Abstract
There is increasing evidence demonstrating the role of the cerebral cortex in human postural control. Modulation of EEG both in voltage and frequency domains has been observed preceding and following self-paced postural movements and those induced by external perturbations. The current study set out to provide additional evidence regarding the role of cerebral cortex in human postural control by specifically examining modulation of EEG as a function of postural sway direction. Twelve neurologically normal subjects were instructed to produce self-paced voluntary postural sways in the anterior-posterior (AP) and medial-lateral (ML) directions. The center of pressure dynamics and EEG both in voltage and frequency domains were extracted by averaging and Morlet wavelet techniques, respectively. The amplitude of movement-related cortical potentials (MRCP) was significantly higher preceding ML sways. Also, time-frequency wavelet coefficients (TF) indicated differential modulation of EEG within alpha, beta and gamma bands as a function of voluntary postural sway direction. Thus, ML sway appear to be more difficult and energy demanding tasks than the AP sway as reflected in differential modulation of EEG. These results are discussed within the conceptual framework of differential patterns of brain activation as a result of postural task complexity.
Keywords: Human, Postural control, Electroencephalography
Traditionally, subcortical pathways, brainstem and spinal cord have been postulated as the core structures involved in human postural control [see 13 for review]. However, more recent studies suggest that cortical processing can also be involved in the initiation and regulation of postural responses. Several brain imaging studies have provided important insights into the cortical neurophysiology related to postural stability. For example, the presence of movement-related cortical potentials (MRCP) in the motor cortex preceding the onset of self-paced postural movement was observed in EEG studies by Saitou [21] and more recently by Slobounov [25]. Specifically, self-paced initiation of postural sway in the sagittal plane was preceded by slow negative DC shift, similar to MRCP accompanying voluntary limb movement. Also, a burst of 40 Hz gamma activity at frontal central areas preceded the initiation of compensatory postural adjustment when balance was challenged [25].
As an alternative strategy, a postural perturbation design has been implemented to induce EEG changes after perturbation (i.e., perturbation-evoked potentials [5, 1, 6, 14], and prior to perturbation [10]. It has been suggested that early (i.e. N1) perturbation-evoked brain responses (PER) may be due to somatosensory afferent input [5, 14] or may represent both somatosensory and vestibular information related to the perturbation [26]. Whereas, the later components of PER may be associated with sensorimotor processing related to the control of balance responses [14] or could possibly relate to specific cognitive events such as the allocation of attention towards novel stimuli or tasks demands. Although the details of this ongoing debate is beyond the scope of our current report, the growing line of recent research may provide an insight into how the human central nervous system (CNS) may process and integrate various sources of information to generate precise motor adjustments to control upright posture.
Several studies have examined the relative contribution of anterior-posterior (AP) versus medial-lateral (ML) postural sway in the regulation of quiet standing both in young adults, elderly and pathological subjects. Overall, AP sway has a higher magnitude than ML sway during spontaneous upright stance in normal young adults [4, 19]. Usually, AP sway has about twice the amplitude of ML sway [7]. This may be due to the fact that ML sway is controlled at the hip by a bigger group of muscles that produce stronger responses [15], whereas AP sway exerted by the smaller group of ankle muscles [4]. It was also reported that the disturbance torque in the ML direction is lower than in the AP direction and the amount of movement at the subtalar joint is restricted when compared with the talucrural joint mechanics that allow the movement in the AP direction [4].
Increased ML sway associated with high risk of falling was observed in young adults during standing on unstable base of support [19], in elderly subjects [18] and in children with cerebral palsy with postural stability deficits [4]. Moreover, patients with cerebellar deficits experienced greater ML sway when either proprioceptive or visual information was distorted [8]. More recently, medial-lateral postural sway has been shown to be larger in subjects suffering from acute lateral ankle sprains [16]. These findings support the notion that CNS may use different postural strategies to control the AP versus ML components of postural stability [28]. However, direct examination of this notion and the neural bases underlying control of voluntary postural sways in the AP versus ML directions has not been reported. Provided that: (a) there are modulations of EEG activity prior to initiation of postural movement similar to those accompanying voluntary limb movement [21, 25]; and (b) there is sensitivity of EEG potentials both in voltage and frequency domains towards directional properties of voluntary limb movement [17, 23, 20], it is feasible to hypothesize that there is differential modulation of cortical activity as a result of voluntary postural sway direction. This study was set-up to address this hypothesis.
Twelve normal volunteers participated in this study (8 males, 4 females aged 21 – 25 years old). Subjects stood upright on the force plate (AMTI, model SGA6-4 amplifier, 6 Channels) with their arms crossed on the chest. Subjects were instructed to have their feet placed apart comfortably on the force plate and produce three self-paced whole-body discrete postural tasks: (a) anterior-posterior (AP) sway; (b) medial-lateral sway to the right (ML-R); (c) medial-lateral sway to the left (ML-L). Subjects were instructed to sway as far as they could to the limits of their respective stability boundary at comfortable speed without moving their feet. Subjects were instructed to produce postural sways approximately once every 10 s. Subjects performed 60 postural sways in every direction per one session. There were 2 test sessions with a break of 10 min to ensure that at least 60 EEG artifact-free trials were acquired for extracting movement-related cortical potentials. Prior to data collection, subjects were provided with visual feedback of the Center of Pressure (COP) displacement while performing postural sways with special emphasis to produce the same amount of sway, predominantly at the ankle joint, regardless of its direction. No visual feedback was provided during actual experimental sessions. The force platform signals were used to compute the coordinates of the center of pressure (COP) trajectory in AP and ML directions. It should be noted that the peaks of posterior sway could not be analyzed because they were too small and inconsistent, both within and between subjects, relative to the other directions under study (forward, ML_L and ML_L). Therefore, in the following analysis only peaks of the anterior sway were considered. Nevertheless, due to the fact that the subjects were instructed to sway forward and back to the initial quiet stance, this action was refereed to as an anterior-posterior (AP) postural task.
The EEG was recorded with Ag/Ag electrodes using a Quik-Cap Electrode Helmet at 19 electrode sites: FP1, FP2, Fz, F3, F4, FCz, FC3, FC4, Cz, C3, C4, CP3, CP4, Pz, P3, P4, O1 and O2 according to international 10–20 system [12]. Linked earlobes served as reference and electrode impedances were kept below 5 KOhm. The signals were measured using a DC coupled SynAmps amplifier (NeuroScan Inc., El Paso, TX). The EEG signals were amplified (gain 1000, recording range set for +/− 55 mV) and band pass filtered in the DC to 100 Hz frequency range. The EEG data were sampled at 500Hz using separate 16-bit analog-to-digital converters for each channel.
EEG electrode DC shift was compensated for off-line by a fourth-order trend correction of each channel over the entire recording epoch (linear detrend option of NeuroScan’s 4.1. software). The baseline was derived from the average of the segment from 1500 to 1200 ms before the trigger point derived from the force plate, as sway initiated, for each channel. Each epoch was visually inspected and those containing artifacts were removed. At least 60 trials were averaged for each postural sway condition. The amplitude of motor-related cortical potentials (MRCP) was measured according to Jahanshahi and Hallett [11] at all of the above mentioned electrode sites representing the frontal, central and parietal cortical areas. For the time-frequency analysis of EEG, the Morlet wavelet was used as implemented via the Matlab wavelet toolbox. Specifically, continuous wavelet transform (CWT) was performed to track the dynamics of EEG power alterations within different frequency clusters approximated with the initiation of postural sway. The CWT is able to resolve both time and scale (frequency) events better than the short Fourier transform (STFT). In mathematics and signal processing, the continuous wavelet transform (CWT) of a function f is defined by:
| (1) |
;
Where τ represents translation, s represents scale which is related to frequency and ψ is the mother wavelet. z̅ is the complex conjugate of z. In this paper the mother wavelet is complex Morlet wavelet, as it has both good time and frequency accuracy. The time-frequency (TF) energy of EEG trials was averaged and the mean time-varying energy of sway induced EEG waveforms in 8–12, 14–24 and 30–50 Hz frequency bands across trials was computed. The absolute values of the wavelet coefficients (C) were plotted, and the clusters of dominant energy distribution within these frequency bands were noted. The scale space of the wavelet transform was windowed separately to cover various frequency bands, to increase the resolution and to stress between-sway direction differences. The major dependent variable for postural data (COP maximum displacement as a function of sway direction AP, ML-L, ML-R) and EEG data (in voltage, MRCP amplitude) and frequency, TF wavelet coefficients as a function of sway direction) were analyzed using within-subject repeated measures ANOVA with direction of sway (n=3) and electrode sites (n=19) as factors. To examine dynamics of EEG wavelet coefficients as a functions of postural task, we have conducted a 3-way ANOVA with direction of postural sway (AP, ML-R and ML_L), electrode sites combining 19 EEG channels into 4 regions of interest: frontal (FP1, FP2, Fz, F3, F4), frontal-central (FCz, FC3, FC4), central (C3, Cz. C4) and central-parietal-occipital (CP3, CP4, Pz, P4, P3, O1, O2) areas, and time relative to phases of sway (3 levels) as factors. The wavelet alpha and beta coefficients were grand averaged within 3 time windows [i.e. −1900 to−2100 ms; −900 to −1100 ms); −100 to +100 ms] and subjected to ANOVA. Whereas the dynamics of EEG wavelet coefficients within gamma frequency clusters was examined relative to peaks of the postural sway. The wavelet gamma coefficients were grand averaged within 3 time windows [i.e., −100 to +100 ms; −900 to −1100 ms; +900 to +1100 ms] and subjected to ANOVA.
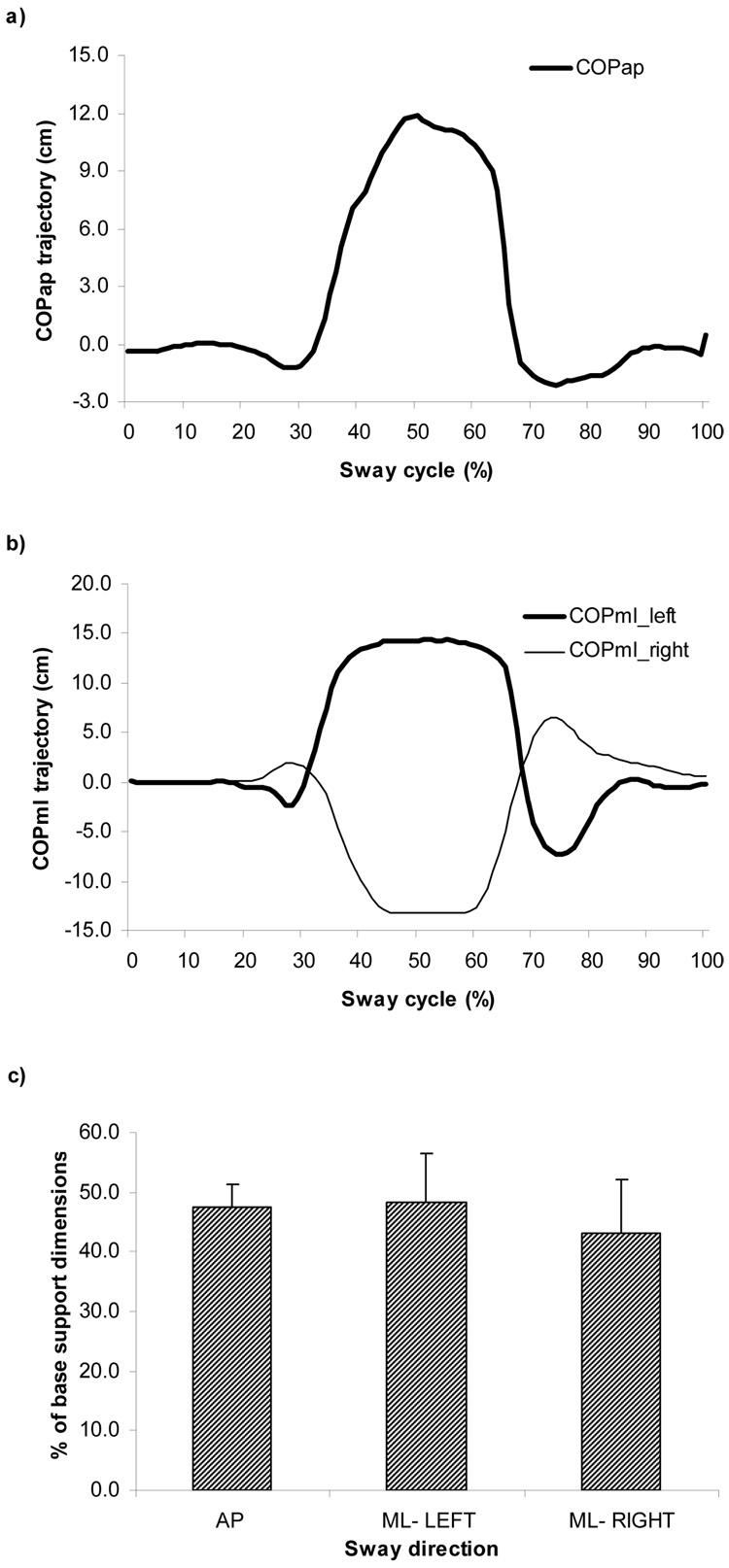
The ANOVA revealed that the main effect of COP displacement normalized among subjects and postural sway conditions (AP, ML-L and ML-R) was not significant, F (11, 22) = 2.345, p> .05. This indicates that subjects, indeed, did follow the instruction to produce postural sways with the same magnitude regardless of its direction. See Fig. 1c for details. Also, as can be seen from Fig 1.a & b, there were no differences between postural tasks in terms of duration of the sway (total sway time), F (11, 22) = 3.234, p > 05. Overall, as instructed, no discernable behavioral differences were observed, both in terms of time and magnitude, during the AP and ML voluntary postural sways.
Fig. 1.
Typical subject’s performance of the anterior-posterior (AP) and medial-lateral (ML) voluntary sways to the left and right directions (a & b). Both panels show the trajectories of COP along a time-normalized cycle. The position of the subject’s feet was measured accurately to define the dimensions of the base of support. The magnitude of the COP displacement was normalized with regard to dimensions of the base of support. For COP (AP), the peak was normalized by the largest length of the feet obtained by a straight line between the heel and the second toe. For COP (ML), the peak was normalized by the distance between the lateral boundaries of the feet. The maximal displacement (peak trajectory) of the COP with respect to the dimensions of base of support during AP and ML sways is shown in Fig.1c.
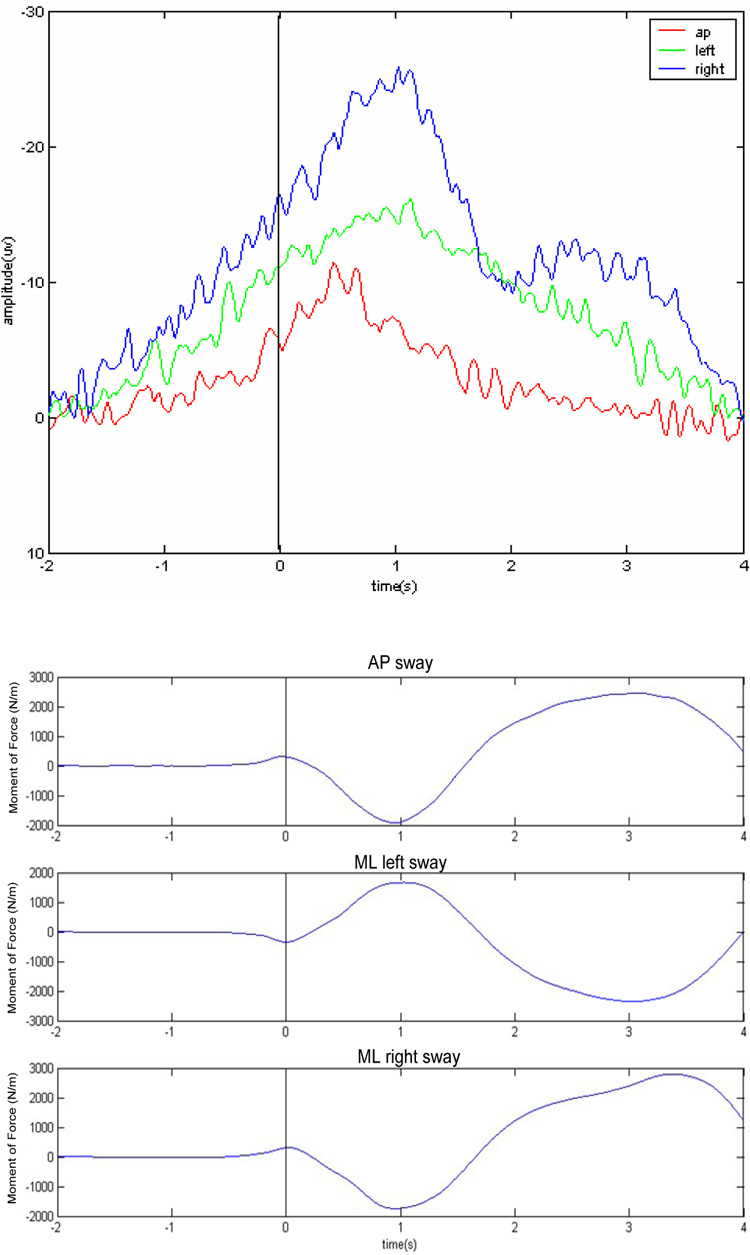
The major EEG finding from this study is that there was a differential modulation of cortical activity as a function of voluntary postural sway direction. First, in terms of EEG voltage, a well-pronounced DC negative shift approximately 1200+/−50 ms prior to initiation of intended postural sway was observed regardless of sway direction. However, peak amplitude of MRCP at initiation of intended sway was significantly higher prior to ML (15+/−4 µV ) than prior to AP (9+/−3 µV ) direction, F (11, 22) = 9.321, p< 001. Post-hoc Turkey test revealed no significant differences between ML-L and ML –R conditions (see also Fig.2). Moreover, there was a main effect of electrode site, F (2, 36) = 11.566, p <.001, suggesting that the most prominent differences were observed at frontal-central electrode sites with maximum values at Cz.
Fig. 2.
(Top) Grand averaged DC slow wave evolution at Cz electrode site preceding the onset of self-paced voluntary AP and ML sways. At least 60 trials were averaged to extract MRCP. Notice, the larger MRCP preceding the ML sways. (Bottom) Typical COP trajectories during AP and ML sways.
In terms of EEG in time-frequency domain, visually there was a well pronounced drop in power of alpha (8–12 Hz) and beta (14–25 Hz) activity just prior to initiation of sway. The alpha and beta power was localized centrally approximately 200 ms prior to its significant drop at sway initiation. Interestingly, there was a burst of 35 > Hz gamma activity localized centrally at the point of maximum deflection of the CP and initiation of sway back to initial position. In terms of alpha power, ANOVA revealed that the main effects of sway direction [F (2,286) = 3.36 p=0.036), electrode site, ROI [F (2,286) = 183.6, p<.0001] and time relative to phase of the sway [F (2,268) = 106.22, p< .0001) were significant as evidenced by TF wavelet coefficients. There was also a significant interaction, between RO1 and time relative to phase of the sway, F (4,286) = 44.4, p< .0001, and between direction of sway and ROI, F(4, 286)=10.83,p< .0001, overall suggesting that the amount of alpha power drop was significantly larger prior to ML sway predominantly at central electrode sites.
Similar significant effects were revealed by ANOVA considering the TF wavelet coefficients within beta power (p<.001), overall suggesting that the amount of beta power drop was significantly larger prior to initiation of ML sway at central electrode sites. Specifically, the main effects of sway direction [F (2,286) = 4.56 p<.001), electrode site, ROI [F (2,286) = 140.3, p<.001] and time relative to phase of the sway [F (2,268) = 86.22, p< .001) were significant. There was also a significant effect of interaction, between RO1 and time relative to phase of the sway, F (4,286) = 33.53, p< .001, and between direction of sway and ROI, F (4, 286) = 8.46, p< .001,
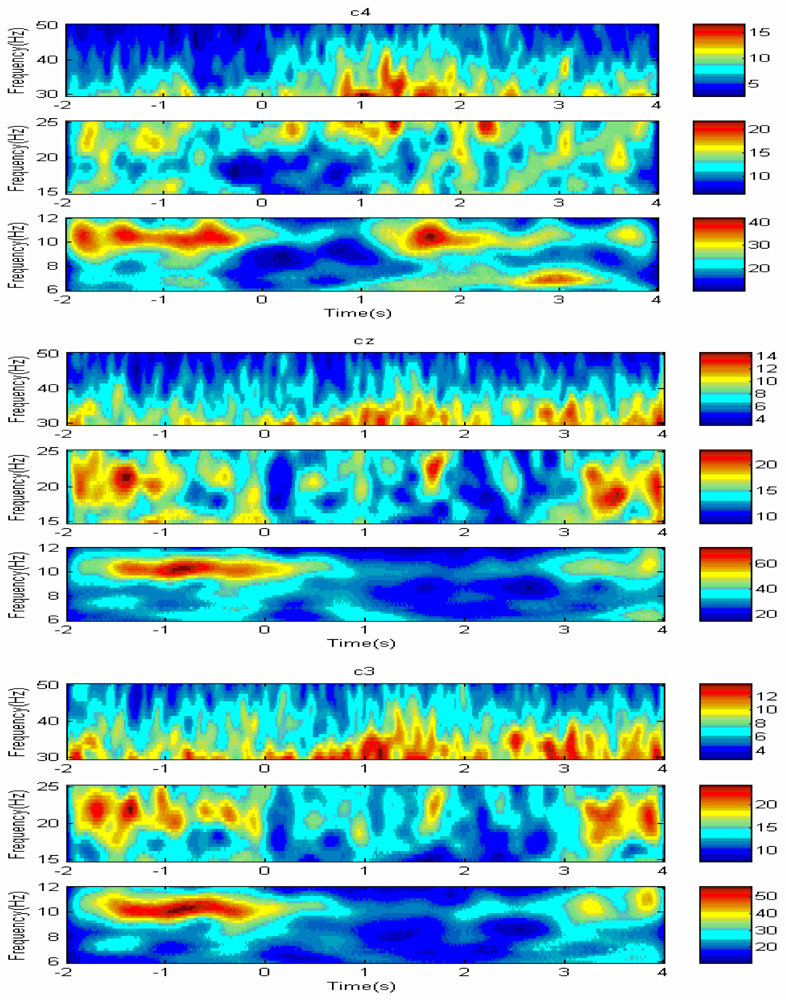
Similarly, 35> Hz gamma power burst was larger at maximum deflection of CP during ML conditions, as evidenced by significant main effect of direction of sway, F (2,286) = 7.58 p<.001, and effect of interaction between the direction of sway and time relative to phase of the sway, F (4, 286)= 46,34, p<.001. Moreover, the main effect of electrode site (ROI) was significant, F (2, 286) = 11.34, p> .001, as well as effect of interaction between RO1 and time relative to phase of the sway, F (4,286) = 21.77, p< .001, suggesting that the most pronounced changes in EEG power within gamma frequency band were observed at central electrode sites (C3, Cz, C4). An example of EEG wavelet maps associated with AP sway is shown in Fig.3.
Fig. 3.
Typical examples of EEG wavelet map at C3, Cz and C4 electrode sites within alpha (8–12 Hz), beta (14–25) and gamma (35> Hz) prior to initiation of AP sway.
There is an indication in the literature regarding independent control of ML versus AP components of postural sway during quiet upright standing [28]. A number of recent studies have also reported that increased ML but not AP component of spontaneous postural sway may cause postural instability in young adults performing more challenging tasks [19] as well as in a neurologically abnormal subjects [8, 4]. Considering these findings collectively, it is feasible to propose that increased “energetic demands” [3, 9] and/or “neural working load” [2] may be necessary to preserve balance in presence of enhanced magnitude of ML component of postural sway.
Clearly, modulation of EEG activity both in voltage (amplitude of MRCP) and time-frequency (power of wavelet TF coefficients within alpha, beta and gamma frequency bands) was more pronounced during ML than those preceding AP voluntary postural sway. Previous EEG research has documented increased MRCP as a function of motor tasks difficulty [17, 24]. Also, alpha power reduction (event-related desynchronization, ERD, reflecting energetic processes in the brain [9, 3] primarily at frontal-central areas due to increasing task complexity has been reported in a number of previous studies [3, 27]. In addition, the functional correlates of EEG gamma enhancement, initially defined as a sign of focused cortical arousal [22] and later on as an index of “neural working load” [2] which accompany challenging cognitive and motor tasks are now widely recognized. Thus, it is feasible to suggest that ML components of postural sway are more complex in terms of control as reflected in increased energetic demands [9] reflected in modulation of EEG patterns. Therefore, due to complexity of control, it is not surprising an increased ML sway associated with high risk of falling in young adults during standing on unstable base of support [19], in elderly subjects [18], in children with cerebral palsy with postural stability deficits [4], an in patients with cerebellar deficits [8].
To conclude, contrasting features of EEG patterns accompanying postural movement suggests that modulation of cortical activity during postural tasks is sway direction-dependent phenomenon. Further, modulation of EEG patterns in voltage (MRCP potentials) and time-frequency (wavelet time-frequency decomposition within alpha, beta and gamma bands) may serve as a neural basis underlying independent control of ML versus AP components of postural sway [28]. Our future research will focus on examining contrasting features of EEG patterns accompanying the AP and ML components of spontaneous versus voluntary postural sways.
Acknowledgments
This research was supported in part by NIH grant R01NS05622701A2 and the NINDS Intramural Program. We thank Alessander Danna dos Santos for his help in postural data collection and preliminary analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackerman H, Diener HC, Dichgans J. Mechanically evoked cerebral potentials and long-latency muscle responses in the evaluation of afferent and efferent long-loop pathways in humans. Neurosci Lett. 1986;66:233–238. doi: 10.1016/0304-3940(86)90024-8. [DOI] [PubMed] [Google Scholar]
- 2.Basar E, Basar-Eroglu C, Karaka S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Intern J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 3.Boiten F, Sergeant J, Geuze R. Event-related desynchronization: the effect of energetic and computational demands. Clin Neurophysiol. 1992;82:302–309. doi: 10.1016/0013-4694(92)90110-4. [DOI] [PubMed] [Google Scholar]
- 4.Bustamante Valles KD, Schneider JM, Long JT, Rieder SA, Johnson ML, Farris GF. Combined sagittal and coronal plane postural stability model; Proceedings of the 28th IEEE, EMBS Annual International Conference; NY, USA. 2006. Aug 30, [DOI] [PubMed] [Google Scholar]
- 5.Dietz V, Quintern J, Berger W. Corrective reactions to stumbling in man: functional signiticance of spinal and transcortical reflexes. Neurosci Lett. 1984;44:131–135. doi: 10.1016/0304-3940(84)90070-3. [DOI] [PubMed] [Google Scholar]
- 6.Dimitrov B, Gavrilenko T, Gatev P. Mechanically evoked cerebral potentials to sudden ankle dorsiflexion in human subjects during standing. Neurosci Lett. 1996;208:199–202. doi: 10.1016/0304-3940(96)12580-5. [DOI] [PubMed] [Google Scholar]
- 7.Gage WH, Winter DA, Frank JS, Adkin AL. Kinematic and kinetic validity of the inverted pendulum model in quiet standing. Gait Posture. 2004;19:124–132. doi: 10.1016/S0966-6362(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 8.Gatev P, Thomas S, Lou JS, Lim M, Hallett M. Effects of diminished and conflicting sensory information on balance in patients with cerebellar deficits. Mov. Disorders. 1996;11(6):654–664. doi: 10.1002/mds.870110610. [DOI] [PubMed] [Google Scholar]
- 9.Gevins A, Smith ME, McEvoy L, Yu DD. High resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing and practice. Cerebral Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs J, Fujiwara K, Tomita H, Furune N, Kunita K, Horak F. Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin Neurophys. 2008 doi: 10.1016/j.clinph.2008.02.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahanshahi M, Hallett M. The bereitschaftpotential: movement-related cortical potentials. NY: Kluger Academic Press/Plenum Press; 2003. [Google Scholar]
- 12.Jasper HH. The ten-twenty electrode system of the International Federation. EEG Clin Neurophysiol. 1958;11:371–375. [PubMed] [Google Scholar]
- 13.Horak FB, Macpherson JM. Balance orientation and equilibrium. In: Shepard J, Rowell L, editors. Handbook of physiology. Exercise: Regulation and integration of multiple systems, section 12, ch.7. NY: Oxford University Press; 1996. pp. 255–292. [Google Scholar]
- 14.Quant S, Adkin AL, Staines WR, McIIroy WE. Cortical activation following a balance disturbance. Exp Brain Res. 2004;155:393–400. doi: 10.1007/s00221-003-1744-6. [DOI] [PubMed] [Google Scholar]
- 15.Kapteyn T. Afterthought about the physics and mechanics of the postural sway. Aggressologie. 1973;14C:27–35. [PubMed] [Google Scholar]
- 16.Kernozek TW, Greany JF, Anderson DR, Van Heel D, Youngdahl RL, Benesh BG, Durall CJ. The effect of immersion cryotherapy on medial-lateral postural sway variability in individuals with a lateral ankle sprain. Physiother Res Int. 2008;30:1122–1133. doi: 10.1002/pri.393. [DOI] [PubMed] [Google Scholar]
- 17.Kristeva R, Chayne D, Lang W, Lindengen G, Deecke L. Movement-related potentials accompanying unilateral and bilateral finger movements with different inertial loads. EEG Clin Nerurophys. 1990;71:41–48. doi: 10.1016/0013-4694(90)90086-y. [DOI] [PubMed] [Google Scholar]
- 18.Maki B, Holliday P, Topper A. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki L, Duatre M, Amadio AC, Zatsiorsky VM, Latash M. Changes in postural sway and its functions in conditions of postural stability. J. Appl. Biomech. 2006;22:51–60. doi: 10.1123/jab.22.1.51. [DOI] [PubMed] [Google Scholar]
- 20.Pfurtscheller G, da Silva Lopes. Event-related EEG/MEG synchronization and desynchonization: basic principles. Clin Neurophysiol. 1999;100:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 21.Saitou K, Washimi Y, Koike Y, Takahashi A, Kaneoke Y. Slow negative cortical potentials preceding the onset of postural adjustment. EEG Clin Neurophysiol. 1996;110:499–455. doi: 10.1016/0013-4694(96)95004-x. [DOI] [PubMed] [Google Scholar]
- 22.Sheer DE. Focused arousal and 40 Hz-EEG. In: Knight RM, Bakker DJ, editors. The neuropsychology of learning disorders. Baltimore: Univ. Park Press; 1976. pp. 71–78. [Google Scholar]
- 23.Slobounov S, Simon R, Tutwiler R, Ray W. EEG correlates of wrist kinematics as revealed by averaging techniques and Morlet wavelet transforms. Motor Contr. 2000;4(3):350–372. doi: 10.1123/mcj.4.3.350. [DOI] [PubMed] [Google Scholar]
- 24.Slobounov S, Johnston J, Chiang H, Ray W. Movement-related EEG potentials are force or end-effector dependent: evidence from a multi-finger experiment. Clin Neurophysiol. 2002;113:1125–1135. doi: 10.1016/s1388-2457(02)00123-2. [DOI] [PubMed] [Google Scholar]
- 25.Slobounov S, Hallett M, Stanhope S, Shibasaki H. Role of cerebral cortex in human postural control: EEG study. Clin Neurophys. 2005;116:315–323. doi: 10.1016/j.clinph.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Staines RW, McIIroy WE, Brooke JD. Cortical representation of whole-body movement is modulated by propriorceptive discharge in humans. Exp Brain Res. 2001;138:235–242. doi: 10.1007/s002210100691. [DOI] [PubMed] [Google Scholar]
- 27.Van der Lubber R, Jaskowsli P, Verleger R. The influence of time pressure on cued finger movements; Psychophysiol. Abstr., 39 Ann Cong. Soc. Psychophysiol Res; Spain. 1999. [Google Scholar]
- 28.Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]