Abstract
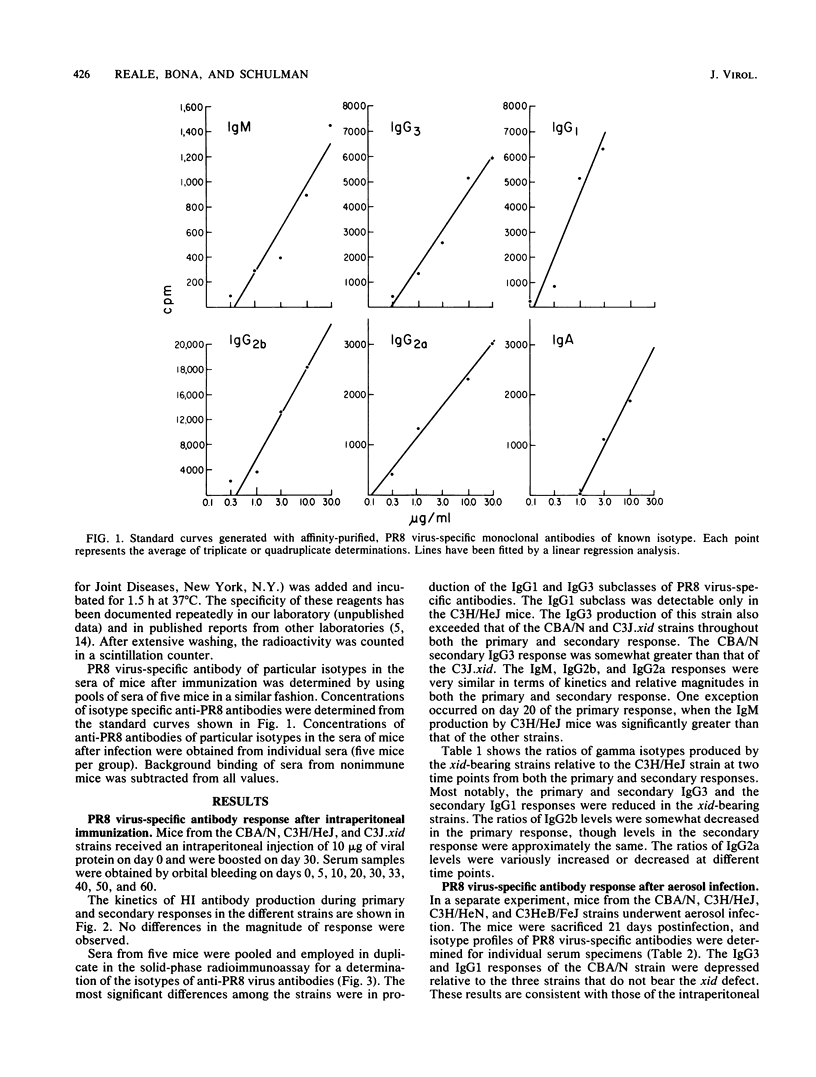
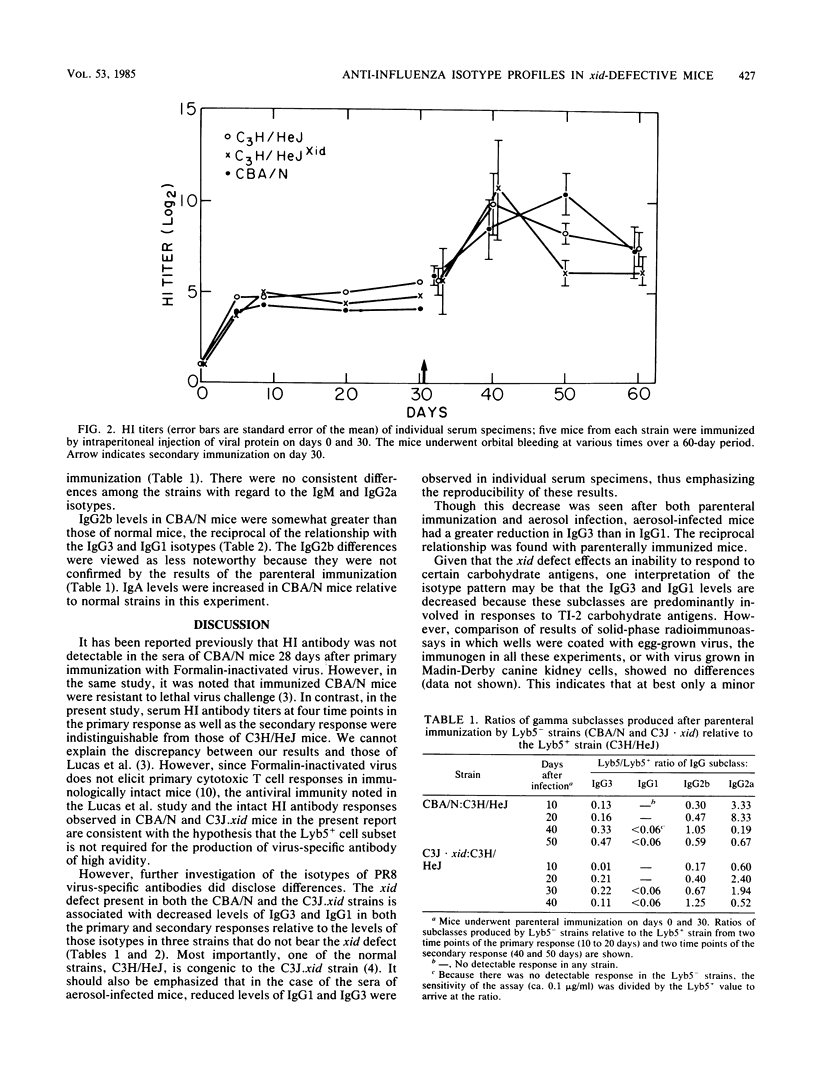
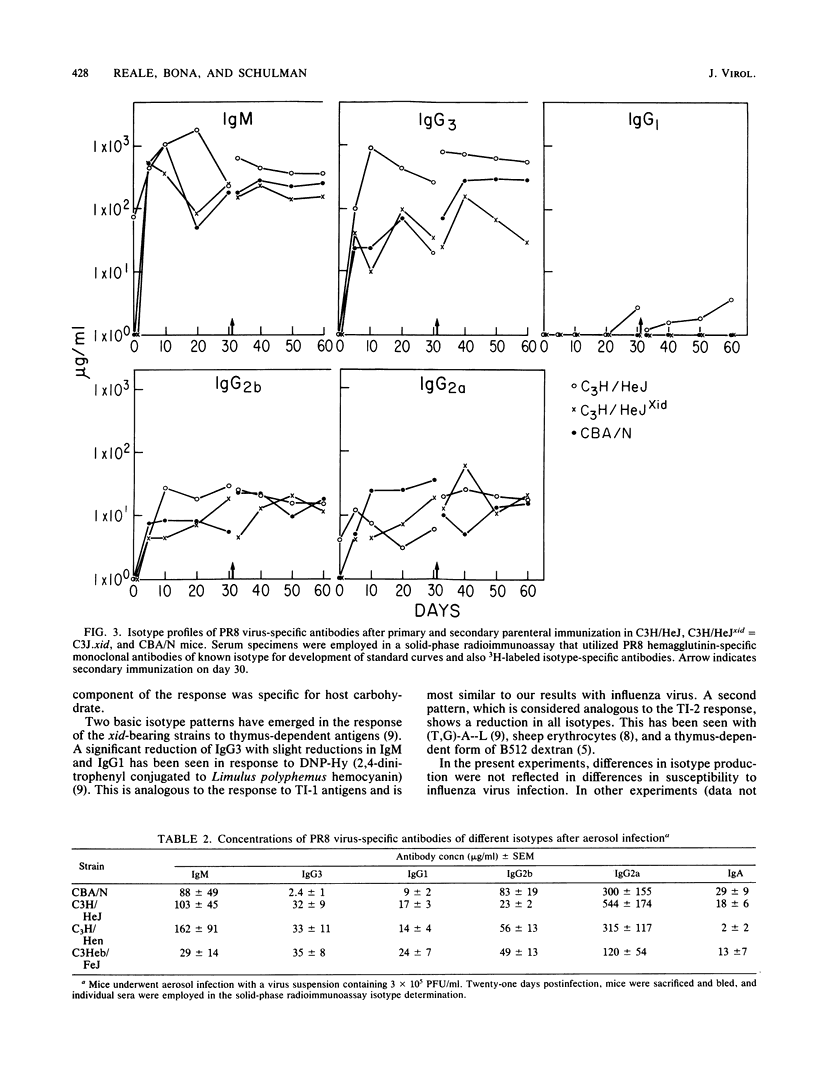
The humoral response to influenza A/PR8 virus was examined in the CBA/N and C3J.xid strains of mice, both of which bear an X-linked genetic defect (xid), and in strains lacking this defect. Hemagglutination-inhibiting antibody titers and measurement of virus-specific antibodies by solid-phase radioimmunoassay indicated that the xid defect does not impair the production of an adequate anti-influenza antibody response. However, investigation of the isotypes of PR8 virus-specific antibodies disclosed a relative decrease in the levels of IgG3 and IgG1 in the xid-bearing strains. This was observed after both intraperitoneal immunization and aerosol infection. The isotype differences were not reflected in the susceptibility of these strains to influenza virus infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bona C., Mond J. J., Paul W. E. Synergistic genetic defect in B-lymphocyte function. I. Defective responses to B-cell stimulants and their genetic basis. J Exp Med. 1980 Jan 1;151(1):224–234. doi: 10.1084/jem.151.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Smith A. H., Scher I., Paul W. E. Abnormal ratio of membrane immunoglobulin classes in mice with an X-linked B-lymphocyte defect. J Exp Med. 1975 Nov 1;142(5):1316–1321. doi: 10.1084/jem.142.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S. J., Barry D. W., Kind P. Antibody production and protection against influenza virus in immunodeficient mice. Infect Immun. 1978 Apr;20(1):115–119. doi: 10.1128/iai.20.1.115-119.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Norton G., Paul W. E., Scher I., Finkelman F. D., House S., Schaefer M., Mongini P. K., Hansen C., Bona C. Establishment of an inbred line of mice that express a synergistic immune defect precluding in vitro responses to type 1 and type 2 antigens, B cell mitogens, and a number of T cell-derived helper factors. J Exp Med. 1983 Nov 1;158(5):1401–1414. doi: 10.1084/jem.158.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini P. K., Stein K. E., Paul W. E. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J Exp Med. 1981 Jan 1;153(1):1–12. doi: 10.1084/jem.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Zitron I. M., Mond J. J., Ahmed A., Scher I., Paul W. E. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol Rev. 1977;37:89–104. doi: 10.1111/j.1600-065x.1977.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. Isolation and characterization of influenza virus recombinants with high and low neuraminidase activity. Use of 2-(3'-methoxyphenyl)-n-acetylneuraminic acid to identify cloned populations. Virology. 1974 Jan;57(1):227–237. doi: 10.1016/0042-6822(74)90123-8. [DOI] [PubMed] [Google Scholar]
- Phillips N. E., Campbell P. A. IgG subclass distribution of anti-sheep red blood cell plaque-forming cells in mice with the CBA/N defect. J Immunol. 1982 May;128(5):2319–2321. [PubMed] [Google Scholar]
- Press J. L. The CBA/N defect defines two classes of T cell-dependent antigens. J Immunol. 1981 Apr;126(4):1234–1240. [PubMed] [Google Scholar]
- Reiss C. S., Schulman J. L. Cellular immune responses of mice to influenza virus vaccines. J Immunol. 1980 Nov;125(5):2182–2188. [PubMed] [Google Scholar]
- Scher I., Ahmed A., Strong D. M., Steinberg A. D., Paul W. E. X-linked B-lymphocyte immune defect in CBA/HN mice. I. Studies of the function and composition of spleen cells. J Exp Med. 1975 Apr 1;141(4):788–803. [PMC free article] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Sieckmann D. G., Scher I., Asofsky R., Mosier D. E., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. II. A thymus-independent response by a mature subset of B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1628–1643. doi: 10.1084/jem.148.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Zopf D. A., Miller C. B., Johnson B. M., Mongini P. K., Ahmed A., Paul W. E. Immune response to a thymus-dependent form of B512 dextran requires the presence of Lyb-5+ lymphocytes. J Exp Med. 1983 Feb 1;157(2):657–666. doi: 10.1084/jem.157.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Kelly K., Largen M., Taylor B. A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978 Feb;120(2):422–424. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]



