Abstract
To explore rearrangements of the reentrant loop HP2 relative to transmembrane domains (TMs) 7 and 8 during transport by the glial glutamate transporter GLT-1/EAAT2, cysteine pairs were introduced at the extracellular ends of these structural elements. The pairs were introduced around 10–15Å “above” the residues, which make contact with substrate in the related archaeal homologue GltPh. Transport by the double mutants M449C/L466C (HP2/TM 8), L453C/I463C (HP2/TM 8), and I411C/I463C (TM 7/TM 8) was inhibited by copper(II)(1,10-phenanthroline)3 (CuPh) and by Cd2+. Inhibition was only observed when the two cysteines were present in the same construct, but not with the respective single cysteine mutants or when only one cysteine was paired with a mutation to another residue. Glutamate and potassium, both expected to increase the proportion of inward-facing transporters, significantly protected against the inhibition of transport activity of M449C/L466C by CuPh. The non-transportable analogues kainate and d, l-threo-β-benzyloxyaspartate are expected to stabilize an outward-facing conformation, but only the latter potentiated the effect of CuPh on M449C/L466C. However, both analogues increased the aqueous accessibility of the cysteines introduced at positions 449 and 466 to a membrane-impermeant sulfhydryl reagent. Inhibition of L453C/I463C by CuPh was protected not only by glutamate but also by the two analogues. In contrast, these ligands had very little effect on the inhibition of I411C/I463C by CuPh. Our results are consistent with the proposal that HP2 serves as the extracellular gate of the transporter and indicate that glutamate and the two analogues induce distinct conformations of HP2.
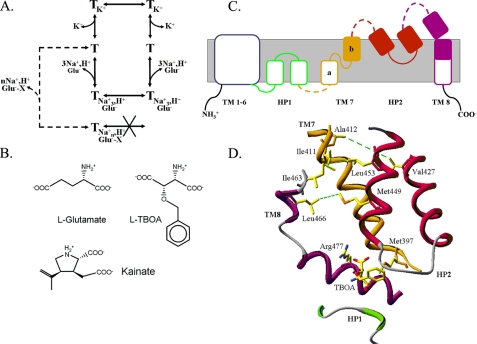
Many neurotransmitters are removed from the synaptic cleft by transporters, which thereby enable efficient synaptic transmission. Many of these transporters belong to the neurotransmitter:sodium:symporter (NSS) family and are sodium- and chloride-dependent. The notable exception is the small family of transporters of glutamate, the major excitatory neurotransmitter in the central nervous system. Glutamate transporters are essential in preventing hyperexcitability and neurotoxicity. Glutamate transport is an electrogenic process (1–3) in which the transmitter is cotransported with three sodium ions and one proton (4, 5), followed by the countertransport of one potassium ion (6–8). The mechanism, involving two half-cycles (Fig. 1A), is supported by the fact that mutants impaired in potassium interaction are locked in an obligatory exchange mode (8, 9). Under physiological conditions, the transporter accumulates the transmitter into the cell against its concentration gradient (1, 4, 5), but elevated external potassium causes reverse transport (6, 10). Thus, an increase in the proportion of transporters in the inward facing conformation, where the binding site is exposed to the cytoplasm, is expected in the presence of either high extracellular potassium or l-glutamate (Fig. 1A). On the other hand, addition of non-transportable glutamate analogues (Fig. 1B) is expected to stabilize an outward-facing conformation (where the binding site is exposed to the extracellular medium) of the transporter (Fig. 1A).
FIGURE 1.
Transport cycle and probable external access pathway of GLT-1. A, transport cycle of GLT-1 and other eukaryotic glutamate transporters. After binding of sodium, glutamate, and a proton from the extracellular medium (left), the outward-facing substrate-loaded translocation complex is formed. After the closing of the external gate and the opening of the internal one, the substrate and cotransported ions dissociate into the cytoplasm (right). Subsequently, intracellular potassium enters the binding pocket. After the internal gate closes the external gate opens, potassium is released into the extracellular medium, and the net influx cycle is completed. Net efflux, observed at elevated extracellular potassium concentrations, proceeds via reversal of the above steps and exchange involves reversible translocation via the glutamate half-cycle and does not require potassium. When a bulky non-transportable glutamate analogue (GluX) binds from the extracellular medium together with sodium (the stoichiometry (n) is unknown), the transporter becomes locked in the outward-facing conformation (dashed line) because transport is impossible. B, structures of l-glutamate and the non-transportable blockers l-TBOA and kainate. C, topology of GLT-1 by analogy to that of GltPh. TM helices 1–8 and hairpins (HP1 and HP2) are labeled. The parts of HP1, TM7 (7b), HP2, and TM8, shown in panel D, are filled or shown as solid lines (tips of HP1 and HP2). D, external access to the binding pocket, based on the TBOA-bound structure of GltPh. Residue numbers are those of its GLT-1 counterpart. Only one of the contact residues with the aspartate moiety, Arg-477, is shown for clarity, alongside with Met-397, which is close to the aromatic ring of TBOA. Also shown are HP2 (red), the extracellular parts of TM 7 (orange), and TM8 (purple), as well as the tip of HP1 (green). Connected by dashed green lines are those residues that are close to each other in the structure, as also evidenced by the inhibition of the paired double cysteine mutants by CuPh.
A crystal structure of the archaeal glutamate transporter homologue GltPh has been published (11). It forms a trimer with a permeation pathway through each of the monomers, indicating that the monomer is the functional unit. This is also the case for the eukaryotic glutamate transporters (12–15). The membrane topology of the monomer is quite unusual (11), but is in excellent agreement with the topology inferred from biochemical studies (16–18). It contains eight transmembrane domains (TM)2 and two oppositely oriented reentrant loops, HP1 and HP2 (11) (Fig. 1C). TMs 1–6 form the outer shell of the transporter monomer, whereas TMs 7 and 8 and the reentrant loops form the binding pocket of GltPh (11). Satisfactorily, the side-chains of most of the counterparts of the eukaryotic transporter residues, inferred to be important for the interaction of sodium (19, 20), potassium (8, 9), and glutamate (21, 22), are facing toward the binding pocket of GltPh (11). It has been suggested that reentrant loop HP2 may form the external gate of the transporter (11), and recent support for this idea has been obtained when the crystal structure of GltPh in complex with the non-transportable analogue d,l-threo-β-benzoylaspartate (TBOA) was solved (23). In the presence of sodium, the overall structure of GltPh was found to be similar in the aspartate-bound, TBOA-bound, and substrate-depleted structures, except that in the latter two, HP2 adopts an “open” conformation (23).
Here we use oxidative cross-linking of engineered cysteine pairs to explore the idea that HP2 moves relative to TMs 7 and 8 during transport in the eukaryotic transporters. Such cross-linking often results in the inhibition of transport (24–26). The inhibition may be due to restrictions imposed by the disulfide cross-link on the conformational changes, which the transporter undergoes during a transport cycle or may be the result of a steric barrier or another distortion introduced by the cross-link. We have introduced cysteine pairs at the extracellular ends of these structural elements of the glial glutamate transporter GLT-1 (27), also known as EAAT2 (28), at positions located around 10–15 Å “above” (closer to the extracellular medium; as opposed to “below”, closer to the cytoplasm) the residues that make contact with aspartate and TBOA in GltPh (23) (Fig. 1D). The double mutants were subjected to conditions of oxidative cross-linking in the presence and absence of transporter ligands. In parallel we have examined the effects of these ligands on the reactivity/accessibility of the individual cysteines to the membrane-impermeant sulfhydryl reagent MTSET.
EXPERIMENTAL PROCEDURES
Generation and Subcloning of Mutants—Mutations were made by site-directed mutagenesis of wild-type (WT) GLT-1 or its cysteine-less version (16) in the vector pBluescript SK(–) (Stratagene) using single-stranded uracil-containing DNA as described previously (29, 30). Briefly, the GLT-1-containing plasmid was used to transform Escherichia coli CJ236 (dut–, ung–). From one of the transformants, single-stranded uracil-containing DNA was isolated upon growth in uridine-containing medium, according to the standard protocol from Stratagene, using helper phage R408. This yields the sense strand, and consequently mutagenic primers were designed to be antisense. The mutants were subcloned into constructs containing either WT or cysteine-less GLT-1 in the vector pBluescript SK(–), using the unique restriction enzymes BsrGI or BsgI and XbaI. The coding and non-coding strands were sequenced between the above restriction sites.
Cell Growth and Expression—HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 200 units/ml penicillin, 200 μg/ml streptomycin, and 2 mm glutamine. Infection with recombinant vaccinia/T7 virus vTF7-3 (31) and subsequent transfection with plasmid DNA, as well as d-[3H]aspartate transport, was done as published previously (32). In all the experiments described, the expression vector was pBluescript SK(–).
Inhibition Studies with the Impermeant Sulfhydryl Reagent MTSET—Before transport measurements, cells adhering to 24-well plates were washed two times with 1 ml of the transport medium containing 150 mm choline chloride instead of NaCl. Each well was then incubated at room temperature with 200 μl of the preincubation medium (the different compositions are indicated in the figure legends) with the indicated concentrations of MTSET (Anathrace, Inc). After 5 min, the medium was aspirated, and the cells were washed twice with 1 ml of the transport solution, followed by d-[3H]aspartate transport. The concentration of MTSET chosen in the different experiments was determined after preliminary titration experiments, optimized not only according to the experimental conditions but also to the mutants used. This was necessary because some mutants are more sensitive than others. The MTSET concentrations used are given in the figure legends. Statistical evaluation of the inhibition of the different mutants by MTSET (or either CuPh or Cd2+) utilized a one-way analysis of variance with a post-hoc Dunnett multiple comparison test, where p < 0.05 or smaller was taken as significant. Results were plotted using normalized data for each mutant, where the untreated activity levels are normalized to 100%.
Inhibition by Copper(II)(1,10-Phenanthroline)3—This was basically done as described (24, 25). HeLa cells transfected with the indicated construct were washed once with choline solution (150 mm choline chloride, 5 mm KPi, pH 7.4, 0.5 mm MgSO4, and 0.3 mm CaCl2) and preincubated with the solutions of different compositions containing the indicated concentrations of CuPh. Again, the optimal concentration of CuPh for each double mutant was determined by preliminary titration experiments. The CuPh stock solution was prepared for each experiment by mixing 0.4 ml of 1.25 m 1,10-phenanthroline in water: ethanol (1:1) and 0.6 ml of 250 mm CuSO4.
Inhibition by Cd2+—HeLa cells transfected with the indicated construct were washed once with choline solution, and the cells were assayed for transport with the indicated concentrations of cadmium chloride in sodium solution (150 mm NaCl, 5 mm KPi, pH 7.4, 0.5 mm MgSO4, and 0.3 mm CaCl2) for 10 min at room temperature (24).
RESULTS
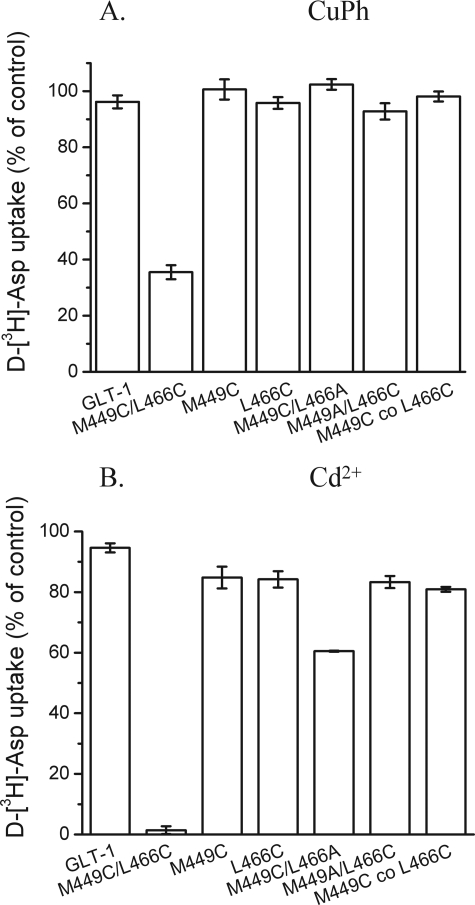
Effects of Thiol Cross-linking and Cd2+ on M449C/L466C-GLT-1—To identify positions in HP2 and TM8, which are potentially close to each other, we used the GltPh structure (11) and a model where the GltPh residues were replaced by their GLT-1 counterparts (using the SPDB Viewer). We identified several pairs of HP2 and TM8 residues that are close in space. Cysteine residues were introduced at these paired positions not only in the Cys-less GLT-1 construct, but also in WT-GLT-1. The latter was done because with the two cysteine pairs, identified by us prior to the publication of the GltPh structure (11, 23), transport activity was only observed when these pairs were introduced in WT (24). The same situation was observed with the M449C/L466C double mutant, which was devoid of activity in the Cys-less GLT-1 background, but exhibited measurable activity in the background of the WT transporter; 14.4 ± 0.6% of that of WT (n = 3). Surface biotinylation experiments showed that the lack of activity of M449C/L466C in the Cys-less GLT-1 background was due to defective maturation and a defective targeting of the double mutant transporter to the plasma membrane (data not shown).
According to the model based on the GltPh structure (11), the distance between the sulfhydryl groups introduced at these positions is 6.6 Å. When HeLa cells, expressing M449C/L466C, were preincubated with the oxidizing agent CuPh (10 μm), a significant inhibition of transport was observed (Fig. 2A). This inhibition was not seen with cells expressing either WT or the single cysteine mutants M449C and L466C (Fig. 2A), indicating that the inhibition of transport by oxidative cross-linking required a cysteine at both positions. No inhibition of transport by 10 μm CuPh was observed when only one cysteine was paired with a mutation to another residue, namely M449C/L466A and M449A/L466C (Fig. 2A). Therefore, it is unlikely that mutation of Met-449 or Leu-466 to a smaller residue has caused the cysteine introduced at either position to become closer to one of the endogenous cysteines of GLT-1. The inhibition by CuPh was only observed when the cysteines at positions 449 and 466 were present on the same polypeptide, but not when the two cysteines resided on two different polypeptides. This was demonstrated by the lack of inhibition by CuPh of transport in cells cotransfected with M449C and L466C (Fig. 2A). This suggests that the cysteines at positions 449 and 466 come into close proximity within the transporter monomer, but not at the interface of two transporter monomers. In contrast to previously characterized double cysteine mutants A412C/V427C (Fig. 1D) and A364C/S440C (tips of HP1 and HP2, respectively) (24), the inhibition of M449C/L466C by CuPh could not be reversed by subsequent incubation of the cells with 20 mm DTT (data not shown). This suggests that the disulfide bond gets occluded upon oxidation of the transporter by compensatory shifting of other parts of the protein. At present, it is not clear what the nature of the underlying conformational change is.
FIGURE 2.
Inhibition of transport of M449C/L466C by CuPh and Cd2+. A, HeLa cells expressing M449C/L466C or the indicated control mutants, all in the background of WT-GLT-1, were preincubated in NaCl-containing medium with 10 μm CuPh for 5 min at room temperature, washed twice with choline chloride-containing solution, and subsequently d-[3H]aspartate transport was assayed as described under “Experimental Procedures.” M449C co L466C represents HeLa cells cotransfected with each of these two constructs. The values shown represent the percentage of activity after treatment with 10 μm CuPh relative to that obtained after preincubation in the absence of CuPh. Values represent the mean ± S.E. of at least three separate experiments each done in triplicates. B, HeLa cells expressing the indicated mutants were washed once with choline chloride-containing solution and assayed for transport in the presence or absence of 500 μm cadmium chloride. Values shown are the percentage activity in the presence of 500 μm cadmium chloride relative to that in its absence. Values represent the mean ± S.E. of at least three separate experiments each done in triplicates.
Independent evidence for proximity of the two cysteines comes from their ability to form a Cd2+ binding site. This divalent cation interacts with cysteinyl side chains (33, 34), and the affinity of the interaction is dramatically increased when the Cd2+ can be coordinated by two cysteines (35). Inhibition of transport by 500 μm Cd2+ was observed with M449C/L466C, but not with WT, the single cysteine mutants or with the M449C/L466A and M449A/L466C double mutants (Fig. 2B). No inhibition of transport by Cd2+ was observed in HeLa cells cotransfected with M449C and L466C (Fig. 2B).
In contrast to treatment with Cd2+, the reaction with CuPh results in the formation of a covalent bond. Therefore it is possible to determine the effect of the external medium on the cross-linking during the pretreatment of the cells with CuPh. When during pretreatment of cells expressing M449C/L466C sodium was replaced by lithium or choline, there was not much change in the extent of inhibition by CuPh (Fig. 3A). However, when sodium was replaced by potassium, a condition expected to increase the proportion of inward-facing transporters (Fig. 1A), a marked reduction in the degree of inhibition by CuPh was observed (Fig. 3A). A similar protection was also observed with l-glutamate (1 mm) (Fig. 3A), another condition expected to increase the proportion of inward-facing transporters (Fig. 1A). The protection by l-glutamate was not seen in the absence of sodium (choline replacement; data not shown) and was not observed with GABA or glycine, which are not substrates of GLT-1 (data not shown). The non-transportable substrate analogues kainate and d,l-TBOA are both expected to increase the proportion of outward-facing transporters (Fig. 1A). Remarkably, these blockers had different effects on the inhibition of transport of M449C/L466C by CuPh; d,l-TBOA caused a clear potentiation of this inhibition, but kainate had no significant effect (Fig. 3A). On the other hand, not only d,l-TBOA, but also kainate, potentiated the inhibition of the M449C and of the L466C mutants by the membrane-impermeable sulfhydryl reagent MTSET, whereas l-glutamate had the opposite effect (Fig. 3, B and C). In these experiments the cysteine mutations were present in the background of Cys-less GLT-1, but similar results were also obtained when the mutations were present in the background of WT-GLT-1 (data not shown). No inhibition by MTSET was observed with the M449A or L466A mutants (data not shown). As compared with lithium and choline, sodium had a protective effect on the inhibition of M449C by MTSET, whereas potassium did not have much of an effect (Fig. 3B). The inhibition of L466C by MTSET was more pronounced in the presence of either sodium or lithium than in the presence of choline (Fig. 3C). Altogether the results presented in this section indicate that kainate does not simply change the accessibility of positions 449 and 466, but also induces a repositioning between them.
FIGURE 3.
Effect of the composition of the external medium on the inhibition of cysteine mutants at positions 449 and 466 by CuPh and by MTSET. HeLa cells expressing M449C/L466C in the background of WT-GLT-1 were preincubated for 5 min in the presence and absence of 10 μm CuPh (A). Cells expressing the single cysteine mutants M449C (B) or L466C (C), both in the background of the Cys-less GLT-1 were preincubated for 5 min in the presence or absence of 0.3 (B) or 1.0 (C) mm MTSET. The indicated preincubation solutions contained NaCl, NaCl + 1 mm l-glutamate, NaCl + 0.2 mm kainate, NaCl + 20 μm TBOA, KCl, choline chloride, or LiCl. Values are given as percent of control (preincubation without CuPh or MTSET) and represent the mean ± S.E. of at least three different experiments done in triplicate.
Effects of Thiol Cross-linking and Cd2+ on L453C/I463C—CuPh (10 μm) also inhibited transport of the paired mutant L453C/I463C (HP2/TM 8) (background of WT), and this inhibition was not seen with the individual cysteine mutants. However, significant inhibition was observed with the L453C/I463S and L453S/I463C mutants: 48.2 ± 2.1% and 41.8 ± 5.6% activity remained after treatment with 10 μm CuPh, respectively, compared with 15.1 ± 2.3% for L453C/I463C (n = 3). This suggests that the introduction of a smaller residue at position 453 or 463 may allosterically promote the cross-linking of the cysteine introduced at the other position with one or more of the cysteines endogenous to WT-GLT-1. However, in contrast to M449C/L466C, low but significant transport activity was observed with the L453C/I463C mutant in the Cys-less background, 13.2 ± 1.2% of that of Cys-less GLT-1 (n = 3). CuPh (10 μm) inhibited transport by the double mutant but not that of Cys-less GLT-1 (Fig. 4A). Two additional double mutants were made in the background of Cys-less GLT-1, L453S/I463C, and L453C/I463S. The activity of the former was only around 1% of that of Cys-less GLT-1 and could not be studied further. The activity of L453C/I463S was 4.4 ± 0.3% of that of Cys-less GLT-1 (n = 3), and this activity was almost insensitive to treatment with 10 μm CuPh with 91.6 ± 2.4% of the activity remaining (n = 3). In contrast to M449C/L466C, the inhibition of transport of L453C/I463C by CuPh could be reversed by a subsequent incubation with 20 mm DTT (data not shown). Again, inhibition was not observed with the single cysteine mutants whether expressed individually or together (Fig. 4A). Similarly, inhibition of transport by Cd2+ was observed only with the double cysteine mutant and only when the two cysteines were introduced in the same construct (Fig. 4B).
FIGURE 4.
Inhibition of transport of L453C/I463C by CuPh and Cd2+. All the mutants were in the background of the Cys-less GLT-1, and the experimental conditions were as described in the legend to Fig. 2 using 10 μm CuPh (A) or 0.5 mm CdCl2 (B).
The effects of transporter ligands on the inhibition of L453C/I463C by CuPh (Fig. 5A) were different than those on M449C/L466C (Fig. 3A). Although potassium also protected against inhibition of transport of L453C/I463C by CuPh, a significant protection was also seen when sodium was replaced by choline and to some extent also by lithium (Fig. 5A). Therefore the effect of potassium on L453C/I463C does not seem to be related with the ability of this ion to increase the proportion of inward-facing transporters (Fig. 1A). In further contrast with M449C/L466C (Fig. 3A), not only l-glutamate but also d,l-TBOA and kainate protected against the inhibition of L453C/I463C by CuPh (Fig. 5A), but GABA and glycine had no effect (data not shown). Except for the effect of lithium, the effects of other ligands on the accessibility of L453C to MTSET, were similar to those observed on the inhibition of transport by L453C/I463C by CuPh, regardless if the cysteine mutation was introduced in Cys-less GLT-1 (Fig. 5B) or in WT-GLT-1 (data not shown). The reactivity to MTSET of the cysteine introduced at position 463, was only modestly affected by transporter ligands and only glutamate, d,l-TBOA and kainate afforded some protection (Fig. 5C).
FIGURE 5.
Effect of the composition of the external medium on the inhibition of cysteine mutants at positions 453 and 463 by CuPh and by MTSET. The mutants were in the background of the Cys-less GLT-1, and the experimental conditions were as described in the legend to Fig. 3 using 10 μm CuPh (A) or MTSET at 0.2 (B) or 0.02 (C) mm.
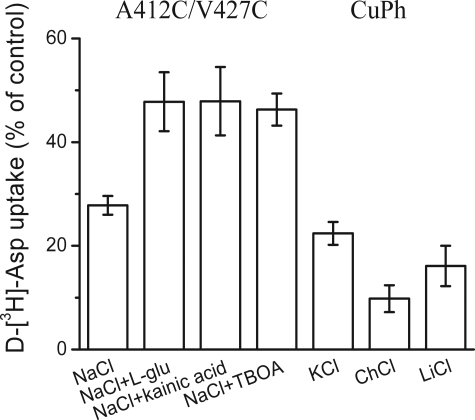
Previously, l-glutamate and kainate were also shown to protect against the inhibition of transport of A412C/V427C (TM 7/HP2) by CuPh (24). A similar protective effect was also observed with d,l-TBOA (Fig. 6). Thus, in contrast to M449C/L466C, TBOA and kainate have similar effects on oxidative cross-linking in L453C/I463C and in A412C/V427C.
FIGURE 6.
Effect of the composition of the external medium on the inhibition of A412C/V427C by CuPh. The mutant was in the background of WT-GLT-1, and the conditions were as described in the legend to Fig. 3A, except that CuPh was used at 100 μm.
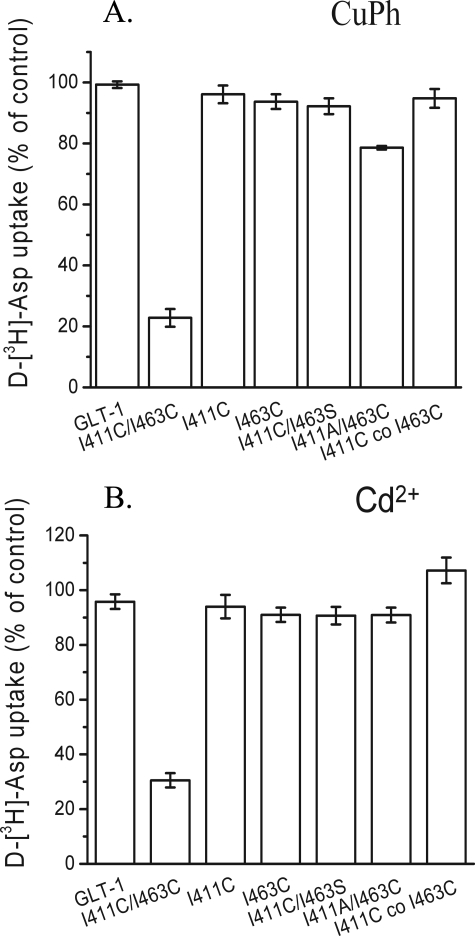
Effects of Thiol Cross-linking and Cd2+ on I411C/I463C—To explore the possibility of movement of TM7 relative to TM8, we used the model to find residues at the extracellular ends of these domains, predicted to be in close proximity to each other. We identified one active double cysteine mutant that was inhibited by CuPh, namely I411C/I463C. The activity of seven other TM7/TM8 double mutants, with cysteines inserted at positions predicted to be close in space, was not inhibited by CuPh (see legend to Fig. 7). Except for I411C/D462C and I411C/S464C, the activity of the five other pairs was sensitive to MTSET. However, preincubation of these five double mutants with CuPh did not protect against MTSET, rendering unlikely the possibility that in these cases CuPh mediated cross-linking without inactivation.
FIGURE 7.
Inhibition of transport of I411C/I463C by CuPh and Cd2+. The mutants were in the background of WT-GLT-1, and the conditions were as described in the legend to Fig. 2, except that CuPh was used at 100 μm. The following TM7/TM8 double cysteine mutants were also tested and found to be basically insensitive to CuPh: F410C/L466C, I411C/D462C, I411C/S464C, F410C/I463C, A412C/I463C, A407C/I463C, and A407C/L466C.
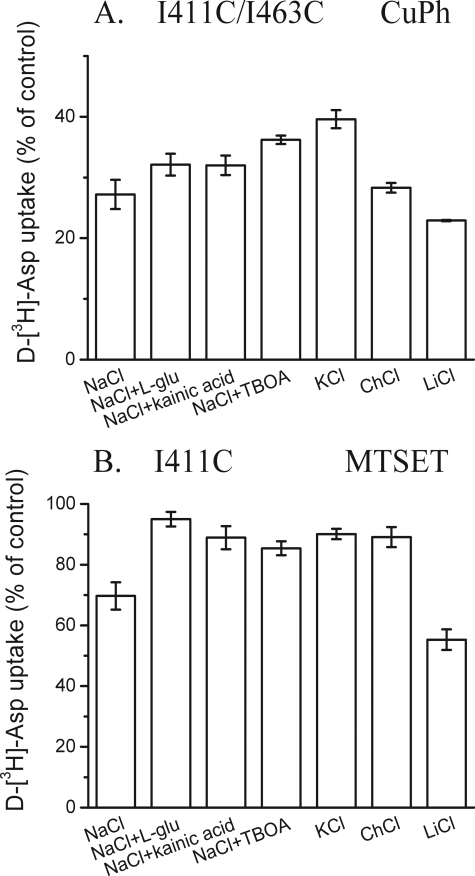
I411C/I463C was active only in the background of WT-GLT-1, but not in that of Cys-less GLT-1 (data not shown). Just as was the case with M449C/L466C, the lack of activity of I411C/I463C in the Cys-less GLT-1 background was due to defective maturation and targeting to the plasma membrane (data not shown). The inhibition of I411C/I463C by CuPh required higher concentrations than in the case of the other two double cysteine mutants, but was nevertheless dependent on the simultaneous presence of cysteines at both positions. The inhibition of I411C/I463C by 100 μm CuPh was not seen with WT, I411C, I463C, and the double mutants I411C/I463S and I411A/I463C (Fig. 7A). Again, inhibition was seen only when the two engineered cysteines were present in the same construct (Fig. 7A), but like in M449C/L466C, the inhibition could not be reversed by DTT (data not shown). Also in the case of I411C/I463C, further evidence for the proximity of the two positions was provided by the characteristics of inhibition of transport by Cd2+ (Fig. 7B). The effects of transporter ligands on the inhibition of I411C/I463C by CuPh were relatively modest (Fig. 8A). The effects of ligands on the reactivity of the cysteine at position 411 to MTSET (Fig. 8B), was even less pronounced than on that of I463C (Fig. 5C). Altogether, the effects of transporter ligands on cross-linking appear to be most pronounced when one of the two cysteine residues resides on HP2.
FIGURE 8.
Effect of the composition of the external medium on the inhibition of cysteine mutants at positions 411 and 463 by CuPh and by MTSET. Effects of the composition of the external medium on inhibition of transport of I411C/I463C (background of GLT-1) by CuPh (A) and of I411C (background of Cys-less GLT-1) by MTSET (B) are shown. The conditions were as described in the legend to Fig. 3, except that CuPh was used at 100 μm and MTSET at 2 mm.
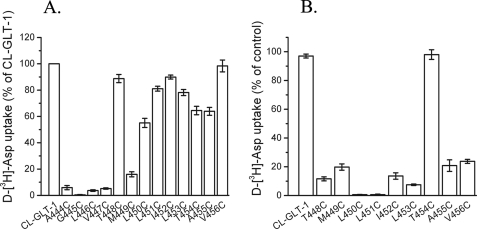
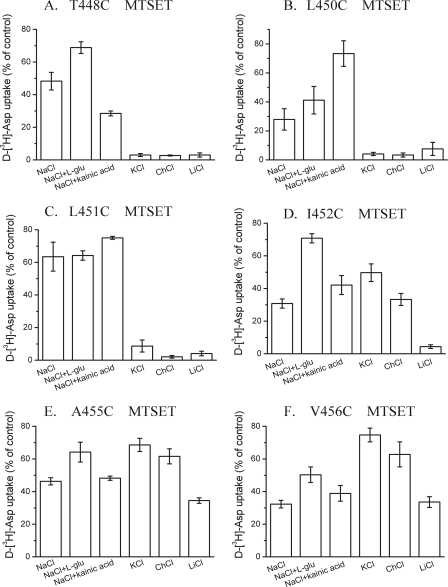
Effect of Transporter Ligands on Inhibition of HP2 Mutants by MTSET—In GLT-1, the reentrant loop corresponding to HP2 of GltPh, extends from position 427 to 456. Previously, we have analyzed the effects of transporter ligands on the sensitivity of cysteine mutants introduced at positions 422–443 (36). This latter stretch encompasses around half of HP2, roughly corresponding to the part of HP2 referred to as HP2a in GltPh (11). The activity of the cysteine mutants at all HP2b positions of the Cys-less GLT-1 was easily detectable, except for positions 444–447 (Fig. 9A). With the exception of T454C, transport by all active single cysteine mutants was potently inhibited by 1 mm MTSET (Fig. 9B). All the cysteine mutants in this stretch showed a conformationally sensitive reactivity pattern. Dependent on the position where the cysteines were introduced, different effects of the ligands were obtained as exemplified in Fig. 10. Relative to other cations, the sensitivity of T448C to MTSET was protected by sodium (Fig. 10A) in an even more pronounced manner than observed with M449C (Fig. 3B). As with M449C, glutamate and kainate had opposite effects on the sensitivity of T448C to MTSET, although in the latter mutant these differences were less pronounced (Figs. 3B and 10A). The sensitivity of L450C to MTSET was also less in the presence of sodium than with other cations, whereas kainate, but not glutamate, afforded a significant protection (Fig. 10B). With L451C a pronounced protection by sodium was observed (Fig. 10C). On the other hand, the sensitivity of V456C to MTSET was largest in the presence of sodium (or lithium) (Fig. 10F). l-glutamate and kainate had modest effects on the sensitivity of L451C, A455C, and V456C (Fig. 10, C, E, and F), but the protective effect of l-glutamate was more pronounced in I452C (Fig. 10D). Our results indicate that the accessibility of many HP2 positions is conformationally sensitive.
FIGURE 9.
Transport activity and the effect of MTSET in HP2b mutants. The Cys-less GLT-1 and the HP2b mutants in the Cys-less GLT-1 background were expressed in HeLa cells, and transport was measured as described under “Experimental Procedures” without any preincubation (A) or after a 5-min preincubation with or without 1.0 mm MTSET in NaCl-containing medium (B). The data are given as percent of activity of Cys-less GLT-1 (A) or as percent of activity of the untreated mutants (B).
FIGURE 10.
Effect of the medium composition on the inhibition of HP2b mutants by MTSET. The conditions were as described in the legend to Fig. 3, B and C using the indicated mutants in the Cys-less GLT-1 background with the MTSET concentrations (mm) used during the preincubation in parenthesis: (A) T448C (0.1), (B) L450C (0.01), (C) L451C (0.03), (D) I452C (0.4), (E) A455C (0.2), and (F) V456C (0.2).
DISCUSSION
Using two lines of evidence, we have identified three new paired positions in the glutamate transporter GLT-1/EAAT2, which behave as if they are close in space. The cysteine pairs were introduced at positions predicted by the GltPh structure to be close to each other, indicating that this structure is a useful model for the study of glutamate transporters in the brain. In each pair, the activity of the double cysteine mutants was inhibited not only by oxidative crosslinking, but also by Cd2+ (Figs. 2, 4, and 7). Because CuPh treatment did not protect against MTSET inactivation of other CuPh-insensitive double cysteine mutants, we conclude that cross-linking did not occur at these sites. This is reasonable, because it can be anticipated that most cross-linking events between structural elements lining the permeation pathway of the transporter are likely to result in inactivation. It is possible that cross-linking without inactivation may be observed in domains of the transporter not directly involved in the permeation pathway.
When cross-linking was done between HP2 and TM8, marked effects by transporter ligands were observed (Figs. 3A and 5A). Because the pairs were introduced around 10–15 Å “above” the residues that make contact with the substrate and the analogue TBOA (11, 23), it is unlikely that the transporter ligands physically interfere with the cross-linking. Thus it appears that the degree of cross-linking between HP2 and TM8 depends on the conformational state of the transporter. The impact of transporter ligands may be due to an effect on the accessibility of the reactive oxygen species to reach the sites of cross-linking and/or on the distance or the relative bond angles of the paired cysteine residues. To evaluate the effect of the ligands on accessibility, we also examined their effect on the reactivity of the corresponding individual cysteines to the impermeant sulfhydryl reagent MTSET. It is important to note that ligand-induced reactivity/accessibility changes also are a consequence of movement, which could either involve movement of the cysteine itself or of that of structural elements surrounding it. In the case of the paired positions 449 and 466, the non-transportable blockers TBOA and kainate each increased the inhibition of transport of M449C and of L466C by MTSET (Fig. 3, B and C), yet in the paired mutant M449C/L466C these blockers had different effects on the ability of CuPh to cross-link the cysteine residues introduced at these positions (Fig. 3A). Therefore it appears that in addition to an effect on accessibility, substrate analogues can cause a relative movement between HP2 and TM8, at least at the latter positions.
The effects of ligands on inhibition of transport of L453C/I463C by CuPh (Fig. 5A) were similar to those on the inhibition of the single mutants by MTSET (Fig. 5, B and C). In this case it is possible that the effects of the ligands in the experiments with CuPh are mostly due to effects on accessibility. Only relatively minor effects of the transport ligands were observed in the case of the pair I411C/I463C (TM7/TM 8) (Fig. 8). On the other hand pronounced effects of the ligands were observed with the previously characterized A412C/V427C (24) (Fig. 6), involving TM7 and HP2. Taken together, our results suggest that the ligand-induced effects on cross-linking of M449C/L466C, are mainly due to a movement of HP2, although we cannot exclude the possibility that TM8 can move as well. Ligand-induced movement of HP2 in GLT-1 is consistent with the proposal, based on the GltPh structure, that HP2 serves as an external gate of the transporter, which moves back and forth to allow substrate into the binding site from the extracellular side (11, 23). A very recently published molecular dynamics simulation study has provided further support for this idea (37).
Glutamate, expected to cause an increase of the proportion of inward-facing transporters (Fig. 1A), protects against MTSET at many positions studied here. This is not only true for HP2 (Figs. 3B, 5B, and 10), but also for positions 463 and 466 of TM8 and 411 of TM7 (Figs. 3C, 5C, and 8B). The decreased accessibility of many HP2 positions by substrate is consistent with the aspartate-bound GltPh structure, where HP2 blocks access to the binding pocket from the extracellular side (11, 23). If HP2 serves as an extracellular lid on the binding pocket also in GLT-1, glutamate would not only be expected to reduce the external accessibility of many HP2 positions, but also of those on TMs 7 and 8, which line the binding pocket.
The substrate analogue kainate, expected to cause an increase of the proportion of outward-facing transporters (Fig. 1A), increased the inhibition by MTSET in HP2 mutants with cysteines introduced at positions 448 (Fig. 10A) and 449 (Fig. 3B) and also at position 443 (36). These observations are in harmony with the TBOA-bound GltPh structure, which represents an “open” conformation where HP2 has moved toward the extracellular side, away from the binding pocket (23). However, in contrast to positions 443, 448, and 449, kainate slows the reactivity of cysteine residues engineered at many other HP2 positions (Figs. 5B and 10B and Ref. 36). It therefore appears that binding of kainate can result in a complex movement of HP2. One part of HP2 may move further outward to open the binding pocket to the extracellular medium, consistent with the observations by Boudker et al. on GltPh (23). However another part of HP2 of GLT-1 may actually close in on the binding pocket in the presence of kainate. The former part of HP2 would include positions 443 (36), 448, and 449 and presumably also the intervening residues, although the latter could not be tested because the lack of activity of the cysteine mutants at positions 444–447 (Fig. 9). Kainate has no effect on crosslinking of M449/L466C (Fig. 3A), despite the ability of this analogue to increase the accessibility of both positions (Fig. 3, B and C). This suggests that kainate may have induced an increase in distance between these positions, possibly through the outward movement of position 449. However, we cannot exclude the possibility that that the effect of kainate on 449–466 is that some other part of the protein moves away from the two cysteines to make them more accessible to MTSET but still close enough to cross-link at the same rate. It is noteworthy that in the aspartate-bound GltPh structure, the positions corresponding to those of GLT-1 predicted to move further outward in the presence of substrate analogues, are indeed closest to the extracellular medium (11). The part of HP2 that may close in on the binding pocket in the presence of substrate analogues is expected to include those positions where the reactivity of introduced cysteines toward MTSET is reduced by kainate. The apparent difference between GLT-1 and GltPh, regarding the movement HP2 induced by substrate analogues, does not seem to be due to the difference between kainate and TBOA, at least for positions 449 (Fig. 3B) and 453 (Fig. 5B).
From our accessibility experiments it appears that the conformations of GLT-1 with and without blocker in the presence of sodium are different (Figs. 3, B and C, 5, B and C, 8B, and 10, A and B). This is different from the situation in GltPh, where no such difference was found between the structures with and without TBOA (23). It is possible that the relatively low resolution of the GltPh structure (23) does not enable to detect subtle sodium-dependent conformational changes induced by TBOA. However, the possibility that the two transporters are different in this regard cannot be excluded.
One of the differences between the eukaryotic glutamate transporters and GltPh is that the former have an additional extracellular domain, which contains the N-linked glycosylation sites. Obviously, its structure and its relationship with the rest of the transporter are as yet unknown, although very recent evidence indicates that this 4B-4C loop may extend to the outer perimeter of the protein (38). In principle, it is possible that glutamate or kainate binding deep in the binding pocket, cause this additional domain to move such as to physically obstruct the external accessibility of part of the HP2 loop. Although this possibility cannot be totally excluded, the new observations do not provide evidence for large scale motions of the 4B-4C loop during transport (38). Moreover, the complex effects of transporter ligands on the reactivity of cysteines introduced at the various HP2 positions to MTSET (Figs. 3B, 5B, and 10 and Ref. 36), render the explanation of an overall a physical blockade of external accessibility of HP2 highly unlikely.
Extracellular potassium is also expected to increase the proportion of inward-facing transporters (Fig. 1A) and therefore to have similar effects on cross-linking and sensitivity of transport to MTSET as glutamate. This expectation is fulfilled in the case of oxidative cross-linking of M449C/L466C (Fig. 3A) and of L453C/I463C (Fig. 5A). This is also the case of sensitivity to MTSET for HP2 mutants I452C, L453C, A455C, and V456C (Figs. 5B and 10, D–F), but potassium actually increases the sensitivity toward the sulfhydryl reagent in T448C, L450C, and L451C (Fig. 10, A–C). However, this potentiation is actually due to the lack of sodium rather than to the presence of potassium because a similar potentiation was also seen when sodium was replaced with lithium or choline (Fig. 10, A–C). Thus this potentiation is not related to the ability of potassium to increase the proportion of inward-facing transporters. In contrast to glutamate, potassium did not protect against cross-linking of A412C/V427C. Thus it appears that the conformation(s) favored by external potassium and glutamate are similar but not identical.
A central finding is that the two substrate analogues, TBOA and kainate differentially affected the cross-linking of the cysteines at positions 449 and 466 (Fig. 3A). Kainate and TBOA also have two carboxyl groups and a primary or secondary amine group (Fig. 1B), which are expected to bind to the same amino acid residues of the transporter as glutamate itself. However the added bulk on each of the two analogues is different. In GltPh, the aromatic ring of TBOA is lodged against Met-311 (equivalent to Met-397 of GLT-1), but the extra bulk of kainate presumably points in the direction of HP1. It is likely that these different additional interactions of the two analogues with the binding pocket result in different allosteric effects on HP2. This can explain the differential effects exerted by the two analogues on the ability of CuPh to cross-link the cysteines at positions 449 and 466. As mentioned above, the allosteric effect, exerted by the substrate itself, is the closure of the entire HP2 gate. In contrast to the complex movement of HP2 induced by substrate analogues, closure by substrate enables the opening of the internal gate, which is a prerequisite for transport.
Acknowledgments
We thank Annie Bendahan for help with surface biotinylation experiments and Elia Zomot for helping with the preparation of the figures.
This work was supported, in whole or in part, by NINDS, National Institutes of Health Grant NS16708. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TM, transmembrane domain; DTT, dithiothreitol; CuPh, copper(II)(1,10-phenanthroline)3; MTSET, (2-trimethylammonium) methanethiosulfonate; WT, wild type; HP, hairpin loop; TBOA, d, l-threo-β-benzoylaspartate.
References
- 1.Kanner, B. I., and Sharon, I. (1978) Biochemistry 17 3949–3953 [DOI] [PubMed] [Google Scholar]
- 2.Brew, H., and Attwell, D. (1987) Nature 327 707–709 [DOI] [PubMed] [Google Scholar]
- 3.Wadiche, J. I., Arriza, J. L., Amara, S. G., and Kavanaugh, M. P. (1995) Neuron 14 1019–1027 [DOI] [PubMed] [Google Scholar]
- 4.Zerangue, N., and Kavanaugh, M. P. (1996) Nature 383 634–637 [DOI] [PubMed] [Google Scholar]
- 5.Levy, L. M., Warr, O., and Attwell, D. (1998) J. Neurosci. 18 9620–9628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanner, B. I., and Bendahan, A. (1982) Biochemistry 21 6327–6330 [DOI] [PubMed] [Google Scholar]
- 7.Pines, G., and Kanner, B. I. (1990) Biochemistry 29 11209–11214 [DOI] [PubMed] [Google Scholar]
- 8.Kavanaugh, M. P., Bendahan, A., Zerangue, N., Zhang, Y., and Kanner, B. I. (1997) J. Biol. Chem. 272 1703–1708 [DOI] [PubMed] [Google Scholar]
- 9.Zhang, Y., Bendahan, A., Zarbiv, R., Kavanaugh, M. P., and Kanner, B. I. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 751–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szatkowski, M., Barbour, B., and Attwell, D. (1990) Nature 348 443–446 [DOI] [PubMed] [Google Scholar]
- 11.Yernool, D., Boudker, O., Jin, Y., and Gouaux, E. (2004) Nature 431 811–818 [DOI] [PubMed] [Google Scholar]
- 12.Koch, H. P., and Larsson, H. P. (2005) J. Neurosci. 25 1730–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grewer, C., Balani, P., Weidenfeller, C., Bartusel, T., Tao, Z., and Rauen, T. (2005) Biochemistry 44 11913–11923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leary, G. P., Stone, E. F., Holley, D. C., and Kavanaugh, M. P. (2007) J. Neurosci. 27 2938–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch, H. P., Brown, R. L., and Larsson, H. P. (2007) J. Neurosci. 27 2943–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunewald, M., Bendahan, A., and Kanner, B. I. (1998) Neuron 21 623–632 [DOI] [PubMed] [Google Scholar]
- 17.Slotboom, D. J., Sobczak, I., Konings, W. N., and Lolkema, J. S. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 14282–14287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunewald, M., and Kanner, B. I. (2000) J. Biol. Chem. 275 9684–9689 [DOI] [PubMed] [Google Scholar]
- 19.Zhang, Y., and Kanner, B. I. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 1710–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borre, L., and Kanner, B. I. (2001) J. Biol. Chem. 276 40396–40401 [DOI] [PubMed] [Google Scholar]
- 21.Bendahan, A., Armon, A., Madani, N., Kavanaugh, M. P., and Kanner, B. I. (2000) J. Biol. Chem. 275 37436–37442 [DOI] [PubMed] [Google Scholar]
- 22.Teichman, S., and Kanner, B. I. (2007) J. Gen. Physiol. 129 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boudker, O., Ryan, R. M., Yernool, D., Shimamoto, K., and Gouaux, E. (2007) Nature 445 387–393 [DOI] [PubMed] [Google Scholar]
- 24.Brocke, L., Bendahan, A., Grunewald, M., and Kanner, B. I. (2002) J. Biol. Chem. 277 3985–3992 [DOI] [PubMed] [Google Scholar]
- 25.Zomot, E., Zhou, Y., and Kanner, B. I. (2005) J. Biol. Chem. 280 25512–25516 [DOI] [PubMed] [Google Scholar]
- 26.Leighton, B. H., Seal, R. P., Watts, S. D., Skyba, M. O., and Amara, S. G. (2006) J. Biol. Chem. 281 29788–29796 [DOI] [PubMed] [Google Scholar]
- 27.Pines, G., Danbolt, N. C., Bjoras, M., Zhang, Y., Bendahan, A., Eide, L., Koepsell, H., Storm-Mathisen, J., Seeberg, E., and Kanner, B. I. (1992) Nature 360 464–467 [DOI] [PubMed] [Google Scholar]
- 28.Arriza, J. L., Fairman, W. A., Wadiche, J. I., Murdoch, G. H., Kavanaugh, M. P., and Amara, S. G. (1994) J. Neurosci. 14 5559–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunkel, T. A., Roberts, J. D., and Zakour, R. A. (1987) Methods Enzymol. 154 367–382 [DOI] [PubMed] [Google Scholar]
- 30.Kleinberger-Doron, N., and Kanner, B. I. (1994) J. Biol. Chem. 269 3063–3067 [PubMed] [Google Scholar]
- 31.Fuerst, T. R., Niles, E. G., Studier, F. W., and Moss, B. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 8122–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pines, G., Zhang, Y., and Kanner, B. I. (1995) J. Biol. Chem. 270 17093–17097 [DOI] [PubMed] [Google Scholar]
- 33.Perez-Garcia, M. T., Chiamvimonvat, N., Marban, E., and Tomaselli, G. F. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glusker, J. P. (1991) Adv Protein Chem. 42 1–76 [DOI] [PubMed] [Google Scholar]
- 35.Benitah, J. P., Tomaselli, G. F., and Marban, E. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 7392–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunewald, M., Menaker, D., and Kanner, B. I. (2002) J. Biol. Chem. 277 26074–26080 [DOI] [PubMed] [Google Scholar]
- 37.Huang, Z., and Tajkhorshid, E. (2008) Biophys. J. 95 2292–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch, H. P., Hubbard, J. M., and Larsson, H. P. (2007) J. Biol. Chem. 282 24547–24553 [DOI] [PubMed] [Google Scholar]