Abstract
Pregnant substance users can benefit significantly from substance abuse treatment but treatment retention can be challenging. Two hundred pregnant substance users entering outpatient substance abuse treatment at 1 of 4 treatment programs were randomized to receive either 3 individual sessions of Motivational Enhancement Therapy for pregnant substance users (MET-PS) or the first 3 individual sessions normally provided by the program. All participants were encouraged to participate in all other treatment offered by the program. Outcome measures included treatment utilization according to clinic records, qualitative urine toxicology measures, and self-report of substance use. One hundred and sixty two participants (i.e., 81%) completed the 1 month active phase. Participants attended 62% of scheduled treatment on average and reported decreased substance use during the first month of treatment, with no differences between MET-PS and treatment as usual participants. There was some evidence that the efficacy of MET-PS varied between sites and that MET-PS might be more beneficial than TAU in decreasing substance use in minority participants. These results suggest that MET-PS is not more effective than treatment as usual for pregnant substance users in general but that there might be particular subgroups or treatment programs for which MET-PS might be more or less effective than treatment as usual.
Keywords: Pregnant, substance abuse, Motivational Enhancement Therapy, clinical trial, randomized
1. Introduction
During pregnancy, an estimated 4% of women use illicit substances, 4% binge drink, and 18% smoke cigarettes, the associated prenatal, neonatal, and postnatal complications of which represent a leading preventable cause of mental and physical problems in children (SAMHSA, 2005). Past research suggests that substance abuse treatment is effective in decreasing substance use and improving birth outcomes for pregnant women who attend treatment (McMurtrie et al., 1999; Jones et al., 2002) but that retaining pregnant women in treatment can be difficult (Haller et al., 1997); hence increasing treatment utilization has been identified as an important goal for programs treating pregnant substance users (Howard and Beckwith, 1996). Past research suggests that treatment retention can be improved by providing case management (Jones et al., 2004) or housing (Haller et al., 1997) to help pregnant women stabilize their living situations and by removing external barriers to treatment, for example, by providing transportation and child care (Weisdorf et al., 1999) but that retention is still problematic (Haller et al., 1997). Thus, in addition to removing external barriers to care it is important to remove internal barriers to care such as ambivalence about giving up substance use, which is common in individuals entering substance abuse treatment (Shaffer and Simoneau, 2001). Relatedly, it has been suggested that best practices for treating pregnant substance users include the use of a nonjudgmental and non-punitive approach as opposed to a confrontational one (Howell et al., 1999). Motivational enhancement therapy (MET), which seeks to reduce ambivalence about change in a non-confrontational, non-judgmental manner, would thus seem to be an ideal treatment for reducing internal barriers to treatment in pregnant substance users.
Several studies have found that the use of brief motivational interventions, such as motivational interviewing (MI) or MET, significantly increases treatment utilization in substance abusing populations (Saunders et al. 1995; Swanson et al., 1999; Martino et al., 2000; Carroll et al., 2001; Carroll et al., 2006). One prior trial has evaluated the efficacy of MI, combined with vouchers for goods earned for negative urine toxicology screens, for increasing treatment retention in pregnant substance users (Jones et al., 2002; Jones et al., 2004). Based on the poor treatment compliance of the first 31 pregnant substance users enrolled, the investigators added a case management component in an effort to improve treatment retention for the subsequent 59 participants enrolled (Jones et al., 2004). While the participants receiving case management attended the first treatment session at a higher rate than those without case management, subsequent treatment retention was not significantly different between the two groups (Jones et al., 2004). The Jones et al. (2004) study highlighted two important characteristics of pregnant substance abusing women: as a group, they have enormous unmet psychosocial needs, frequently including the basic needs of food and shelter and, second, retaining this population can be extremely difficult. However, since the Jones et al. (2004) study was conducted in a research setting in which treatment was limited to brief interventions and case management, it is difficult to delineate any additional implications for substance abuse community treatment programs (CTPs), which offer a wider range of treatment options. Perhaps in the context of more comprehensive treatment services, MI or MET might be effective in increasing pregnant substance users’ utilization of these services.
The use of brief motivational interventions in pregnant substance users is also of interest in terms of their potential efficacy in reducing substance use. In the Jones et al. study (2002), the subgroup of participants receiving all 4 MET sessions, compared to those not receiving all 4 sessions, had significantly less substance use and better birth outcomes, suggesting that MET might have some efficacy in reducing substance use. The possible efficacy of motivational interventions in reducing substance use in pregnant women is also suggested by a pilot study of MI in reducing alcohol consumption in a primary care sample of pregnant women (Handmaker et al., 1999). The findings suggest that a 1-hour motivational interview, compared with letters informing control participants of the risks of drinking during pregnancy, seemed to significantly benefit the women who had reported drinking the most during the initial interview. Specifically, this subgroup of women assigned to the MI condition reported significantly lower levels of intoxication at the 2 month follow up than did comparable participants in the control group (Handmaker et al., 1999).
The primary objective of the present trial was to evaluate the efficacy of a 3 session MET intervention for pregnant substance users (MET-PS), compared to treatment as usual (TAU), in increasing treatment utilization and decreasing substance use during the first month of treatment. Evaluating the longer-term efficacy of MET-PS, relative to TAU, was an important secondary objective. This randomized, parallel, two group trial included a 1 month active study phase with 2 follow-up assessments completed at 1 and 3 months, respectively, following the end of the active study phase. It was predicted that MET-PS, compared to TAU, would significantly increase treatment utilization and decrease substance use during the first month of treatment. In addition, it was predicted that MET-PS, compared to TAU, would significantly increase treatment utilization and decrease substance use through the entire 4 month study period.
2. Materials and methods
2.1 Study Overview
This study was conducted by the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN). The CTN was established in 1999 in response to an Institute of Medicine report (Lamb et al. 1998) delineating the relative lack of information exchange between substance abuse researchers and substance abuse community treatment providers, resulting in the minimal impact of research findings on treatment practice. In CTN, substance abuse researchers work together with treatment providers to identify promising treatment approaches, to design clinically meaningful trials, and to implement the clinical trials in the “real world” settings of the CTPs, utilizing the existing staff as interventionists and clinic patients as participants. The present trial was thus conceived and designed by a team of researchers and substance abuse treatment providers from CTPs with programs for pregnant women. One of the important study design decisions resulting from this collaboration was the choice of the control condition. Based on the treatment providers’ expressed interest in understanding how MET-PS compared to their current treatment practices, as opposed to a standard control condition, it was decided, in the interest of clinical relevance, to compare MET-PS to treatment as usual (TAU) at the participating CTPs.
2.2 Study Sites
A total of 12 CTPs expressed interest in participating as study sites, of which the 4 sites treating the greatest number of pregnant women in outpatient treatment were selected; one additional site had been selected but closed for fiscal reasons prior to study initiation. Each of the four community treatment programs randomizing participants into this study were women-specific programs. The Horizons Program in North Carolina is a comprehensive treatment program for substance-using women and their children. The program offers several levels of outpatient treatment (including IOP) as well as specialized services for specific populations including a prenatal clinic, a residential program for women and children, and a licensed childcare facility. At the time of participation in this study, Horizons was admitting approximately 75 –100 women per year, of which approximately 55% were pregnant. The Milagro Program in New Mexico is a perinatal substance abuse prevention and treatment project that provides an array of services to alcohol and other drug dependent pregnant or postpartum women and their children. These services include high risk prenatal care, outpatient and residential substance abuse treatment, opioid replacement therapy, nursing care management (prenatal and post partum), delivery, inpatient care, parenting classes, and pediatric follow up. Milagro serves approximately 150 women per year, all of whom are pregnant at time of admission.
Project Home at Midtown Community Mental Health Center in Indiana provides a home-based outpatient treatment program with comprehensive services to pregnant and parenting women who use substances, and to their families. Project Home includes individual, group and family therapy as well as education that focus on perinatal substance use, parenting, vocational goals, life skills and independent living. The program also operates a transitional housing program for clients and their children. At the time of participation in the study, Project Home maintained a census of approximately 60 active cases. Overall, Midtown CMHC serves approximately 90 pregnant substance using women per year across all its outpatient services, including Project Home. Project Link at JADAC in Kentucky serves over 100 pregnant substance-using women per year, using a case management model for treatment engagement and retention. Through ongoing assessment and case management, Project Link clients are linked to substance abuse treatment modalities at JADAC, including women’s IOP, substance abuse education, detoxification, and residential substance abuse treatment. Case managers also link clients to other community resources as appropriate.
2.3 Participants
Participants for the study were recruited from intakes to the pregnant women treatment programs of the 4 participating CTPs. Study staff worked to increase clinic intakes by using advertising and direct community promotions, such as networking with community professionals and presenting at community health fairs, to promote the clinic. All participants were given a thorough explanation of the study and signed an informed consent form that was approved by the Institutional Review Boards of the participating sites.
Eligible participants were at least 18 years of age and pregnant (as confirmed by a pregnancy test) and not planning to terminate the pregnancy. To be eligible, participants were required to be identified as needing substance abuse treatment via the CTP’s usual screening procedure and to have a living arrangement of sufficient stability to allow for outpatient treatment. Participants were excluded from the study if they required residential or inpatient treatment (other than detoxification), were more than 32 weeks pregnant, planned to relocate from the area within 4 months of signing the study consent form, had pending legal charges that might lead to incarceration (other than those requiring the participant to attend treatment), or were a significant suicidal/homicidal risk. A total of 733 were pre-screened, 204 signed consent and 200 were randomized into the trial. Of the 533 participants excluded, 82 (15.4%) were not pregnant, 151 (28.3%) did not need to enter substance abuse treatment, either due to not using substances or to already being in treatment, 20 (3.8%) were under 18 years of age, 32 (6%) were not interested in participating, 9 (1.7%) had unstable living arrangements, 10 (1.9%) had plans to relocate within 4 months, 9 (1.7%) had pending legal charges, 110 (20.6%) self-reported having conditions that required inpatient/residential treatment, 4 (<1%) were a significant suicidal/ homicidal risk, 38 (7.1%) were more than 32 weeks pregnant, and 68 (12.8%) were ruled out for other reasons (e.g., planning to terminate the pregnancy, refused treatment services, etc.).
2.4 Clinicians
The study clinicians were 23 substance abuse counselors from the 4 participating CTPs. If required by the site IRB, the clinicians signed informed consent. Clinicians were eligible to participate if they had experience working with pregnant substance users and/or had been trained to work with pregnant substance users, were willing to learn and implement a manualized version of MET-PS, were willing to participate in the study as a clinician (e.g., willing to be randomly assigned to either the MET-PS or TAU condition, willing to have their sessions audiotaped, etc.), and were approved by administrative/ supervisory staff as appropriate for the study. Clinicians were excluded if they had received credentialing as a MET trainer or had served as a MET clinician in a prior clinical trial, with the exception of the last clinician added to the trial, who was the only staff person available to replace a MET-PS clinician and who had received credentialing as a MET trainer. To help ensure the comparability of the clinicians administering MET-PS and TAU, all eligible clinicians were randomly assigned to the MET-PS or TAU condition. As a group, the clinicians were primarily female (91.3%), racially diverse, with 52% Caucasians, 39% African Americans, and 9% Hispanics, and had a mean age of 40 (SD=10.8). The majority of clinicians had a masters degree (74%), with the remaining 26% having a bachelors degree. Six of the clinicians (26.1%) were licensed or certified in social work, 4 were licensed professional counselors (17.4%), 1 clinician was a nurse (4.3%), 1 was a marriage and family therapist (4.3%), and 1 clinician was a certified alcohol and drug counselor (4.3%). Participants were assigned to clinicians on the basis of availability.
2.5 Study Treatments
2.5.1 Motivational Enhancement Therapy for Pregnant Substance Users (MET-PS)
Intervention
The MET-PS intervention is comprised of the brief motivational techniques described by Miller and colleagues (Miller and Rollnick, 1991; Miller, 1999), modified specifically for pregnant substance users. The MET-PS intake session began with developing rapport through the use of open ended questions including discussion of the participant’s feelings about her pregnancy, reflective listening, affirming the participant, and summarizing what the participant said. In addition, the participant’s perceived pros and cons of using substances, including possible adverse effects on the fetus, were explored. The remainder of the session was devoted to the clinic’s usual assessment and intake procedures. The second session was devoted to reviewing the participant’s individualized personal feedback report concerning the consequences of substance use for both the participant and her pregnancy and the degree to which she was engaging in activities to promote a healthy pregnancy. The third session was devoted to developing a change plan for participants who expressed a readiness to change and strengthening commitment to change in participants who were not yet ready to change. These 3 sessions replaced the intake session and the first 2 individual treatment sessions typically offered by the CTP. The first MET-PS session was approximately 1.5 to 2 hours in length while the other 2 sessions were approximately an hour in length. Participants in the MET-PS condition were encouraged to participate in the other treatment services typically offered at the CTP (e.g., group treatment, case management, etc.).
Training
Clinicians randomly assigned to MET-PS completed 20 hours of training with a MET Expert that included a lecture format as well as role playing exercises involving scenarios with pregnant substance users. Three MET Experts provided training during the course of the trial; two were members of the Motivational Interviewing Network of Trainers (MINT), while the third was Dr. Nancy Handmaker, the Principal Investigator for the pilot study of MI in reducing alcohol consumption in pregnant women (Handmaker et al., 1999). Following training, each MET-PS clinician completed at least 1 training case, working with a pregnant substance user from the CTP. The training cases were supervised by a protocol MET-PS supervisor via ratings of the audiotaped sessions for clinician adherence and competence, utilizing a system used in previous multi-site clinical trials (Carroll et al., 2006). The inter-rater reliability of the 2 MET-PS supervisors was evaluated for a randomly selected sample of ten tapes. The intraclass correlation coefficients (ICCs), calculated according to Shrout and Fleiss (1979), revealed that the supervisors evidenced an excellent level of inter-rater reliability for the MET adherence (.97) and skill (.90) scales. Clinicians receiving ratings of at least “average” on half of the MET items for both adherence and skill were then eligible to be assigned study participants. Otherwise, the clinician completed additional training cases and supervision until meeting this criterion.
Treatment Integrity
Clinician adherence and competence was monitored via the ongoing review of randomly selected audiotaped sessions by the protocol MET-PS supervisors; 62% of the sessions were reviewed. Any clinician identified as performing below the set standards (i.e., at least “average” on half of the MET items for both adherence and skill) was given additional training and more intense supervision until he or she met or exceeded standards.
2.5.2 Treatment As Usual
Participants assigned to TAU were offered the treatment typically provided at the CTP with the constraint that they receive at least 3 individual sessions with a clinician, including the intake session. For 3 of the sites, this did not require any modification to their normal program. The fourth site, which has a primary focus on case management and typically offers individual counseling only on a monthly basis, modified its program to include 2 weekly individual counseling sessions in addition to the standard intake. All study participants were offered the other services typically provided by the CTP (e.g., group treatment, additional individual treatment, case management, etc.). The 3 individual sessions were audiotaped to allow for an evaluation of the discriminability between TAU and MET-PS sessions. As in the MET-PS intervention, the first TAU session was approximately 1.5 to 2 hours in length while the other 2 sessions were approximately an hour in length.
2.5.3 Treatment Discriminability
In order to test the efficacy of MET-PS compared to TAU, it must be established that the MET-PS and TAU sessions could be distinguished from one another. This check on the discriminability of the MET-PS/TAU sessions was completed by having a randomly selected sample of tapes reviewed by 3 raters who were blind to clinician treatment assignment and who had no other involvement in the study except to rate the sessions. The current study utilized the adherence and competence instruments utilized in previous multi-site clinical trials (Carroll et al., 2006). Specifically, 10 items each were used to assess the clinician’s utilization of MET (e.g., avoiding confrontation, asking open-ended questions, reflective listening) general substance abuse counseling (e.g., substance use assessment, discussion of pregnancy and substance use, treatment planning), and strategies inconsistent with MET (Anti-MET; e.g., confrontation of denial, asserting the authority of the therapist). Each item was rated for adherence (1: not present in the session to 7: extensively) and skill (1: very poor to 7: excellent).
Following training, including feedback on rated tapes, the inter-rater reliability of the 3 independent raters was evaluated for a randomly selected sample of 6 tapes. The intraclass correlation coefficients (ICCs), calculated according to Shrout and Fleiss (1979), revealed that the raters evidenced a good level of inter-rater reliability for the adherence scales for MET (.83), General Counseling (.91), and Anti-MET (.81) and for the skill scales for MET (.86), General Counseling (.89), and Anti-MET (.82). A total of 434 sessions (228 TAU and 206 MET-PS) were completed during the study, of which 167 were rated by the independent raters.
2.6 Participant Measures
The primary outcome measure was treatment utilization defined as the ratio of the number of outpatient (including intensive outpatient) treatment hours attended to the number of hours scheduled. Secondary outcome measures of treatment utilization included the number of weeks in which at least 1 treatment session was attended and the number of weeks until treatment dropout, defined as failure to attend any treatment provided by the CTP for 3 consecutive weeks, while the participant was still pregnant; the 3-week time-frame was selected a-priori based on a consensus among the substance abuse treatment providers on the study design team regarding how they would define a “drop out” in their treatment programs. The 3 measures of treatment utilization were based on clinic records of treatment attendance.
The secondary outcome measures of substance use included self-report of substance use (i.e., alcohol and illicit drugs) and qualitative urine toxicology results. Urine samples were collected and tested for opiates, cocaine, methamphetamines, benzodiazepines, and marijuana at screening, weekly during the active study phase, and at the 2 follow-up visits using the OnTrak TesTcup®. The Substance Use Calendar, a self-report assessment of the participant’s use of substances for each day of the study, was completed at each research visit using the Timeline Follow-Back procedure (Sobell and Sobell, 1992; Fals-Stewart, 2000). The University of Rhode Island Change Assessment (URICA) (DiClemente & Hughes, 1990) was used to assess the participants’ motivation to change their substance use behavior. The URICA was completed at baseline and at the end of the active study phase. Four scales are derived from the URICA: Pre-contemplation, Contemplation, Action and Maintenance; a readiness score which combines these scales (Pantalon et al., 2002) was used for the present study.
2.7 Procedures
Pregnant women identified by CTP clinical staff as needing substance abuse treatment and who expressed a willingness to learn more about the study were referred to the research assistant (RA). After signing the Informed Consent Form, the study candidate completed screening and baseline assessments. Ineligible individuals continued into the CTP’s standard intake assessment and treatment program. Eligible participants were assigned to MET-PS or TAU via urn randomization to balance on 3 dichotomous variables: pressure to attend treatment, self-report of drug and alcohol use, and need for methadone maintenance. Pressure to attend treatment was defined as having jail, the removal of a child (or children), or the removal of housing as the consequence of not attending treatment. The self-report of use variable was operationalized as the number of days in the past 28 that the participant reported using alcohol and/or drugs (<10 or ≥10 days of use in the last 28 days). Need for methadone maintenance was determined by the participant’s self-report of substance use and the treatment referral practices followed at the CTP.
The active study phase was 4 weeks in duration. During this time, participants in both treatment conditions were offered at least 3 individual sessions with a clinician. An attempt was made to have the study participant attend the initial treatment session (MET-PS or TAU) immediately following randomization but, in practice, the initial session typically occurred within the first week of randomization. There was a 28-day time frame, starting from randomization, for the participant to receive the 3 MET-PS/TAU sessions with the constraint that no more than 2 sessions occurred in a single week. Participants in both conditions were encouraged to participate in the other treatment services offered by the CTP (e.g., group treatment, case management, etc.) During the first month of treatment, participants were scheduled to meet with the RA on a weekly basis. In addition, the participants were scheduled to meet with the RA at the 2 and 4 month post-randomization follow-up visits. Study participants received $30 in retail scrip or vouchers for each of the 5 research visits that were relatively long (i.e., the 2 baseline visits, the end of active phase visit, and the 2 follow-up visits) and $25 for each of 3 research visits that were relatively brief.
2.8 Data Analysis
Per the study statistical analysis plan, the primary outcome measure, the ratio of attended to scheduled treatment hours during the first month of treatment, was analyzed using a Cox proportional hazards model with the log of the number of scheduled hours and the type of patient (intensive outpatient or outpatient) as covariates. The present study was powered to detect differences between the treatment groups when the data were pooled across sites, and, thus, the protocol-specified analyses did not include an evaluation of site by treatment interaction effects; however, analyses evaluating such effects were included as exploratory analyses; specifically, the three sites with sufficient sample size were included in these analyses.
In addition, the statistical analysis plan did not specify the inclusion of treatment group baseline differences as possible covariates in the statistical models. There were a number of significant baseline differences between the MET-PS and TAU groups (see section 3.1). The baseline differences that were deemed most likely to be related to treatment outcomes were: 1. the greater proportion of participants with cocaine as the primary drug of choice in the MET-PS group; 2. the greater proportion of participants with marijuana as the primary drug of choice in the TAU group; 3. the greater proportion of participants in the TAU group with pressure to attend treatment. In addition, since a meta-analysis revealed that the effect sizes of MI were larger in ethnic minorities (Hettema et al., 2005), we also evaluated the importance of the group baseline difference of significantly more minority participants in the MET-PS group. The importance of these baseline differences was tested for each outcome measure by introducing the covariate into the model as the lone covariate and retaining those covariates with a p value less than .10 in the final analysis of the outcome variable.
Several statistical analyses were utilized for the secondary outcome measures, depending upon the nature of the data. Secondary analyses involving repeated measures data were analyzed using Generalized Estimating Equations (GEE) first with a regression including week as an effect, and if no week effects were found, a second regression testing for a treatment effect was completed without week in the model. In addition, for the repeated measures data, two sets of analyses were conducted; the first included data from the first month of treatment only while the second included the data from randomization through 4 month follow-up. Non-longitudinal binomial data were analyzed with logistic regression using a quasi-likelihood approach to accommodate possible over-dispersion. The survival analysis of time to drop out while pregnant was completed with a Cox proportional hazards model in which data points with a value of 106 or more days were censored as were the data from 6 participants who withdrew consent. All analyses were conducted for both the ITT sample (i.e., all 200 randomized participants) as well as for the evaluable sample, which was comprised of the 171 participants who received at least 1 MET-PS or TAU session; results for the evaluable sample are reported where they differed from those of the ITT sample.
3. Results
3.1 Sample Characteristics
The sample characteristics by site and by treatment group are provided in Table 1. The participants were, on average, 26 years of age and 20 weeks pregnant at the time of randomization. The majority of study participants were unmarried, were unemployed and had, on average, a high school education. As can be seen in Table 1, the sample was fairly diverse in terms of race and ethnicity. There were significant site differences on many participant characteristics including age, race/ethnicity, marital status, number of weeks pregnant at time of randomization, primary drug of choice, days of substance use, need for methadone maintenance, and pressure to attend treatment. This suggests that the study was successful in including a diverse set of sites. There were a number of significant baseline differences between the MET-PS and TAU groups, including significantly older participants in the MET-PS group, significantly more minority participants in the MET-PS group, significantly more years of education in the MET-PS group, significantly more participants with cocaine as a primary drug of choice in the MET-PS group, and significantly more participants with marijuana as a primary drug of choice in the TAU group. In addition, there was a trend (p=.07) for more participants with pressure to attend treatment in the TAU group.
Table 1.
Participant demographic and baseline characteristics by site and by treatment group
| Site A | Site B | Site C | Site D | Site Analysis | TAU | MET | Group Analysis | |
|---|---|---|---|---|---|---|---|---|
| N=61 | N=74 | N=55 | N=10 | X2 or F | N=98 | N=102 | X2 or t | |
| Age (Years) | 23.9 (5.0) | 27.9 (5.7) | 26.6 (4.1) | 25.9 (6.8) | 10.67** | 25.1 (4.8) | 27.3 (5.8) | 3.02** |
| Race/Ethnicity (%)+ | 20.53** | 3.85* | ||||||
| African-American | 39.7 | 54.4 | 0.0 | 55.6 | 31.1 | 38.1 | ||
| Caucasian | 56.9 | 44.1 | 15.4 | 33.3 | 46.7 | 33.0 | ||
| Hispanic | 1.7 | 1.5 | 65.4 | 11.1 | 14.4 | 24.7 | ||
| Other | 1.7 | 0.0 | 19.2 | 0.0 | 7.8 | 4.1 | ||
| Marital Status (%) | 13.53** | 0.52 | ||||||
| Married | 1.6 | 14.9 | 23.6 | 40.0 | 16.3 | 12.7 | ||
| Separated/Divorced | 18.0 | 18.9 | 10.9 | 0.0 | 15.3 | 15.7 | ||
| Not Married | 80.3 | 66.2 | 65.5 | 60.0 | 68.4 | 71.6 | ||
| Education (Years) | 11.7 (1.5) | 11.2 (1.8) | 11.7 (1.7) | 10.7 (2.4) | 1.58 | 11.2 (1.4) | 11.7 (1.9) | 2.33* |
| Employed Full/Part time (%) | 29.5 | 20.3 | 25.5 | 20.0 | 1.55 | 27.6 | 21.6 | 0.97 |
| Weeks Pregnant | 19.1 (7.5) | 23.0 (7.8) | 19.1 (7.3) | 14.9 (7.7) | 6.01** | 19.9 (7.5) | 20.7 (8.2) | 0.74 |
| Primary drug used (%) | ||||||||
| Alcohol | 11.5 | 6.8 | 10.9 | 30.0 | 1.05 | 11.2 | 9.8 | 0.11 |
| Cocaine | 8.2 | 33.8 | 21.8 | 50.0 | 12.72** | 17.3 | 29.4 | 4.05* |
| Marijuana | 65.6 | 21.6 | 7.3 | 20.0 | 51.06** | 37.8 | 24.5 | 4.10* |
| Opiates | 8.2 | 4.1 | 32.7 | 0.0 | 24.25** | 12.2 | 13.7 | 0.10 |
| Methamphetamine | 4.9 | 4.1 | 16.4 | 0.0 | 7.67* | 6.1 | 8.8 | 0.53 |
| Benzodiazepines | 1.6 | 1.4 | 0.0 | 0.0 | 0.85 | 1.0 | 1.0 | 0.00 |
| Other | 0.0 | 28.4 | 10.9 | 0.0 | 22.78** | 14.3 | 12.7 | 0.10 |
| Days of use (past 28) | 8.0 (10.8) | 6.7 (9.1) | 16.0 (12.4) | 6.0 (8.5) | 13.23** | 9.0 (10.9) | 10.2 (11.5) | 0.44 |
| Need methadone (%) | 1.6 | 2.7 | 40.0 | 0.0 | 48.84** | 12.2 | 12.7 | 0.01 |
| Pressure to attend treatment (%) | 23.0 | 24.3 | 1.8 | 20.0 | 13.08** | 22.4 | 12.7 | 3.26 |
Note. Where not specifically indicated, numbers represent means (standard deviations).
Comparisons are for Caucasian to minority participants.
p<.05
p<.01.
3.2 Study Retention
A total of 162 study participants (81%) completed the 4 week active study phase. Study completion rates did not differ significantly between the MET-PS (79.4%) and TAU (82.7%) treatment groups (X2=0.34, df=1, p>.05). Fifty-five percent of early terminations were participants who failed to attend the clinic visits and could not be reached, with no significant difference between the MET-PS (10/102, 10%) and TAU (11/98, 11%) groups (X2=0.11, df=1, p>.05). Eighteen percent of early terminations were participants who reported that they were unable to attend visits due to practical problems (e.g., issues with transportation, childcare, etc.), with no significant difference between the MET-PS (3/102, 3%) and TAU (4/98, 4%) groups (X2=0.19, df=1, p>.05). Sixteen percent of early terminations were participants who withdrew consent, with no significant difference between the MET-PS (5/102, 5%) and TAU (1/98, 1%) groups (X2=2.59, df=1, p>.05). Another 8% of terminations resulted from participants being incarcerated, with no significant difference between the MET-PS (3/102, 3%) and TAU (0/98, 0%) groups (X2=2.93, df=1, p>.05). Finally, 1 TAU participant reported that she did not need treatment and so discontinued study participation.
3.3 Treatment Fidelity and Discriminability
If MET-PS and TAU are distinguishable, and if MET-PS were implemented correctly, one would expect the MET-PS, compared to TAU, sessions to receive significantly higher ratings on the MET adherence and skill scales and to receive significantly lower ratings on the Anti-MET adherence and skill scales. Analysis of variance (ANOVA) was conducted for the adherence and skill ratings for the MET and General Counseling scales, with the natural log used for the adherence ratings on General Counseling to make the data more normal. A Cox model was used for the adherence and skill ratings on the Anti-MET scale due to the extreme non-normality of the data. These analyses revealed that the MET-PS, compared to the TAU, sessions were rated significantly higher on the MET adherence (F=59.13, df=1, p<.001) and skill (F= 37.94, df=1, p<.001) scales. The analysis also revealed that the TAU, compared to the MET-PS, sessions were rated significantly higher on the Anti-MET scales for both the adherence (F=25.08, df=1, p<.001) and skill (F=24.79, df=1, p<.001) scales, suggesting that elements antithetical to MET were less likely to occur in the MET-PS compared to the TAU sessions. A significant site by treatment effect was found for Anti-MET skill; post hoc tests revealed that this significant effect suggests that, overall, MET-PS clinicians had less skill on the Anti-MET scale compared to TAU clinicians but that this difference was diminished at Site B. Somewhat surprisingly, the TAU sessions, compared to the MET-PS sessions, were rated more highly on the adherence (F=11.64, df=1, p<.001) and skill (F=9.89, df=1, p<.01) scales for general counseling.
3.4 Treatment Utilization
3.4.1 Ratio of attended to scheduled hours
The primary outcome analysis, which did not include baseline covariates or site effects, revealed no significant Treatment effect (X2=0.52, df=1, p>.05) on the proportion of scheduled hours attended during the first month of treatment. As can be seen in Table 2, the participants, on average, attended approximately 62% of their scheduled treatment hours, which translates into an average of 9.0 hours (SD=11.4) attended for TAU and 6.2 hours (SD=10.9) for the MET-PS participants. The exploratory analysis, which included baseline covariates (see section 2.8) and site in the model, revealed no significant Treatment effects, including Site × Treatment interaction effects.
Table 2.
Treatment Utilization as a function of Treatment Group and Site
| Site A | Site B | Site C | Site D | All Sites | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAU | MET | TAU | MET | TAU | MET | TAU | MET | TAU | MET | |||||||||||
| n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | |
| Scheduled hours attended (%) | 30 | 64% | 30 | 75% | 36 | 63% | 38 | 64% | 26 | 61% | 26 | 47% | 5 | 63% | 3 | 62% | 97 | 62% | 97 | 62% |
| Days to drop out while pregnant | 30 | 53.2 (34.8) |
31 | 52.3 (33.5) |
36 | 44.2 (33.8) |
38 | 49.7 (34.7) |
27 | 64.6 (43.3) |
28 | 41.3 (31.4) |
5 | 65.8 (47.4) |
5 | 36.8 (45.3) |
98 | 53.7 (38.0) |
102 | 47.5 (33.8) |
| Treatment weeks attended | ||||||||||||||||||||
| First month | 30 | 3.1 (0.8) |
31 | 2.8 (1.1) |
36 | 2.3 (1.4) |
38 | 2.3 (1.2) |
27 | 2.9 (1.5) |
28 | 2.2 (1.4) |
5 | 2.6 (1.1) |
5 | 1.0 (1.4) |
98 | 2.7 (1.3) |
102 | 2.4 (1.3) |
| Three Month Follow-up | 19 | 6.2 (3.7) |
25 | 4.6 (3.6) |
29 | 5.1 (2.8) |
29 | 5.2 (3.5) |
23 | 7.0 (4.3) |
18 | 5.3 (4.3) |
4 | 6.3 (3.8) |
3 | 5.7 (4.0) |
75 | 6.0 (3.6) |
75 | 5.0 (3.7) |
Note. Where not specifically indicated, numbers represent means (standard deviations).
3.4.2 Days until Treatment Drop Out
The analysis of the number of days until drop-out while the participants were still pregnant revealed a non-significant Treatment effect (X2=0.22, df=1, p>.05) and a significant Site × Treatment interaction effect (X2=10.15, df=2, p<.05). Contrast analyses revealed that Site C, at which the MET-PS participants dropped out of treatment more quickly than the TAU participants, differed significantly from Sites A (X2=5.20, df=1, p<.05) and B (X2=9.51, df=1, p<.01), at which this effect was not seen. An analysis of each site separately revealed no significant Treatment effect for either Sites A (X2=0.53, df=1, p>.05) or B (X2=2.21, df=1, p>.05) but did reveal a significant Treatment effect for Site C (X2=6.6, df=1, p<.05), suggesting that MET-PS participants dropped out of treatment significantly earlier at this site compared to TAU participants. The differential in days to drop-out between MET-PS and TAU, as a function of site, can be seen in Table 2.
3.4.3 Number of Weeks in which treatment attended
A secondary measure of treatment utilization was the number of weeks in which at least 1 treatment session was attended during the first month of treatment and during the entire 4-month study period. The analyses revealed no significant Treatment effects, including Site by Treatment interaction effects, for the first month of treatment or for the entire 4 month treatment period. As can be seen in Table 2, the MET-PS participants attended an average of 2.4 weeks of treatment during the first month of treatment while the TAU participants attended an average of 2.7 weeks. During the 3 month follow-up period, the MET-PS participants attended an average of 5.0 weeks of treatment compared to an average of 6.0 weeks by TAU participants.
3.5 Substance Use
3.5.1 Urine Toxicology Results
The overall proportion of positive urine toxicology screens as a function of study phase, treatment group, and site can be seen in Table 3. The analysis of the urine toxicology results revealed a significant Treatment × Week × Site effect for the first month of treatment (X2=7.65, df=2, p<.05) and a marginally significant Treatment × Week × Site effect for the entire 4-month period (X2=5.50, df=2, p=0.064); contrasts revealed that this effect represented a significant difference between Sites A and C for both the first month of treatment (X2=6.11, df=1, p<.05) and the entire 4-month period (X2=5.43, df=1, p<.05), with MET-PS participants evidencing a greater decrease in the number of positive urine toxicology samples compared to TAU at Site A and the reverse true at site C. When this analysis was repeated for the evaluable sample, the results revealed an additional site effect: for the first month of treatment, Site A was significantly different than site B (X2=3.97, df=1, p<.05), with MET-PS participants evidencing a greater decrease in the number of positive urine toxicology samples compared to TAU at Site A, an effect not seen at site B at which MET-PS and TAU were more equivalent.
Table 3.
Substance use by Site and Treatment Group
| Site A | Site B | Site C | Site D | All | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAU | MET | TAU | MET | TAU | MET | TAU | MET | TAU | MET | |||||||||||
| n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | |
| Positive Urine Toxicology % | ||||||||||||||||||||
| Baseline | 27 | 51.9% | 30 | 46.7% | 33 | 30.3% | 38 | 29% | 26 | 50% | 27 | 33.3% | 5 | 20% | 5 | 0 | 91 | 41.8% | 100 | 34% |
| First month | 27 | 42.6% | 27 | 32% | 32 | 12.3% | 36 | 14.9% | 23 | 31.7% | 19 | 36.5% | 5 | 25% | 3 | 10% | 87 | 27.8% | 85 | 25.4% |
| Three Month Follow-up | 25 | 34.8% | 27 | 26.5% | 30 | 15.6% | 30 | 17% | 22 | 25% | 18 | 37.2% | 4 | 21.7% | 3 | 38.7% | 81 | 14.3% | 78 | 16.7% |
| Days of use+: Alcohol/Drug | ||||||||||||||||||||
| Baseline | 30 | 8.93 (11.13) |
31 | 7.03 (10.63) |
36 | 5.75 (8.78) |
38 | 7.66 (9.37) |
27 | 14.37 (12.29) |
28 | 17.57 (12.46) |
5 | 3.60 (4.39) |
5 | 8.4 (11.33) |
98 | 8.99 (10.94) |
102 | 10.23 (11.54) |
| First month | 30 | 1.77 (3.05) |
28 | 1.86 (3.39) |
34 | 0.71 (1.38) |
35 | 1.49 (2.76) |
25 | 13.52 (12.76) |
23 | 13.78 (12.87) |
5 | 0.40 (0.89) |
3 | 2.00 (1.73) |
94 | 4.44 (8.72) |
89 | 4.80 (8.74) |
| Three Month Follow-up | 28 | 2.70 (5.08) |
28 | 1.29 (2.97) |
33 | 1.51 (4.34) |
31 | 0.91 (2.44) |
24 | 12.21 (12.73) |
22 | 13.17 (12.97) |
4 | 0.00 (0.00) |
3 | 1.22 (1.26) |
89 | 4.70 (8.87) |
84 | 4.26 (8.73) |
Note. Where not specifically indicated, numbers represent means (standard deviations).
Average monthly days of use.
Within site analyses revealed no significant Treatment effects for either Sites A or B for either the first month of treatment or the entire 4 month study period. For Site C, there was a marginally significant Treatment by Week effect for the first month of treatment (Z=1.92, p=0.054) in the ITT sample, which was significant in the evaluable sample (Z=2.14, p<.03). For the entire 4 month study period, the analysis for Site C revealed a significant Treatment by Week effect (Z=2.28, p<.05), suggesting that MET-PS, compared to TAU, participants had a higher proportion of positive urine toxicology results.
3.5.2 Self-report of drug and alcohol use
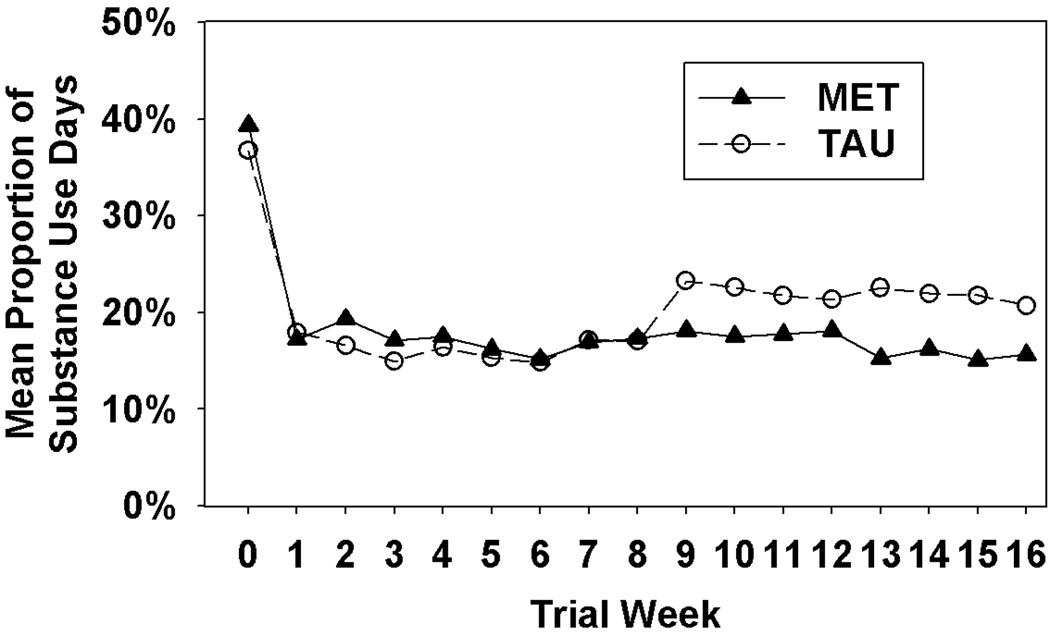
Analysis of the days of drug or alcohol use during the first month of treatment found no significant Treatment (Z=1.19, p>.05), Treatment × Week (Z=0.56, p>.05), or Site × Treatment × Week (X2=2.15, df=2, p>.05) effects but did reveal a significant Week effect (Z=−2.40, p<.05). Review of Figure 1 suggests that both groups evidenced a significant decrease in drug/alcohol use during the first month of treatment. To determine whether the significant Week effect could be explained by the drop-out of the non-completers, we re-ran the analysis including completion status as a covariate and the results were equivalent (i.e., the Week effect was Z=−2.23, p<.05). Analysis of the days of drug or alcohol use during the entire 4-month period revealed no significant Treatment (Z=1.11, p>.05), Treatment × Week (Z=−0.95, p>.05), Week (Z=0.02, p>.05), or Site × Treatment × Week (X2=0.68, df=2, p>.05) effects.
Figure 1.
Mean proportion of alcohol or illicit drug use days as a function of treatment group and study week.
3.6 Readiness to Change
Analysis of the URICA-derived Readiness score revealed a significant treatment effect (X2=5.77, df=1, p<.05). The mean change from baseline to end of treatment was −3.7 (SD=13.7) for TAU compared with 0.3 (SD=9.8) for MET-PS, indicating that the TAU group reported less readiness to change at the end of treatment, compared to the baseline, while the MET-PS group’s level of readiness did not change.
3.7 Minority Analyses
In accordance with NIH guidelines, we completed analyses to determine whether treatment was differentially effective for individuals from a minority group. Our sample size only allowed for analyses evaluating minority vs. non-minority status as opposed to analyses for particular minority groups. The minority analyses followed the same data analytic strategies as those outlined in section 2.8 with the exception that each model included Minority (minority vs. non-minority) and Minority by Treatment effects in the model.
3.7.1 Treatment Utilization
A Chi-square analysis revealed that minority, compared to non-minority, participants were significantly less likely to have received at least 1 MET-PS/TAU session and, thus, to be in the evaluable sample (X2=10.4, df=1, p<.01). Specifically 78.8% of minority participants included in the ITT sample were also in the evaluable sample compared to 95.1% of non-minority participants. This effect was largely due to Site C, which had 62% of minority participants in the evaluable sample compared to 100% of non-minority participants (X2=5.47, df=1, p<.05). Site B also had fewer minority (83%) compared to non-minority (94%) participants in the evaluable sample but this was not statistically significant (X2=2.08, df=1, p>.05). At Site A, 97% and 96% of non-minorities and minorities, respectively, were in the evaluable sample.
Analysis of the primary outcome measure, ratio of attended to scheduled hours, revealed no significant Minority or Minority by Treatment effects. The number of days until drop out while pregnant also revealed no significant Minority or Minority by Treatment effects. However, number of weeks in which treatment was attended revealed a significant Minority effect (X2=8.64, df=1, p<.01) when site was included in the model, with minority individuals attending significantly fewer weeks of treatment during the first 28 days of treatment; this effect was not significant when the entire 4 month period was included (X2=1.03, df=1, p>.05).
3.7.2 Substance Use
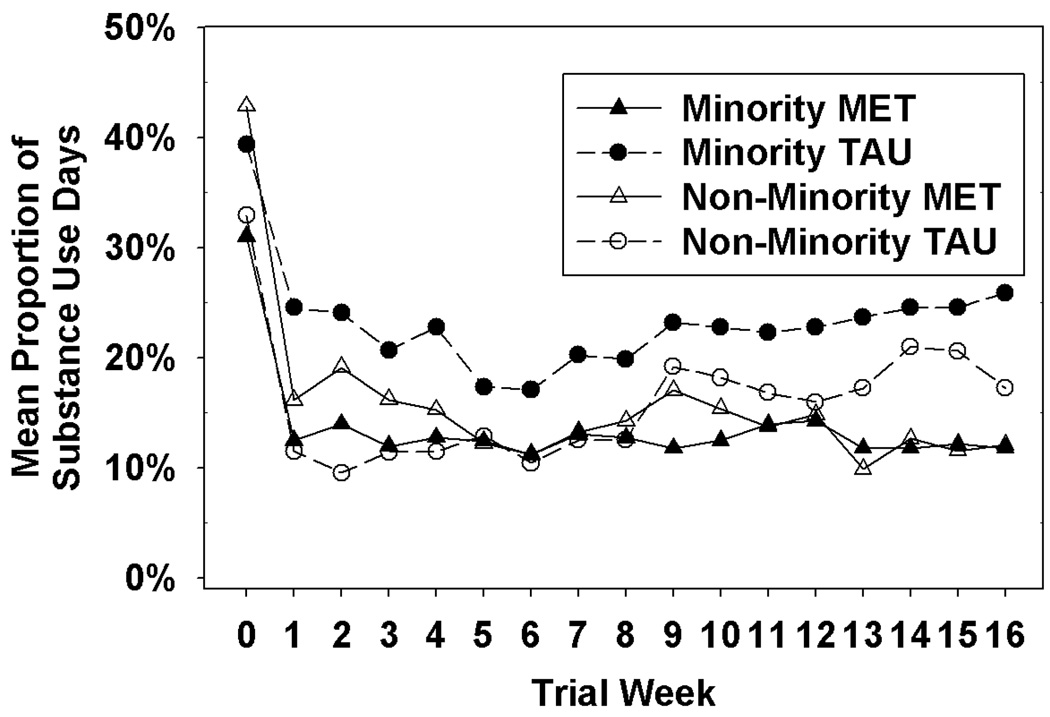
Analysis of the urine toxicology data revealed no significant Minority or Minority × Treatment effects. Analysis of self-report of alcohol or illicit drug use during the first month of treatment revealed no significant Minority or Minority × Treatment effects while self-report for the entire 4-month period revealed a significant Minority effect (Z=−2.08, p<.05) and a marginally significant Minority × Treatment effect (Z=1.96, p=0.0504) in ITT, which was significant in the evaluable sample (Z=2.24, p=.0253). As can be seen in Figure 2, which provides the data from the evaluable sample, minority participants in MET-PS reported a greater decrease in substance use compared to minority participants in the TAU condition.
Figure 2.
Mean proportion of alcohol or illicit drug use days as a function of minority status, treatment group, and study week
3.7.3 Readiness to Change
Analysis of the URICA readiness data revealed a significant Minority by Treatment effect (X2=5.99, df=1, p<.05). Review of the graph (data not shown) suggests that this effect was primarily due to non-minority TAU, compared to MET-PS, participants reporting less readiness to change at the end of treatment, compared to baseline, while this effect was not evident for minority participants.
4. Discussion
This randomized controlled trial of MET for pregnant substance users revealed no evidence that MET-PS was more effective than TAU in increasing treatment utilization or decreasing substance use in the study sample as a whole. The failure to find an effect for MET-PS on treatment utilization is consistent with a study conducted by Jones et al. (2004) in which MI plus behavioral incentives had minimal effect in increasing treatment retention in substance using pregnant women. The failure to find an effect on substance use is inconsistent with the findings from a pilot study with pregnant alcohol drinkers which found that a 1 hour MI session, compared to letters informing participants of the risk of alcohol use during pregnancy, significantly lowered self-reported levels of intoxication in the subgroup of women who were using alcohol most heavily at baseline (Handmaker et al., 1999). There is a growing literature to suggest that the benefits of MET for primary drug abusers in substance abuse treatment might be more inconsistently observed compared to the benefits of MET for primary alcohol abusers (Ball et al., 2007). While the Handmaker et al. (1999) study included all alcohol users, the present trial included only 21 participants (i.e., 10.5% of the study sample) who reported alcohol as the primary drug of choice; this difference in the two study samples could account for the differences in the study findings. Moreover, the control condition included in the present trial was much more intensive than the control utilized by Handmaker et al. (1999), which could also account for the discrepant findings.
Alternatively, the failure to find an effect for MET-PS in the sample as a whole might have stemmed from several other possible causes. First, the present study was conducted in community treatment programs with clinic staff serving as the interventionists, which raises the question of whether the clinicians were able to master the MET-PS intervention to the extent to which MET-PS sessions were discriminable from TAU sessions. An analysis of the independent tape ratings revealed that the sessions were, indeed, discriminable, with the TAU sessions rated significantly higher on the use of techniques antithetical to MET and the MET-PS sessions rated significantly higher on the use of MET techniques. In addition, the finding that the MET-PS participants did not experience the decrease in readiness to change evidenced by the TAU participants suggests that at least one of the therapeutic effects of MET-PS, promoting readiness to change, was operational. Another possible explanation for the lack of a MET-PS effect is the fact that we modified the MET manual used in an earlier MET trial for use with pregnant substance users. This included the addition of discussions of the participant’s feelings about her pregnancy, the provision of information about how substance use impacts pregnancy outcomes, and information about the degree to which the participant was engaging in healthy pregnancy behaviors (e.g., exercise, proper diet, etc.) as part of the personal feedback report. The modified content might have made the MET intervention less effective. However, research suggests that adherence to the basic principles of MET (e.g., being non-judgmental, asking open-ended questions, reflective listening etc.) is more important than particular content in improving patient outcome (Moyers et al., 2005) and so this is unlikely to explain the lack of effect.
The lack of a MET-PS effect might also have been due to a difference between the relative counseling skills of the MET-PS and TAU clinicians. Specifically, an analysis of the independent tape ratings revealed that the TAU sessions, compared to the MET-PS sessions, were rated higher for both adherence and skill for items assessing general counseling techniques. Finally, it is possible that MET-PS is simply not more effective than treatment as usual (TAU) in increasing treatment utilization and decreasing substance use in pregnant substance users. It should be noted that both the MET-PS and TAU participants reported significant decreases in alcohol/drug use during the first month of treatment. This suggests that the treatment as usual offered by the participating sites in this study was effective in decreasing substance use; MET-PS might not have been able to evidence efficacy beyond that evidenced by TAU. Still, it is important to note that TAU, as studied, likely differs from TAU as normally conducted in community treatment programs. First, one of the sites participating in this trial actually modified its normal treatment to include 2 weekly individual counseling sessions that normally would not have been provided. Second, the assessments required for a research study, as well as the additional attention and follow-up that both the clinic and study staff devoted to the study participants, represent an enhancement of TAU that might have increased its efficacy.
The study results did reveal several significant Site by Treatment interaction effects but, since this trial was not powered to compare MET-PS to TAU within site, it is difficult to determine the importance of the Site by Treatment interactions observed. In addition, 3 sites had a sufficient sample size to be included in the analyses of site effects with one site typically revealing a trend towards better outcomes for MET-PS, one with better outcomes for TAU, and one varying between trends toward better outcomes for MET-PS or TAU depending upon the outcome variable. Given this pattern of results, it is difficult to speculate about what site characteristics, if any, might have contributed to the results observed. The finding of site effects is consistent with a CTN multisite study of MET, which also found evidence of site effects (Ball et al., 2007).
An evaluation of the impact of minority status on outcome revealed that minority participants were less likely to receive an initial MET-PS/TAU session and attended fewer weeks of treatment during the initial month of treatment, compared to non-minorities. This finding, which suggests that minority pregnant substance users might be particularly susceptible to being lost early in treatment, is consistent with that of a trial with cocaine dependent individuals, which found that African-American participants were more likely to drop out after the intake visit compared to Caucasians (Siqueland et al., 2002) and with a retrospective chart review of women in treatment finding that African American women were more likely to drop out of treatment (Scott-Lennox et al., 2000). The minority analyses also revealed a significant Minority by Treatment interaction effect for self-report of alcohol/drug use, with minority participants in MET-PS reporting a greater decrease in use compared to minority participants in the TAU group. This finding is consistent with the results of a meta-analysis of MI studies which found larger effect sizes for MI in ethnic minority populations (Hettema et al., 2005). If the present finding is replicated in a future trial it might be of interest for culturally-based treatment approaches for pregnant substance users.
The present study had several strengths, including the use of a randomized controlled study design, which is the gold standard for clinical trials. In addition, this study included an unusually large number of randomized participants for the pregnant substance using population and, to our knowledge, is the largest randomized clinical trial with this population to date. Third, this study was conducted in the “real world” settings of substance abuse community treatment programs in which the clinic patients were the study participants and clinic staff members were the clinicians, and, thus, the results of this trial should be generalizable to other community treatment programs. In addition, the check on treatment discriminability suggests that the clinic staff randomized to MET-PS were able to implement MET-PS to the degree that it was readily and significantly discriminable from TAU. The present study also had several limitations. First, the MET-PS and TAU groups were not balanced on several potentially important baseline characteristics. This might have been due to problems with the urn randomization programs used at the sites, although a check on program functioning during the course of the trial indicated that the programs were operating within expected parameters. It might have been that our attempt to balance on 3 factors resulted in an imbalance on other factors. While we attempted to correct for the imbalances statistically by including the variables as covariates, the imbalance might have impacted the results in a mannerthat could not be accounted for statistically. A second limitation is that, despite attempts to have participants receive their first session of MET-PS/TAU as soon as possible, only 85.5% of participants received at least 1 MET-PS or TAU session.
In conclusion, the results of this trial suggest that MET for pregnant substance users was not more effective than TAU in increasing treatment utilization or decreasing substance use in the study sample as a whole. There was some evidence that the effect of MET-PS did vary between sites, but, given the relatively small sample sizes within sites and the overall small number of sites, it is difficult to speculate as to the import of these findings. Finally, there was some indication that MET-PS might have been more effective than TAU in decreasing self-reported use of alcohol and illicit drugs in minority, compared to non-minority, participants; this finding would need to be replicated in order to establish its importance.
Acknowledgments
This study was supported by a series of grants from NIDA as part of the Cooperative Agreement on National Drug Abuse Treatment Clinical Trials Network (CTN) in the Ohio Valley Node (U10DA013732), the North Carolina Node (U10DA013711), and the Southwest Node (U10DA015833). The authors wish to acknowledge the valuable contributions made to this project by the faculty and staff at the study sites: Jefferson Alcohol and Drug Abuse Center, Louisville, KY; Midtown Community Mental Health Center, Indianapolis, IN; Horizons, Chapel Hill, NC; and Milagro Clinic, Albuquerque, NM. In particular we wish to thank the following for their work and dedication to the study: Primary Research Staff - Stephanie Kapp, Ann Whetzel Nevar, Mindi Kirklin, Tracey Vann, Lydia Montoya; Study Clinicians - Stephania Lovell, Nicholas Johnson, Irene McCluskey, Stacee Barney, Rhonda Battles, Marissa Gordon, Angela Short, Janvier Snead, Debra Wells, Karen Cummings, Addie Ferguson, Karen Hagan, LaTonda Camp, Kiarni Hall-Blakemore, Karen Spetz, Beverly Allen, Marcia Ware, Kendall Brown, Veronica Hill, Sandra Garriott-Stejskal, Michael Brennan, Ani Bisono, Rebecca Frock, Patricia Lake, Madelaine Pinkerton, Sylvia Price, Shirley Cherino; MET Supervisors - Steven Ondersma, Doug Polcin; Node Staff - Tamara Dowd Owens, Emily DeGarmo, Rebecca DeFevers. We also thank William R. Miller for his helpful comments on the manuscript. A portion of this report was presented at the American Psychological Association August 2007 Convention in San Francisco, California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball SA, Martino S, Nich C, Frankforter TL, Van Horn D, Crits-Christoph P, et al. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. Journal of Consulting and Clinical Psychology. 2007;75(4):556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Ball SA, Nich C, Martino S, Frankorter TL, Farentinos C, Kunkel LE, Mikulich-Gilbertson SK, Morgenstern J, Obert JL, Polcin D, Snead N, Woody GE. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Dependence. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Libby B, Sheehan J, Hyland N. Motivational interviewing to enhance treatment initiation in substance abusers: an effectiveness study. American Journal on Addictions. 2001;10(4):335–339. doi: 10.1080/aja.10.4.335.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Rosengren DB, Downey L, Cox GC, Sloan KL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96:1149–1160. doi: 10.1046/j.1360-0443.2001.96811498.x. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Haller D, Knisely J, Elswick R, Dawson K, Schnoll S. Perinatal substance abusers: factors influencing retention. Journal of Substance Abuse Treatment. 1997;14:513–519. doi: 10.1016/s0740-5472(97)00119-0. [DOI] [PubMed] [Google Scholar]
- Handmaker NS, Miller WR, Manicke M. Findings of a pilot study of motivational interviewing with pregnant drinkers. Journal of Studies on Alcohol. 1999;60:285–287. doi: 10.15288/jsa.1999.60.285. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Howard J, Beckwith L. Issues in subject recruitment and retention with pregnant and parenting substance-abusing women. In: Rahdert E, editor. Treatment for drug exposed women and their children: Advances in research methodology. 1996. pp. 52–67. NIDA Research Monograph No. 165, DHHS Publication No. 96-3632. [PubMed] [Google Scholar]
- Howell E, Heiser N, Harrington M. A review of recent findings on substance abuse treatment for pregnant women. Journal of Substance Abuse Treatment. 1999;16:195–219. doi: 10.1016/s0740-5472(98)00032-4. [DOI] [PubMed] [Google Scholar]
- Jones HE, Svikis D, Rosado J, Tuten M, Kulstad JL. What if they do not want treatment?: lessons learned from intervention studies of non-treatment-seeking, drug-using pregnant women. American Journal on Addictions. 2004;13(4):347–357. doi: 10.1080/10550490490483008. [DOI] [PubMed] [Google Scholar]
- Jones HE, Svikis DS, Tran G. Patient compliance and maternal/infant outcomes in pregnant drug-using women. Substance Use and Misuse. 2002;37(11):1411–1422. doi: 10.1081/ja-120014084. [DOI] [PubMed] [Google Scholar]
- Lamb S, Greenlick MR, McCarty D, editors. Bridging the gap between practice and research, forging partnerships with community-based drug and alcohol treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- Martino S, Carroll KM, O’Malley SS, Rounsaville BJ. Motivational interviewing with psychiatrically ill substance abusing patients. American Journal on Addictions. 2000;9:88–91. doi: 10.1080/10550490050172263. [DOI] [PubMed] [Google Scholar]
- McMurtrie C, Rosenberg KD, Kerker BD, Kan J, Graham EH. A Unique Drug Treatment Program for Pregnant and Postpartum Substance-Using Women in New York City: Results of a Pilot Project, 1990–1995. The American Journal of Drug and Alcohol Abuse. 1999;25(4):701–713. doi: 10.1081/ada-100101887. [DOI] [PubMed] [Google Scholar]
- Miller WR. SAMHSA Treatment Improvement Protocol Series Volume 35, DHHS Publication No. (SMA) 99-3354. Rockville, MD: Substance Abuse and Mental Health Services Administration Center for Substance Abuse Treatment; 1999. Enhancing motivation for change in substance abuse treatment. (Consensus chair) [PubMed] [Google Scholar]
- Miller WR, Yahne CE, Tinigan JS. Motivational interviewing in drug abuse services: a randomized trial. Journal of Consulting and Clinical Psychology. 2003;71:754–763. doi: 10.1037/0022-006x.71.4.754. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford; 1991. [Google Scholar]
- Moyers TB, Miller WR, Hendrickson SM. How does motivational interviewing work? Therapist interpersonal skill predicts client involvement within motivational interviewing sessions. Journal of Consulting and Clinical Psychology. 2005;73:590–598. doi: 10.1037/0022-006X.73.4.590. [DOI] [PubMed] [Google Scholar]
- Pantalon MV, Nich C, Frankforter T, Carroll KM. The URICA as a measure of motivation to change among treatment-seeking individuals with concurrent alcohol and cocaine problems. Psychology of Addictive Behaviors. 2002;16:299–307. [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration), Office of Applied Studies. [Accessed on July 27, 2007];Substance Use During Pregnancy : 2002 and 2003 Update. 2005 June 2; ( http://www.oas.samhsa.gov/2k5/pregnancy/pregnancy.htm)
- Saunders B, Wilkinson C, Phillips M. The impact of a brief motivational intervention with opiate users attending a methadone programme. Addiction. 1995;90:415–424. doi: 10.1046/j.1360-0443.1995.90341510.x. [DOI] [PubMed] [Google Scholar]
- Scott-Lennox J, Rose R, Bohlig A, Lennox R. The impact of women's family status on completion of substance-abuse treatment. Journal of Behavioral Health Services Research. 2000;27:366–379. doi: 10.1007/BF02287819. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Simoneau G. Reducing resistance and denial by exercising ambivalence during the treatment of addiction. Journal of Substance Abuse Treatment. 2001;20(1):99–105. doi: 10.1016/s0740-5472(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–429. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop B, Gastfriend D, Lis J, Frank A, Griffin M, Blaine J, Luborsky L. Who starts treatment: engagement in the NIDA Collaborative Cocaine Treatment Study. American Journal on Addiction. 2002;11(1):10–23. doi: 10.1080/10550490252801602. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow back: A technique for assessing self-reported ethanol consumption. In: Allen J, Lit-ten R, editors. Techniques to Assess Alcohol Consumption. New Jersey: Humana Press, Inc.; 1992. pp. 19–28. [Google Scholar]
- Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. The Journal of Nervous and Mental Disease. 1999;187:630–635. doi: 10.1097/00005053-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Weisdorf T, Parran TV, Graham A, Snyder C. Comparison of pregnancy-specific interventions to a traditional treatment program for cocaine-addicted pregnant women. Journal of Substance Abuse Treatment. 1999;16:39–45. doi: 10.1016/s0740-5472(98)00006-3. [DOI] [PubMed] [Google Scholar]