Abstract
Defects in anti-tumor immune responses have been associated with increased release of prostaglandin E2 (PGE2) as a result of over-expression of cyclooxygenase (COX-) 2 by tumors. In this report, we examine the effects of PGE2 upon anti-tumor CD8+ T cell responses generated both by cross-presenting dendritic cells (DC) and by direct priming by tumor cells. Our data show that PGE2 inhibits DC maturation, resulting in the abortive activation of naive CD8+ T cells, and is dependent on IL-10 production by DC. Interaction of tumor cells with naïve CD8+ T cells in the presence of PGE2 in vitro results in the induction of CD8+ CD28- T cells, which fail to proliferate or exhibit effector function. In vivo over-expression of COX-2 by tumor cells results in a decrease in number of tumor-infiltrating DC and confers the ability of tumor cells to metastasize to the TDLN.
Keywords: CD8+ T cell responses, tumor cells, COX-2, PGE2, antigen presentation, cross-presentation, dendritic cells, peripheral tolerance
Introduction
Activation of naïve tumor-specific CD8+ T cells occurs either through direct interaction with metastatic tumors in the tumor draining lymph nodes (TDLN), or indirectly via dendritic cells (DC), cross-presenting tumor-derived antigens to naïve CD8+ T cells within the TDLN. Deficiencies in DC recruitment and APC function have been observed in many human cancers, resulting in reduced T cell proliferation and effector function(1), (2). In some cases these defects in DC function were associated with the release of tumor-induced factors, including IL-10 and prostaglandin E2 (PGE2)(3), (4), (5). IL-10 production can be up-regulated by PGE2(6), (7) and has been shown to have anti-inflammatory activity, dampening CD4+ and CD8+ T cell responses by inhibiting maturation(8) and cytokine release(9) by DC resulting in T cell anergy(2), (10). Over-expression of PGE2 has been demonstrated in many cancers(5), (11), causing reduced number of tumor-infiltrating DC, and a reduction in their APC function(12), (13). PGE2 has been shown to shift the profile from anti-tumor Th1 responses to Th2 responses, characterized by reduced IL-12 and increased IL-10 secretion by DC(14). Furthermore, PGE2 increases tumor cell survival(15), motility(16) and angiogenesis(17); thus allowing them to metastasize to the TDLN. Although metastasis provides a means of direct CD8+ T cell activation(18), migration of COX-2 over-expressing tumors to the TDLN fails to induce anti-tumor T cell responses(19).
In order to investigate the effects of PGE2 upon anti-tumor CD8+ T cell responses, we have adapted a murine Renal Cell Carcinoma (Renca) model in which the hemagglutinin antigen (HA) of influenza virus A/PR8 H1N1 is expressed as a neo-antigen(20)). RencaHA cells were transfected with murine COX-2 cDNA (T3), resulting in the over-expression of COX-2 and PGE2. CD8+ T cell responses to these tumor cell lines were investigated using transgenic CL4 CD8+ T cells expressing TCRs with high affinity for the immunodominant KdHA-restricted epitope(21). Our data clearly show that PGE2 abrogates anti-tumor specific CTL responses in vivo and in vitro by down-regulating both direct antigen presentation and cross-presentation pathways.
Materials and Methods
Mice
6-8 week old BALB/c and BALB/c CL4 TCR transgenic mice(21) were bred and maintained under specific pathogen-free conditions at the University of Bristol Animal Services Unit. All experiments were conducted in accordance with U.K. Home Office guidelines.
Cell lines
The RencaNT cell line was single cell-cloned from a population of Renca cells(22). The RencaHA(20) COX-2 (T3) cell line was established by transfecting the RencaHA cell line. Briefly, a full length COX-2 cDNA PCR product containing flanking NotI restriction sites was generated using the following primers: 5′-ATAAGAATGCGGCCGCATGCTCTTCCGAGCTGTGC-3′ and 5′-ATAGTTTAGCGGCCGCTCATTACAGCTCAGTTGAACGCC (Source plasmid was kindly provided by Prof. R. Dubois, Vanderbilt University Medical Center and Vanderbilt-Ingram Cancer Center; Nashville, TN, USA), and cloned into the NotI restriction site of the expression vector pIRESpuro2 (Clontech, Mountain View, USA). RencaHA cells were plated in 6 well plates prior transfection. When 70-90% confluent, cells were incubated with 10μg of Midiprep DNA (Qiagen Hilden, Germany) mixed with lipofectamine™ 2000 reagent (Invitrogen Paisley, UK), according to the manufacturer’s instructions. Cells were then incubated in the absence of antibiotics penicillin/streptomycin (Invitrogen) and FCS (Invitrogen) for 72 hrs, after which cells were washed with HBSS (Invitrogen), and grown in media supplemented with 1μg/ml puromycin (Sigma-Aldrich, Dorset, UK) and 0.1mg/ml geneticin (Invitrogen). Resulting colonies were expanded and assayed for integrated COX-2 cDNA by PCR and COX-2 mRNA expression by RT-PCR and for PGE2 production by ELISA. RencaNT cells were maintained in complete medium (RPMI 1640 (Sigma-Aldrich) supplemented with 10% (vol/vol) FCS, 2mM glutamine, 50U/ml penicillin/streptomycin and 5×10-5 M 2-ME, all obtained from Invitrogen). RencaHA cells were grown in complete media supplemented with 0.1mg/ml geneticin (Invitrogen), and T3 were maintained in complete media containing 0.1mg/ml geneticin and 1μg/ml of puromycin (Sigma-Aldrich).
Bone marrow-derived dendritic cells (BMDC)
BMDC were generated from hematopoetic progenitor cells(23). Briefly bone marrow cells were harvested aseptically from the leg bones of BALB/c mice by flushing out the lumen of the bones with complete RPMI using a 25 gauge needle (Tyco healthcare, Basingstoke, U.K.), in plastic petri dishes. Cells were filtered through a 40 μm cell strainer. Macrophages were depleted after 3 hrs incubation of bone marrow cells in GM-CSF medium (Complete RPMI containing 10% (vol/vol) GM-CSF obtained from supernatant of GM-CSF-secreting X63 hybridoma cell line; a gift from Dr. F. Ronchese, Malaghan Institute of Medical Research, Wellington University School of Medicine, Wellington, New Zealand) in T75 flask at 37° C in a humidified incubator with 5% (vol/vol) CO2. After 3 hrs incubation supernatant was centrifuged at 1800rpm for 5 mins and cells were resuspended at 2×106 cells/ml. 2×106 cells/well were cultured in 6 well plates in GM-CSF media, for 10 days at 37°C in a humidified incubator. Media was changed on days 3, 6 and 7, by replacing 2 ml of medium with fresh GM-CSF media. Matured DC (mDC) were generated by addition of LPS (1μg/ml) (E. coli 026:B6, Sigma-Aldrich) to the iDC culture for 18 hrs. Where mentioned on day 7 or 9 immature DC were incubated with 1μM PGE2 (Sigma-Aldrich) followed by 1μg/ml LPS (Sigma-Aldrich) on day 9. On day 10, non-adherent cells were harvested from the wells, washed thoroughly, irradiated with 3000 rad and used as APC, as described below. Where mentioned, DC were treated with 15 μg/ml of purified anti-IL-10R mAb (1B1.3 clone(24)) or normal rat IgG isotype (Invitrogen) on day 9.
ELISA
The concentration of IL-10 in the supernatant of BMDC was measured using standard ELISA protocols. Plates were coated with purified anti-IL-10 mAb (Biosource Oxford Biosystems, UK), blocked with 1% BSA in PBS, and incubated with supernatants for 2 hrs, followed by biotinylated anti-IL-10 mAb (Biosource). Bound biotinylated mAb was detected by incubation with ExtrAvidin-HRP (Sigma-Aldrich) followed by incubation with tetramethylbenzidine substrate (Sigma-Aldrich). The reaction was stopped with 2M H2SO4. Absorbance was read at 450 nm with a 595 nm reference. Supernatant from RencaHA and T3 tumor cell lines were analyzed for PGE2 by ELISA, using the PGE2 EIA kit (Assay Designs Cambridge, UK) according to the manufacturer’s instructions. Briefly, tumor cells left untreated or treated with 5μg/ml NS-398 (Sigma-Aldrich; dissolved in 10% (vol/vol) DMSO) for 72hrs, were pretreated with 44 μM Arachidonic acid (Sigma-Aldrich) for 45 mins before the supernatant was collected and frozen at -80°C. The number of cells for each tumor was counted and the concentration of PGE2 obtained from ELISA was normalized to 1×106 cells.
Enrichment of CL4 CD8+ T cells
Single cell suspensions were generated from CL4 TCR transgenic mice by positive selection using anti-CD8 MACS beads on midiMACS (Miltenyi Biotec Bisely, UK) and LS separation columns, according to manufacturer’s instructions. This routinely gave a purity of CD8+ T cells >95% as determined by flow cytometry. In some experiments, CL4 CD8+ T cells were labeled with 5μM CFSE (Molecular Probes Inc., Eugene, Oregon) in accordance with described protocols(23).
In vivo proliferation assays
BALB/c mice were injected subcutaneously (s.c.) with 1×106 RencaHA or T3 on day 0. On day 11 mice were injected intravenous (i.v.) with 3x106 purified, naïve CFSE-labeled CL4 CD8+ T cells. For COX-2 inhibition, mice were injected intraperitoneally (i.p.) with 10 mg/kg NS-398 (Sigma-Aldrich) dissolved in 10% (vol/vol) DMSO. The drug was administered before tumor injection and then 3 times per week for the duration of the experiment. Tumor growth was determined on days 16 and 21(20). Tumors were measured with calipers and the growth evaluated applying the following formula (a2 × b/2), where a = horizontal diameter and b = vertical diameter of the tumor. For assessment of CL4 accumulation in the TDLN and tumor, mice were sacrificed on days 16 and 21, respectively.
Co-culture and CL4 CD8+ T cell proliferation in vitro
CL4 CD8+ T cell proliferation assays were performed as described previously(23). Briefly, 1×104 naïve CL4 CD8+ T cells were co-cultured with 1×104 irradiated BMDC in 96 well plates in the presence of 1 ng/ml KdHA peptide (IYSTVASSL), at 37°C, 5% (vol/vol) CO2 for 72 hrs. For the final 8 hrs, cells were pulsed with 1 μCi/well 3H-thymidine (Amersham Life Sciences Buckinghamshire, UK). 3H-thymidine incorporation was measured using a 1450 Microbeta liquid scintillation counter with Microbeta for Windows 2.7 (Wallac-Oy). For co-culture assays, 1×106 naïve CFSE-labeled CL4 CD8+ T cells were cultured with 1×105 irradiated BMDC, splenocytes, or tumors in 24 well plates. For experiments using BMDC and splenocytes, 1 ng/ml KdHA (IYSTVASSL) peptide was added to the culture at the time of incubation with CL4 CD8+ T cells. After 48-72 hrs at 37°C, 5% (vol/vol) CO2, CL4 CD8+ T cells were examined by flow cytometry for intracellular IFN-γ or for the expression of cell-surface molecules. For IL-10R blocking experiments, CL4 CD8+ T cells were co-cultured with DC in the presence of 15 μg/ml anti-IL-10R mAb(24) or rat IgG1 isotype (Invitrogen) for 72 hrs.
Flow cytometry
Single cell suspensions derived from BMDC were stained with anti-CD11c.PE mAb in the presence of supernatant from anti-FcγIII mAb-secreting 2.4G2 cell line. CD11c+ cells were analyzed for cell surface molecules using anti-H2Kd.bio, CD80.bio, CD86.bio, CD40.bio mAbs or isotype controls followed by SA.APC. CL4 CD8+ T cells were stained by anti-Thy1.1.PE, CD69.bio, CD28.bio and Ly6C.bio followed by SA.APC. All these mAbs were purchased from BD Oxford, UK. Dead cells were stained with 7-Amino-actinomycin D (7AAD; Sigma-Aldrich) and gated out in the analyses. Intracellular IFN-γ and CTLA-4 expression in CL4 CD8+ T cells were detected using the BD Perm/fix kit with Golgi plug (BD) according to the manufacturer’s instructions and stained with anti-IFN-γ.APC mAb or anti-CTLA-4.PE (BD). The expression of all surface and intracellular molecules except of CTLA-4 was measured after 72 hrs culture. Expression of the intracellular CTLA-4 in CL4 CD8+ T cells was measured after 48 hrs culture. RencaHA, T3 and RencaNT cell lines were stained for HA, using 37/38 mAb followed by goat anti-mouse IgG-FITC (Sigma-Aldrich) secondary Ab, MHC Class I expression in RencaHA and T3 cell lines was detected by anti-H2Kd.bio mAb followed by SA.APC. Cells were acquired on a FACSCalibur flow cytometer with CellQuest software (BD Cytometry Systems Oxford, UK).
PCR and RT-PCR
For HA RT-PCR, TDLN from mice with T3 or RencaHA were isolated and total RNA was extracted from single cell suspension using TRIzol reagent (Invitrogen) following manufacturer’s instructions(20). cDNA was synthesized using a cDNA synthesis kit (Invitrogen). For the HA PCR the following primers were used: 5′-CAATTGGGGAAATGTAACATCGCCG-3′, 5′-AGCTTTGGGTATGAGCCCTCCTTC-3′; Cycling conditions were 94°C/5 mins, 28 cycles for 94°C/30 s, 61°C/30 s (HA). The stable integration of the COX-2 cDNA into the T3 genome was examined by PCR. COX-2 RT-PCR was performed on single cell suspensions of RencaHA and T3 cell lines from in vitro cultures using the RETROscipt® reverse transcription kit (Ambion Austin Texas), according to the manufacturer’s instructions. The COX-2 primer sequences used were: 5′-ATGCTCTTCCGAGCTGTGC-3′ and 5′-TTACAGCTCAGTTGAACGCC-3′. The HPRT primers used were: 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ and 5′-GAAGGGTAGGCTGGCCTATAGGCT-3′. For COX-2, the PCR cycle conditions were 94°C/2 mins and 30 sec, 94°C/30 sec, 57°C/30 sec, 72°C/30 sec for 30 cycles and finally 72°C/7 mins. For HPRT, the PCR cycle conditions were 94°C/2 mins and 30 sec, 94°C/30 sec, 60°C/30 sec, 72°C/30 sec for 28 cycles and finally 72°C/7 mins.
Statistical analysis
P values were calculated with the student t-test using the Prism 4.03 software (GraphPad Software, Inc La Jolla, CA), with two tailed distribution and two-sample equal variance parameter.
Results
PGE2 inhibits DC maturation in vitro
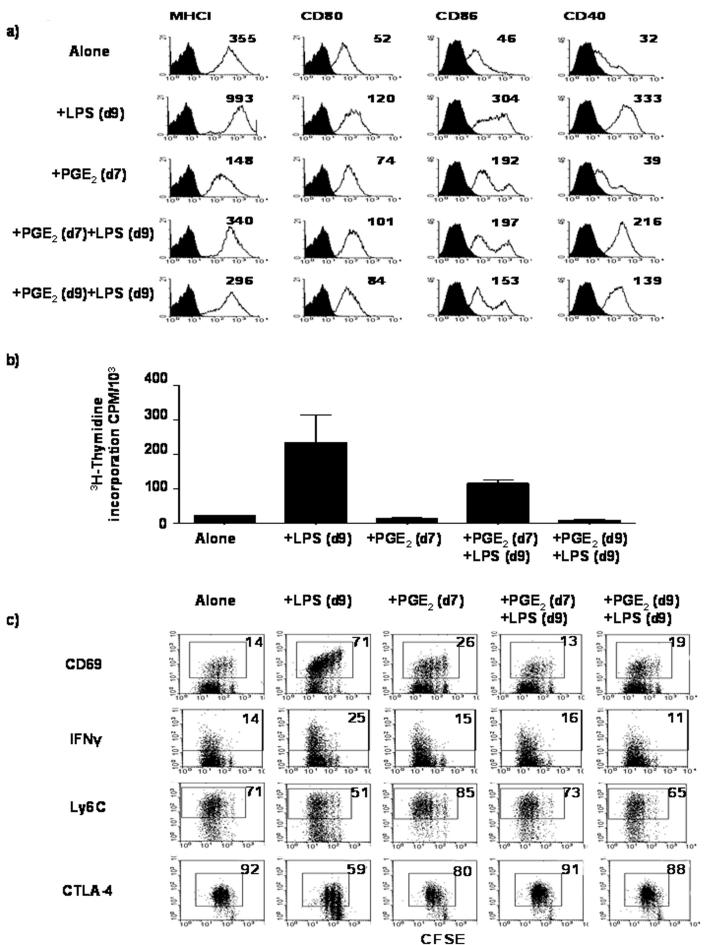
Previous studies in vitro have shown that PGE2 exerts different effects on the cytokine profile of DC depending upon their stage of differentiation and maturation(25). We wished to determine whether or not DC maturation was altered if PGE2 was present during differentiation. Immature dendritic cells (iDC) were generated in vitro from BM stem cells in the presence or absence of exogenous PGE2; either before or during LPS maturation (Figure 1a). Treatment of iDC cultures with LPS on day 9 of differentiation resulted in dendritic cell maturation to form matured dendritic cells (mDC); as evidenced by increased expression of MHC class I, CD80, CD86 and CD40, compared with iDC. However, when iDC were differentiated in the presence of PGE2, expression of MHC class I was reduced as compared to untreated iDC, yet up-regulation of CD80 and CD86 was observed. Importantly, treatment of iDC with PGE2 at the same time as LPS prevented increased expression of MHC class I, CD80, CD86 and CD40 compared with untreated LPS-matured DC. Similarly, the presence of PGE2 during differentiation into iDC reduced their ability to mature. However, CD40 and CD86 expression was higher amongst DC differentiated in the presence of PGE2, and matured with LPS, compared with DC matured in the presence of PGE2. Taken together, these data suggest that PGE2 renders iDC refractory to maturation signals when present either during differentiation, or during maturation, with the latter being more susceptible to the effects of PGE2.
Figure 1. PGE2-treated DC induce abortive activation of CL4 cells in vitro.
a) iDC were either left untreated (Alone), treated with 1μg/ml LPS for 18hrs on day 9 (+LPS(d9)), treated with PGE2 (10-6 M) alone on day 7 (+PGE2(d7)), or treated with PGE2 (10-6 M) on day 7 followed by LPS on day 9 (+PGE2(d7)+LPS(d9)). Cells were alternatively treated with a mixture of PGE2-LPS on day 9 (+PGE2(d9)+LPS(d9)). Cells harvested on day 10 were analyzed by flow cytometry, for cell surface receptor expression as indicated in the figure (open histograms). Filled histograms represent isotype controls. The data are from one of eight performed experiments. Numbers in each histogram represents the mean fluorescence intensity (MFI). b) CL4 proliferation as measured by 3H-thymidine incorporation after co-culture with iDC treated as described in a. The data are mean ± SD, from one of three performed experiments, each with triplicates. c) Phenotypic analyses of Thy1.1+ CFSE labeled CL4 cells after co-culture with iDC treated as described in a). Numbers indicate the percentage of divided CL4 cells expressing the receptors as indicated in the figure. The data are from one of two performed experiments.
PGE2 present during DC maturation abrogates CL4 T cell activation in vitro
To evaluate the consequences following priming of naïve CD8+ T cells by PGE2-conditioned DC, purified naïve CL4 cells were co-cultured with PGE2-treated, KdHA peptide-pulsed DC, and their ability to proliferate and exhibit CTL effector function was analyzed in vitro (Figure 1b & c). Co-culturing naïve CL4 cells with mDC resulted in a 10-fold increase in proliferation, as compared with co-culturing in the presence of iDC. PGE2 treatment of iDC did not affect proliferation of cells compared with untreated iDC. However, co-culturing CL4 cells with LPS-matured DC treated with PGE2 during their differentiation reduced CL4 proliferation compared with CL4 cells co-cultured with LPS-matured DC. Critically, treatment of iDC with PGE2 at the same time as LPS abrogated CL4 cell proliferation. These data suggest that conditioning of DC with PGE2, suppresses their APC function, which is greatest during DC maturation.
Following naïve CL4 cell co-culture with iDC, few CL4 cells increased expression of CD69 and elaborated IFN-γ upon re-stimulation with KdHA peptide. Furthermore, greater than 70% and 90% of CL4 cells were Ly6Chi and CTLA-4hi respectively (Figure 1c). Such a phenotype is consistent with the cell-surface phenotype of CL4 CD8+ T cells undergoing abortive activation and peripheral tolerance induction(23), (26), (27). When naïve CL4 cells were co-cultured with LPS-matured DC, greater than 70% of CL4 cells expressed CD69, the number of IFN-γ+ cells increased, and only around half of the cells were Ly6Chi and CTLA-4hi; suggesting that these cells were undergoing productive activation to form effector CTL(23). Following PGE2 treatment, iDC were still able to induced abortive activation of CL4 cells, however PGE2 treatment prevented productive activation by mDC. The shift in response in favor of abortive activation occurred regardless of whether DC were treated with PGE2 during differentiation or maturation.
Blocking IL-10R in vitro reverses the inhibitory effect of PGE2 on DC function
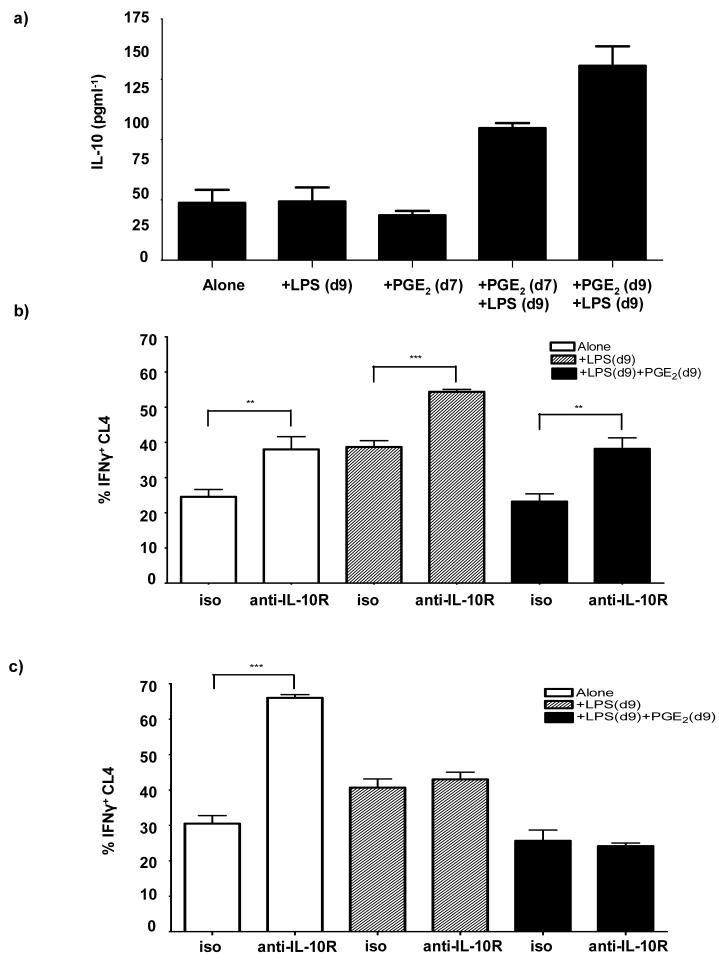
Thus far, the data suggest that PGE2 exerts immunosuppressive effects on DC function. PGE2 is known to cause increased production of IL-10 by DC (28), which has been shown to reduce DC maturation(8), (29), and dampen productive activation of CD8+ T cell by DC(30). Therefore, we wished to evaluate the levels of IL-10 production by DC during differentiation and LPS-maturation in the presence or absence of PGE2 by ELISA (Figure 2a). IL-10 production was approximately equal amongst both iDC and LPS-matured DC. Treatment of iDC with PGE2 alone had no effect on IL-10 production compared with either untreated iDC or with mDC (Figure 2a). However, when PGE2 was present during DC differentiation followed by LPS maturation, IL-10 production increased by 2 fold compared to LPS-matured DC. Moreover, when PGE2 was present during LPS maturation on day 9, IL-10 production increased by greater than 3-fold compared to DC matured without PGE2.
Figure 2. Blocking IL-10 in vitro reverses the inhibitory effects of PGE2 on DC function.
a) iDC were either left untreated (Alone), treated with 1μg/ml LPS for 18hrs on day 9 (+LPS(d9)), treated with PGE2 (10-6 M) alone on day 7 (+PGE2(d7)), or treated with PGE2 (10-6 M) on day 7 followed by LPS on day 9 (+PGE2(d7)+LPS(d9)). Cells were alternatively treated with a mixture of PGE2-LPS on day 9 (+PGE2(d9)+LPS(d9)). Histogram represents IL-10 production by DC treated as indicated in the figure. The data are mean ± SD, from one of two performed experiments, each with triplicates. b) iDC left untreated (Alone), treated with LPS alone on day 9 [+LPS(d9)], or PGE2 and LPS on day 9 [+PGE2(d9)+LPS(d9)] were further treated with purified anti-IL-10R mAb or an isotype control (iso) on day 9; before being washed and co-cultured with CFSE-labeled, naïve Thy1.1+, CL4 cells. Histograms represents %IFN-γ+ CL4 cells isolated from the DC co-cultures, as indicated in the figure. Data, represent the average from two experiments ± SD, performed in triplicate wells. c) Alternatively, iDC left untreated (Alone), treated with LPS alone on day 9 [+LPS(d9)], or PGE2 and LPS on day 9 [+PGE2(d9)+LPS(d9)] were cultured with naïve, CFSE labeled Thy1.1+ CL4 cells and the DC-CL4 cell co-cultures were treated with the anti-IL-10R mAb or the isotype control (iso). Histograms represent %IFN-γ+ CL4 cells isolated from DC co-cultures as indicated in the figure. Data, represent the average from two experiments ± SD, performed in triplicate wells. ** P between 0.001 & 0.01; *** P< 0.001
To investigate whether or not the production of IL-10 by DC has a role in abortive activation, iDC were treated with anti-IL-10R mAb during maturation with LPS in the presence or absence of PGE2, and used as APC for CL4 CD8+ T cell activation in vitro. Subsequently, CL4 cells isolated from the cultures were analyzed for IFN-γ production. Treatment of iDC and mDC with the anti-IL-10R mAb, resulted in an increase in IFN-γ+ CL4 cells compared to those co-culture with isotype-treated iDC and mDC, respectively (Figure 2b). Similarly, anti-IL-10R mAb-treated iDC, that were conditioned with PGE2 during maturation, significantly increased the %IFN-γ+ CL4 cells to the same level as the isotype-treated mDC cultures. To examine whether or not blocking IL-10 during DC-CL4 cell interaction enhances CL4 effector function, CL4 cells were cultured with DC in the presence of the anti-IL-10R mAb or an isotype control mAb, and then analyzed for IFN-γ production. Figure 2c shows that treatment of mDC-CL4 cell co-cultures with anti-IL-10R mAb did not alter the proportion of IFN-γ+ CL4 cells as compared with isotype-treated mDC co-cultures. However, blocking of IL-10R in iDC co-cultures, resulted in a significant increase in %IFN-γ+ CL4 cells above that of both isotype-treated and anti-IL-10R mAb-treated mDC co-cultures. Regardless of the presence or absence of anti-IL-10R mAb, co-culturing of naïve CL4 with iDC conditioned with PGE2 during maturation, did not result in an increase in proportion of IFN-γ+ CL4 cells compared with co-culturing with mDC (Figure 2c).
COX-2 over-expressing RencaHA (T3) tumor cells do not prime naive CL4 cells in vivo
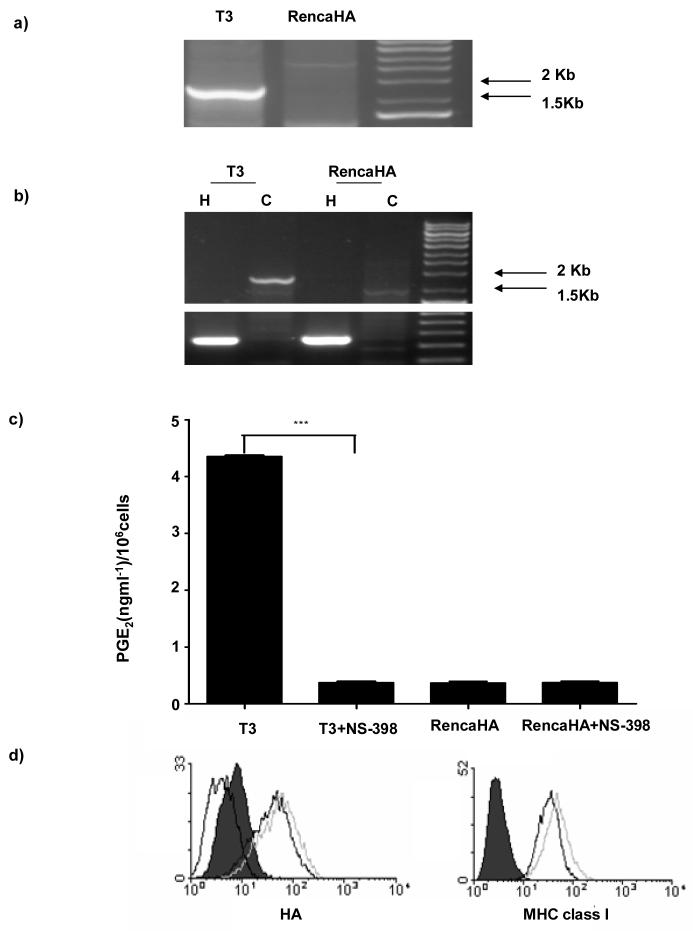
Previous studies in our laboratory have shown that cross-presentation of RencaHA cell-derived KdHA epitopes by BMDC results in priming of naïve KdHA-specific CL4 CD8+ T cells(21), within the TDLN of conventional BALB/c mice; giving rise to the formation of CTL effector and tumor infiltrating T cells (TIL)(20). To examine the effects of COX-2 over-expression upon the induction of tumor-specific CTL responses in vivo, RencaHA cells were transfected with a murine COX-2 cDNA to establish a COX-2 over-expressing cell line (T3) (Figure 3a & b), which resulted in a significant increase in PGE2 release by T3 cells in vitro (Figure 3c). Importantly, treatment of T3 cells with the COX-2-specific inhibitor: NS-398, decreased PGE2 production by T3 to the same level as that produced by RencaHA, indicating that increased PGE2 production by T3 was due to the over-expression of COX-2. Importantly, flow cytometric analyses showed there were equivalent levels of cell-surface HA protein and MHC class I expression amongst the RencaHA and T3 cell lines (Figure 3d). In addition, both tumors were able to grow at the same rate in BALB/c mice (data not shown).
Figure 3. Characterization of RencaHA and T3 cell lines.
a) PCR, showing the presence of the transfected COX-2 cDNA, and b) RT-PCR, showing expression of COX-2 by RencaHA and T3 cell lines. H represents the house keeping gene hypoxanthine phospho-ribosyl transferase (HPRT) and C represents COX-2. c) PGE2 produced by T3 and RencaHA cell lines in vitro left untreated or treated with 5μg/ml NS-398, as measured by ELISA. d) HA and MHC Class I expression by T3 and RencaHA was measured by flow cytometry. Black line represents expression on RencaHA cells, grey line represents expression on T3 cell line and dotted line (HA expression only) represents expression on RencaNT cell line. Filled histogram represents control secondary mAb alone. The data are from one of six performed experiments. *** P< 0.001.
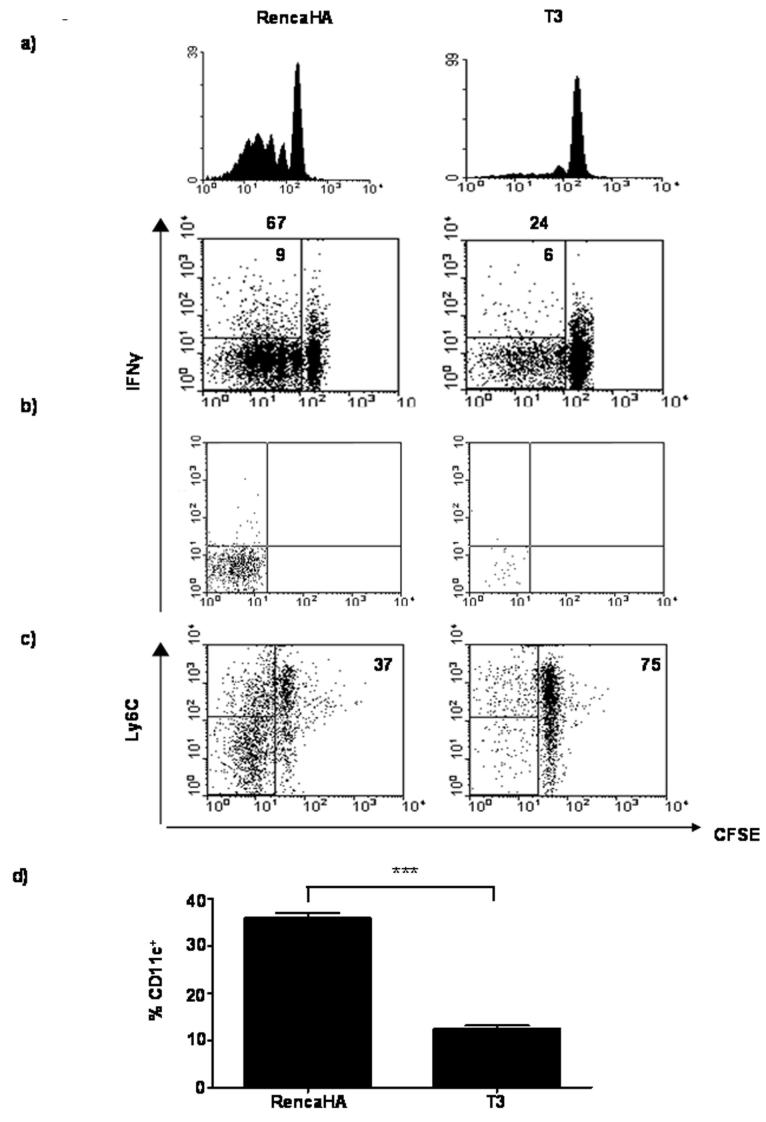
To examine the effect of COX-2 over-expression on anti-tumor CL4 cell responses, Thy1.2+ BALB/c mice were injected s.c. with 1×106 RencaHA or T3 cells on day 0 followed by CFSE-labeled naïve Thy1.1+ CL4 cells on day 11. On days 16 and 21, TDLN and tumors were removed, respectively, and CL4 cell infiltration was assessed by flow cytometric analyses (Figure 4). In mice given RencaHA cells, CFSE-labeled Thy1.1+ CL4 cells proliferated in the TDLN, elaborated IFN-γ upon re-stimulation with KdHA peptide in vitro and formed TIL. However despite both T3 and RencaHA tumors growing to a similar size in vivo, few Thy1.1+ CL4 cells proliferated and produced IFN-γ in the TDLN of T3 tumor-bearing mice, and as a consequence very few CL4 cells were present amongst all of the TIL isolated from the tumors. The lack of CL4 cell proliferation in the TDLN was dependent upon COX-2 over-expression, as the use of NS-398, restored CL4 cell proliferation in the TDLN of T3 tumor-bearing mice to the same level as that in the TDLN of RencaHA tumor-bearing mice (Table 1). Taken together these data suggest that COX-2 over-expression by T3 tumors prevents the formation of both tumor-specific CTL and TIL.
Figure 4. CL4 cells fail to proliferate in response to T3 in vivo.
Thy1.2+ BALB/c mice were injected s.c. with RencaHA or T3 tumor cells. On day 11, mice were given 3×106 naïve, CFSE-labeled Thy1.1+ CL4 cells i.v. and on day 16, or 21 cells obtained from the TDLN or the tumor, respectively, were stained for Thy1.1. Data represent proliferation and IFN-γ among CL4 cells isolated from TDLN (a), and intracellular straining of IFN-γ among CL4 cells isolated from the tumor (b). Histograms and dot plots are gated on Thy1.1+ CL4 cells collected from equal number of lymphocytes and total TIL. Numbers above the plots indicate the percentage of divided CL4 cells, and in the top left quadrants, the percentage of CL4 cells that are IFN-γ+. The data are from one of three performed experiments. c) Dot plots represent Ly6C expression on divided CL4 cells isolated from the TDLN of T3 and RencaHA tumor bearing mice. Plots are gated on Thy1.1+ CL4 cells. The data are from one of two performed experiments which utilized three animals per group. d) Accumulation of DC within the tumors extracted from the T3 or RencaHA bearing mice as described in (a). Histogram shows percentage of CD11c+ cells amongst total cells collected. The data are from one of two performed experiments ± SD, which utilized three mice per group. *** P< 0.001
Table 1. Use of COX-2 inhibitor, NS-398, up-regulates CL4 CTL responses to T3 tumor cells.
Thy1.2+ BALB/c mice were injected s.c. with 1×106 RencaHA or T3 tumor cells. On day 11, mice were given 3×106 CFSE-labeled, purified, naïve Thy1.1+ CL4 cells i.v. and on day 16 cells obtained from the TDLN were stained with antibodies against Thy1.1. Mice were injected i.p. with 10 mg/kg NS-398 before tumor injection and then 3 times per week for the duration of the experiment, or left untreated. Numbers are indicative of the percentage of the divided cells (CFSElo) and of IFN-γ+ amongst divided cells (CFSEloIFN-γ+). The data are from one of two performed experiments, which utilized three animals per group
| RencaHA | T3 | T3+NS-398 | |
|---|---|---|---|
| CFSElo | 20% | 13% | 22% |
| CFSElo IFNγ+ | 5% | 1% | 7% |
CL4 cells undergo abortive activation in response to T3 tumor in vivo
To determine whether or not COX-2 over-expression by T3 induces abortive activation of CL4 CD8+ T cells in vivo, CFSE-labeled CL4 cells recovered from the TDLN of RencaHA and T3 tumor-bearing mice were characterized for the expression of Ly6C(23). Examination of the TDLN of tumor bearing mice revealed that, whereas the majority of dividing CL4 cells in the TDLN of RencaHA tumor-bearing mice were Ly6Clo, the majority of the dividing CL4 cells in the TDLN of T3-bearing mice were Ly6Chi (Figure 4c). These data suggest that over-expression of PGE2, due to the presence of the COX-2 transgene, promotes abortive activation and tolerance induction amongst naïve CL4 cells in the TDLN.
Fewer DC are present within T3 tumors
Data in figure 2 suggest that PGE2 influences DC function by promoting IL-10 production. Other studies have shown that IL-10 over-expression by tumor cells reduces accumulation of DC within the tumor(31). Therefore, flow cytometric analyses of RencaHA and T3 tumors were carried out to determine the absolute numbers of tumor-infiltrating CD11c+ DC(32). Figure 4d shows a significant increase in the absolute numbers of CD11c+ cells within the RencaHA tumors as compared with T3 tumors; suggesting that COX-2 over-expression, and increased PGE2 production that occurs as a result, may influence the accumulation of DC in the tumor.
Exogenous PGE2 abrogates direct activation of CL4 cells by tumor cells
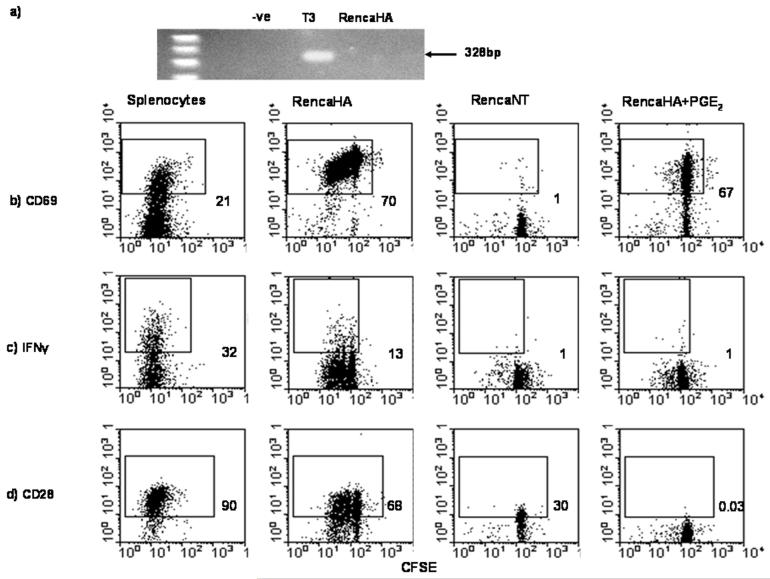
The data indicate that PGE2 over-production by T3 cells may interfere with cross-presentation by DC, preventing activation of anti-tumor CL4 CTL responses in vivo. However, other studies have shown that over-expression of COX-2 by tumors increases motility and angiogenesis amongst tumor cells in vivo(16), (17); allowing them to form metastases in the local TDLN. The formation of metastases may provide a mechanism for activation of the anti-tumor CD8+ T cell responses directly by tumors(18). Therefore, we investigated whether or not tumor cells were present in the TDLN of BALB/c mice given T3 or RencaHA. On day 16, the TDLN from each group of mice were isolated and cDNA prepared for RT-PCR analyses. Whilst, HA mRNA was not detected in any of the TDLN from RencaHA bearing mice (Figure 5), HA mRNA could be detected in the TDLN of T3-bearing mice, suggesting that T3 tumor cells had migrated to these LN.
Figure 5. Exogenous PGE2 abrogates direct activation of CL4 cells by tumor cells.
TDLN of mice injected with RencaHA or T3 tumor cells followed by naïve CL4 cells, as described in Figure 4, were excised on day 16 and analysed for the presence of HA by RT-PCR (a). RencaHA, RencaNT or peptide-pulsed splenocytes, in the presence or absence of PGE2, were co-cultured with naïve, CFSE-labeled Thy1.1+ CL4 cells for 72hrs and b) CD69 expression, c) IFN-γ production and d) CD28 expression by CL4 cells isolated from in vitro cultures were examined by flow cytometry. Dot plots are gated on Thy1.1+ CL4 cells and numbers indicate percentage divided CL4 cells expressing the receptor indicated in the figure. The data are from one of three performed experiments.
Previous studies in our laboratory have shown that RencaHA cells are able to directly prime CL4 CTL responses in vitro; leading to tumor cell lysis(20). To investigate whether or not the presence of PGE2 during this interaction interferes with naive CL4 CTL priming, RencaHA cells were co-cultured for 72 hrs with CFSE-labeled CL4 cells in the presence or absence of PGE2. Following co-culture, CL4 cells were isolated and analyzed for the expression of the activation markers CD69, CD28 and intracellular IFN-γ. RencaHA and KdHA peptide-pulsed splenocytes induced up-regulation of CD69, production of intracellular IFN-γ, and CD28 expression by CL4 cells, indicating the activation of these T cells (Figure 5). As previously shown, CL4 cells cultured with HA negative control RencaNT cells failed to up-regulate expression of any of these molecules, thus indicating that CL4 T cell responses to RencaHA cells are KdHA-specific(20). Significantly, compared with CL4 cells cultured with RencaHA cells alone, proliferation, intracellular expression of IFN-γ and cell surface expression of CD28 was abrogated amongst CL4 cells cultured with RencaHA in the presence of PGE2. However, the fact that these undivided CL4 cells up-regulated CD69 indicated that initial priming had occurred without inducing CL4 CTL responses (Figure 5b, c & d).
Discussion
In this report we show that PGE2 abrogates both cross-presentation and direct presentation pathways involved in the priming of naïve tumor-specific CL4 CD8+ T cells responses. The data show that, whereas, priming of naïve CL4 T cells by KdHA peptide-pulsed mDC resulted in their productive activation, co-culturing with PGE2-treated mDC resulted in reduced CL4 effector function, similar to that which occurs following priming by iDC. This finding is consistent with other studies which showed reduced APC function of DC, treated with PGE2(25). It is likely that the lack of productive activation of naïve CL4 T cells results from the immature phenotype of PGE2-treated DC that were used as APC. Other in vivo studies from several laboratories, including our own, have shown that, while the interaction of antigen-loaded mDC with naïve CD8+ T cells activates effector CD8+ T cell responses, cross-presentation of antigens by iDC to CD8+ T cells has been shown to induce tolerance(23), (33), (34). Furthermore, the abortive activation phenotype, displayed by CL4 cells within the TDLN of mice bearing T3 tumors, is consistent with the increased ability of PGE2-treated iDC to migrate to TDLN (35). Although not tested in this study it has been shown that increased migration of iDC to the TDLN is associated with an increase in the expression of CCR7 following PGE2 treatment of iDC.
Our studies show that PGE2 treatment of DC, during their differentiation or maturation increases IL-10 production compared with mDC. This is in accordance with previous studies where PGE2 was shown to induce IL-10 production by DC in vitro(6). Importantly, such an increase in IL-10 release by DC after exposure to PGE2, may account for the lower numbers of TIDC in T3 tumors compared with RencaHA tumors(31). The importance of IL-10 in PGE2-mediated inhibitory effects on DC was notable, as blocking of IL-10R during DC maturation reversed the effects of PGE2 on APC function of DC. Consistent with other studies, fully matured DC were resistant to the effects of IL-10(8), (25). We hypothesise that the type of CL4 CD8+ T cell response generated by PGE2-treated DC, is determined at the site of DC maturation in the tumor microenvironment through increased production of IL-10. In addition, the data presented here may indicate a possible role of IL-10 in iDC-mediated abortive activation of CL4 cells, and that blocking of IL-10R during iDC-CL4 interaction counters the ability of iDC to induce tolerance.
In vivo COX-2 over-expression by T3 tumors rendered CL4 cells unable to proliferate, produce IFN-γ, or form TIL. Previous studies in our laboratory showed that in vivo, CL4 cell responses to RencaHA tumors rely upon cross-presentation of HA antigen by BMDC(36). Data presented in this report show that over-expression of COX-2 enables T3 tumor cells to migrate to the TDLN; indicating that T3 cells could also directly activate CL4 cells. However, the lack of CL4 cell activation in the TDLN of T3 tumor-bearing mice suggests that direct activation by T3 cells does not occur. Indeed, when RencaHA were cultured with CL4 cells in vitro in the presence of PGE2 at a concentration comparable with that found within the tumor micro-environment of COX-2 over-expressing tumors(37), CL4 CTL responses were abolished. However, undivided CD8+ CL4 cells lost CD28 expression. The presence of CD8+ CD28- T cells with regulatory functions has been observed in patients with different types of cancer(38), (39). Thus in our system, a second stage of CD8+ T cell tolerance could occur through direct interaction of T3 with CL4 cells.
Overall, our data suggest that COX-2/PGE2 over-expression by tumors regulates both direct and indirect antigen presentation to naive CD8+ T cells. We propose that PGE2 within the tumor microenvironment both reduces the accumulation of TIDC, thus inhibiting Ag uptake, and maintains TIDC in an immature state which induces CD8+ T cell tolerance upon migration to the TDLN. In addition, COX-2/PGE2 over-expression by tumors allows migration of tumors to TDLN, also resulting in CD8+ T cell-tolerance induction. These findings provide us with an understanding of how PGE2 over-producing tumor cells can regulate anti-tumor CD8+ T cell responses. Critically, we and others have shown that DC which have already undergone maturation are refractory to the effects of PGE 2 and IL-10(25). Therefore, immunotherapeutic strategies which utilize ex vivo-generated mDC, rather than iDC(40), loaded with tumor-antigen, would be preferable in order to prime protective anti-tumor CD8+ CTL response in patients with cancer. Furthermore, the use of PGE2 inhibitors in patients with cancer would also be greatly desirable in promoting productive activation of anti-tumor CTL cell responses potentially generating fewer side effects than COX-2 specific inhibitors(41).
ACKNOWLEDGMENTS
We thank Dr. Lindsay Nicholson and Dr. Ben Raveney for critical review of this manuscript.
Footnotes
This work was supported in part by Cancer Research UK Project Grant C1484
- COX-2
- Cyclooxygenase-2
- PGE2
- Prostaglandin E2
- CL4
- Clone 4
- HA
- hemagglutinin glycoprotein from PR8
- BMDC
- Bone marrow-derived DC
- iDC
- immature DC
- mDC
- mature DC
- HPRT
- hypoxanthine phosphoribosyltransferase
- 7AAD
- 7-Amino-actinomycin D
References
- 1.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 2.Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–16. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–8. [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–10. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 5.Menetrier-Caux C, Bain C, Favrot MC, Duc A, Blay JY. Renal cell carcinoma induces interleukin 10 and prostaglandin E2 production by monocytes. Br J Cancer. 1999;79:119–30. doi: 10.1038/sj.bjc.6690021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–63. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 7.Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004;173:4352–9. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 8.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 9.McBride JM, Jung T, de Vries JE, Aversa G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol. 2002;215:162–72. doi: 10.1016/s0008-8749(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 10.Dercamp C, Chemin K, Caux C, Trinchieri G, Vicari AP. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 2005;65:8479–86. doi: 10.1158/0008-5472.CAN-05-1319. [DOI] [PubMed] [Google Scholar]
- 11.Smyth GP, Stapleton PP, Barden CB, et al. Renal cell carcinoma induces prostaglandin E2 and T-helper type 2 cytokine production in peripheral blood mononuclear cells. Ann Surg Oncol. 2003;10:455–62. doi: 10.1245/aso.2003.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4:585–93. [PubMed] [Google Scholar]
- 13.Menetrier-Caux C, Montmain G, Dieu MC, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–91. [PubMed] [Google Scholar]
- 14.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 15.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–90. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 16.Gately S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 2000;19:19–27. doi: 10.1023/a:1026575610124. [DOI] [PubMed] [Google Scholar]
- 17.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–91. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 18.Ochsenbein AF, Sierro S, Odermatt B, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–64. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 19.Soumaoro LT, Uetake H, Takagi Y, et al. Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its association with lymph node metastasis. Dis Colon Rectum. 2006;49:392–8. doi: 10.1007/s10350-005-0247-x. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson SR, Williams NA, Morgan DJ. The role of intercellular adhesion molecule-1/LFA-1 interactions in the generation of tumor-specific CD8+ T cell responses. J Immunol. 2005;174:3401–7. doi: 10.4049/jimmunol.174.6.3401. [DOI] [PubMed] [Google Scholar]
- 21.Morgan DJ, Liblau R, Scott B, et al. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–83. [PubMed] [Google Scholar]
- 22.Murphy GP, Hrushesky WJ. A murine renal cell carcinoma. J Natl Cancer Inst. 1973;50:1013–25. doi: 10.1093/jnci/50.4.1013. [DOI] [PubMed] [Google Scholar]
- 23.Raveney BJ, Morgan DJ. Dynamic Control of Self-Specific CD8+ T Cell Responses via a Combination of Signals Mediated by Dendritic Cells. J Immunol. 2007;179:2870–9. doi: 10.4049/jimmunol.179.5.2870. [DOI] [PubMed] [Google Scholar]
- 24.O’Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. Embo J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 26.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–17. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–6. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 28.Harizi H, Gualde N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell Mol Immunol. 2006;3:271–7. [PubMed] [Google Scholar]
- 29.Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–83. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 30.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 31.Qin Z, Noffz G, Mohaupt M, Blankenstein T. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony-stimulating factor gene-modified tumor cells. J Immunol. 1997;159:770–6. [PubMed] [Google Scholar]
- 32.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 33.Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–20. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 34.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 35.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 36.Janicki CN, Jenkinson SR, Williams NA, Morgan DJ. Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res. 2008;68:2993–3000. doi: 10.1158/0008-5472.CAN-07-5008. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Yamagata N, Yadav R, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–35. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meloni F, Morosini M, Solari N, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- T cells. Int Immunol. 1998;10:775–83. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 40.Gilboa E. DC-based cancer vaccines. The Journal of Clinical Investigation. 2007;117:1195–203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chell S, Kaidi A, Williams AC, Paraskeva C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim Biophys Acta. 2006;1766:104–19. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]