector (residues 161–543; SptPphos)14 and assayed export in early export-locked and wild-type equivalent pathways. As expected, FlgDsig–SptPphos fusion was exported in both early-locked (expE) and wild-type (expL + E) pathways (Fig. 4c). In contrast, the FliCsig–SptPphos and FlgKsig–SptPphos fusion proteins were exported only in the wild-type pathway (expE + L) and not by the early export-locked pathway (expE) (Fig. 4c). This supports our view that the N-terminal regions contain sufficient information to determine sorting as a late subunit. While there is primary sequence similarity among the N-terminal regions of rod subunits, there seems to be little identity between these and other early subunits25,26 and no obvious identity distinguishing the N-terminal regions of late proteins. This suggests that subunit sorting might rely on the recognition of structural features specific to each subunit class.
Acknowledgements
We thank Vasillis Koronakis for SptP antisera. This work was supported by a Wellcome Trust Programme grant (C.H.) and a Biotechnology and Biological Sciences Research Council studentship (P.D.).
Edited by I. B. Holland
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2007.09.080
Appendix A. Supplementary Data
Supplementary Fig. 1.
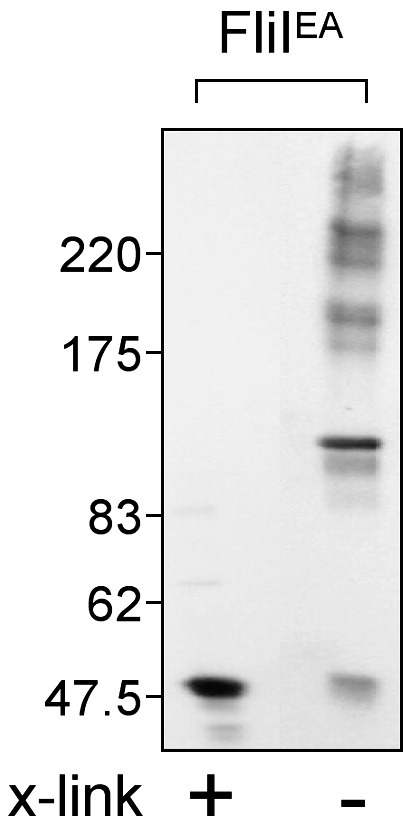
In vitro oligomerisation of FliIE211A ATPase (FliIEA). Purified variant FliI ATPase (0.5 μM) was incubated in cross-linking buffer (20 mM Hepes pH 8.0, 0.1 M NaCl, 0.1 mM ethylenediaminetetraacetic acid and 1 mM DTT) in the presence of E. coli liposomes, with (+) or without (−) 0.1 mM disuccinimidylglutamate). Aliquots were precipitated (10% trichloroacetic acid), subjected to electrophoresis through an SDS 4–10% acrylamide gradient gel and Coomassie stained.
References
- 1.Macnab R.M. Flagella and motility. In: Neidhart F.C., editor. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. American Society for Microbiology; Washington, DC: 1996. pp. 123–145. [Google Scholar]
- 2.Macnab R.M. How bacteria assemble flagella. Annu. Rev. Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 3.Namba K., Vonderviszt F. Molecular architecture of bacterial flagellum. Q. Rev. Biophys. 1997;30:1–65. doi: 10.1017/s0033583596003319. [DOI] [PubMed] [Google Scholar]
- 4.Kutsukake K., Ohya Y., Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soutourina O.A., Bertin P.N. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 2003;27:505–523. doi: 10.1016/S0168-6445(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 6.Fraser G.M., Hirano T., Ferris H.U., Devgan L.L., Kihara M., Macnab R.M. Substrate specificity of type III flagellar protein export in Salmonella is controlled by subdomain interactions in FlhB. Mol. Microbiol. 2003;48:1043–1057. doi: 10.1046/j.1365-2958.2003.03487.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T., Minamino T., Namba K., Macnab R.M. Substrate specificity classes and the recognition signal for Salmonella type III flagellar export. J. Bacteriol. 2003;185:2485–2492. doi: 10.1128/JB.185.8.2485-2492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano T., Yamaguchi S., Oosawa K., Aizawa S. Roles of FliK and FlhB in determination of flagellar hook length in Salmonella typhimurium. J. Bacteriol. 1994;176:5439–5449. doi: 10.1128/jb.176.17.5439-5449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano T., Shibata S., Ohnishi K., Tani T., Aizawa S. N-terminal signal region of FliK is dispensable for length control of the flagellar hook. Mol. Microbiol. 2005;56:346–360. doi: 10.1111/j.1365-2958.2005.04615.x. [DOI] [PubMed] [Google Scholar]
- 10.Aizawa S.I., Kubori T. Bacterial flagellation and cell division. Genes Cells. 1998;3:625–634. doi: 10.1046/j.1365-2443.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas J., Stafford G.P., Hughes C. Docking of cytosolic chaperone–substrate complexes at the membrane ATPase during flagellar type III protein export. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3945–3950. doi: 10.1073/pnas.0307223101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiebel E., Driessen A.J., Hartl F.U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- 13.Thanabalu T., Koronakis E., Hughes C., Koronakis V. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 1998;17:6487–6496. doi: 10.1093/emboj/17.22.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akeda Y., Galan J.E. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- 15.Evans L.D., Stafford G.P., Ahmed S., Fraser G.M., Hughes C. An escort mechanism for cycling of export chaperones during flagellum assembly. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17474–17479. doi: 10.1073/pnas.0605197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auvray F., Ozin A.J., Claret L., Hughes C. Intrinsic membrane targeting of the flagellar export ATPase FliI: interaction with acidic phospholipids and FliH. J. Mol. Biol. 2002;318:941–950. doi: 10.1016/S0022-2836(02)00172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamino T., Gonzalez-Pedrajo B., Yamaguchi K., Aizawa S.I., Macnab R.M. FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol. Microbiol. 1999;34:295–304. doi: 10.1046/j.1365-2958.1999.01597.x. [DOI] [PubMed] [Google Scholar]
- 18.Bennett J.C., Thomas J., Fraser G.M., Hughes C. Substrate complexes and domain organization of the Salmonella flagellar export chaperones FlgN and FliT. Mol. Microbiol. 2001;39:781–791. doi: 10.1046/j.1365-2958.2001.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser G.M., Bennett J.C., Hughes C. Substrate-specific binding of hook-associated proteins by FlgN and FliT, putative chaperones for flagellum assembly. Mol. Microbiol. 1999;32:569–580. doi: 10.1046/j.1365-2958.1999.01372.x. [DOI] [PubMed] [Google Scholar]
- 20.Ehrbar K., Winnen B., Hardt W.D. The chaperone binding domain of SopE inhibits transport via flagellar and SPI-1 TTSS in the absence of InvB. Mol. Microbiol. 2006;59:248–264. doi: 10.1111/j.1365-2958.2005.04931.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.H., Galan J.E. Salmonella type III secretion-associated chaperones confer secretion-pathway specificity. Mol. Microbiol. 2004;51:483–495. doi: 10.1046/j.1365-2958.2003.03840.x. [DOI] [PubMed] [Google Scholar]
- 22.Auvray F., Thomas J., Fraser G.M., Hughes C. Flagellin polymerisation control by a cytosolic export chaperone. J. Mol. Biol. 2001;308:221–229. doi: 10.1006/jmbi.2001.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber-Sparenberg C., Poplau P., Brookman H., Rochon M., Mockel C., Nietschke M., Jung H. Characterization of the type III export signal of the flagellar hook scaffolding protein FlgD of Escherichia coli. Arch. Microbiol. 2006;186:307–316. doi: 10.1007/s00203-006-0146-0. [DOI] [PubMed] [Google Scholar]
- 24.Sorg J.A., Blaylock B., Schneewind O. Secretion signal recognition by YscN, the Yersinia type III secretion ATPase. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16490–16495. doi: 10.1073/pnas.0605974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homma M., Kutsukake K., Hasebe M., Iino T., Macnab R.M. FlgB, FlgC, FlgF and FlgG. A family of structurally related proteins in the flagellar basal body of Salmonella typhimurium. J. Mol. Biol. 1990;211:465–477. doi: 10.1016/0022-2836(90)90365-S. [DOI] [PubMed] [Google Scholar]
- 26.Homma M., DeRosier D.J., Macnab R.M. Flagellar hook and hook-associated proteins of Salmonella typhimurium and their relationship to other axial components of the flagellum. J. Mol. Biol. 1990;213:819–832. doi: 10.1016/S0022-2836(05)80266-9. [DOI] [PubMed] [Google Scholar]
- 27.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichiacoli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi K., Ohto Y., Aizawa S., Macnab R.M., Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J. Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]