Abstract
In order to better understand the molecular and cellular determinants of tumor cell intravasation, our laboratory has generated a pair of congenic human HT-1080 fibrosarcoma variants (i.e. HT-hi/diss and HT-lo/diss) differing 50–100-fold in their ability to intravasate and disseminate. To investigate the molecular differences underlying the distinct dissemination capacities of these HT-1080 variants, we performed a comparative analysis of the cell surface proteomes of HT-hi/diss and HT-lo/diss. Cell membrane proteins were enriched by biotinylation and avidin precipitation and analyzed by tandem mass spectrometry employing multidimensional protein identification technology. By this approach, 47 cell surface-associated molecules were identified as differentially expressed between the HT-1080 intravasation variants. From these candidates, four targets (i.e. TIMP-2, NCAM-1, JAM-C, and tissue factor (TF)) were selected for further biochemical validation and in vivo functional verification. Western blot analysis of the cell surface enriched fractions confirmed the proteomic array data, demonstrating that, in vitro, TIMP-2 protein was increased in the HT-lo/diss variant, whereas NCAM-1, JAM-C, and TF levels were increased in the HT-hi/diss variant. Corresponding in vivo differences in levels of TIMP-2, JAM-C, and TF were demonstrated in primary tumors grown in the chick embryo. Finally, functional inhibition of one selected protein (i.e. TF) by small interfering RNA silencing or ligation with a function-blocking antibody significantly reduced HT-hi/diss intravasation, thus clearly implicating TF in the early steps of tumor cell dissemination. Overall, our cell surface proteomic analysis provides a powerful tool for identification of specific cell membrane molecules that contribute functionally to intravasation and metastasis in vivo.
One of the early and possibly rate-limiting steps in cancer progression from a localized tumor to systemic metastatic disease is intravasation (i.e. the entry of metastatic cells into the vasculature) (1–5). Molecules involved in intravasation represent attractive therapeutic targets, since preventing or inhibiting this process would confine tumor cells to their primary site and provide a more focused target for clinical intervention (6). To identify cellular attributes that functionally contribute to tumor cell intravasation and metastasis, including escape from the primary site, invasion of local stoma, and entry into the vasculature, we have employed a pair of congenic human fibrosarcoma HT-1080 cell variants, differing 50–100-fold in their ability to intravasate and disseminate (HT-hi/diss and HT-lo/diss) while having similar capacities to form primary tumors (7). These cell variants display a distinct differential during spontaneous metastasis but behave comparably in experimental metastasis models where cells are inoculated intravenously and only the later steps of the metastatic cascade are recapitulated. Therefore, comparative analysis of the HT-hi/diss and HT-lo/diss variants can be useful for identification of molecules specifically contributing to early metastatic events.
Previously, we have employed activity-based protein profiling (8) to identify molecules that might underlie the differential intravasation potential of the HT-1080 cell variants. This proteomic approach implicated urokinase activation as a key step in HT-hi/diss dissemination (9). Since many groups of proteins functionally linked to cancer progression are cell surface molecules, such as growth factor receptors, transmembrane signaling molecules, and cell-cell or cell-matrix adhesion proteins, we suggested that HT-hi/diss and HT-lo/diss might differentially express cell surface molecules that facilitate tumor cell intravasation and contribute to early steps of cancer dissemination.
Membrane-tethered proteins are present in relatively low abundance and therefore are often overlooked or not identified in broad spectrum, whole cell, or tissue arrays. Cell surface biotinylation followed by avidin precipitation is a widely used method to enrich membrane proteins (10–14). One major caveat of this approach is a high level of nonspecific intracellular protein contamination in avidin pull-downs. Our initial attempt with a commercially available cell surface labeling kit (Pierce) was disappointing, since it yielded an overwhelming number of known intracellular proteins but few cell surface molecules. Several previous studies involving gel-based detection for protein identification have also been hampered by limited sensitivity of the method (12–14).
To increase the specificity and sensitivity of the cell surface proteomic approach, we have introduced essential modifications to standard cell labeling procedures and used a non-gel mass spectrometry approach employing multidimensional protein identification technology (MudPIT)2 (15–17). This approach was used to identify proteins differentially expressed between the tumor cell intravasation variants by comparing the cell surface proteomes of HT-hi/diss and HT-lo/diss. To link the proteomic data to the process of actual in vivo metastasis, we selected several candidate proteins that were identified by the array as being enriched in one cell variant over the other and verified the differential levels of the selected candidates in cell lysates and primary tumors by Western blotting. Finally, we analyzed the functional role of one of the identified proteins, tissue factor (TF), in HT-1080 intravasation by employing the human tumor-chick embryo spontaneous metastasis model. In this assay, the ability of human tumor cells to intravasate is determined by quantifying the number of human cells arrested in the chorioallantoic membrane (CAM), which serves as a repository of cells that have escaped from primary tumors and entered the vasculature (18, 19). By down-regulating TF function via siRNA silencing or ligation with a function-blocking antibody, we have demonstrated that TF positively contributes to HT-hi/diss intravasation, thereby validating our cell surface proteomic approach.
EXPERIMENTAL PROCEDURES
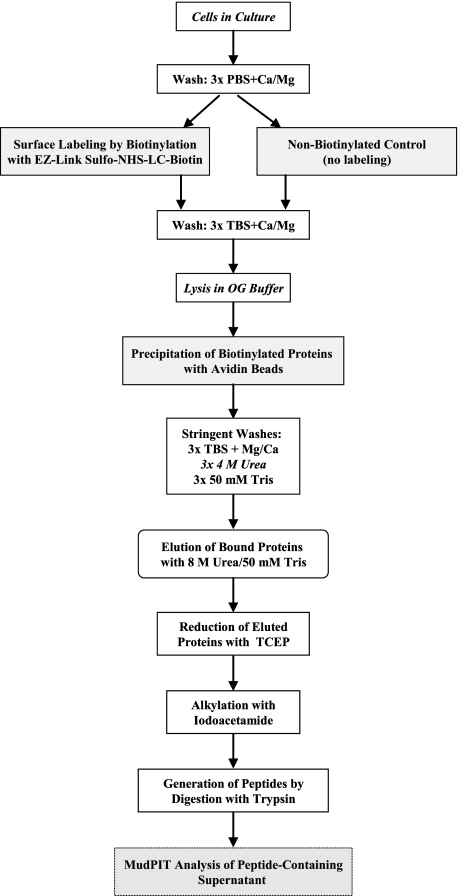
Cell Surface Protein Labeling, Enrichment, and Digestion—A schematic of the labeling and enrichment procedure is presented in Fig. 1. HT-hi/diss and HT-lo/diss cells, generated from the HT-1080 human fibrosarcoma cell line (7), were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (Hyclone, Logan, UT) and 10 μg/ml gentamicin (Invitrogen) (D-10). Cells were harvested by trypsinization and seeded in D-10 at 4 × 106 cells/150-mm dish to achieve 90–100% confluence 24 h later. Cell layers were then washed three times with ice-cold PBS, 1 mm CaCl2, 1 mm MgCl2 (PBS-Ca/Mg) and then incubated in 0.2 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce) for 45 min at room temperature with gentle swirling every 10 min. Nonbiotinylated control plates were incubated in PBS-Ca/Mg alone. After washing three times with TBS-Ca/Mg (pH 7.4) to quench and remove remaining biotinylation reagent, the cells were scraped in TBS, pelleted by centrifugation, and lysed in 50 mm octyl-β-d-glucopyranoside in TBS-Ca/Mg supplemented with protease inhibitors (aprotinin, leupeptin, and pepstatin, each at 10 μg/ml, and phenylmethylsulfonyl fluoride at 1 mm) for 30 min at 4 °C with rocking. Lysates were clarified by centrifugation at 1,000 × g for 2 min and mixed with 300 μl of avidin beads (Sigma). After incubation for 1 h at room temperature while rotating, the beads were pelleted by centrifugation at 200 × g for 3 min and washed three times with TBS-Ca/Mg, three times with 4 m urea in TBS, and finally three times with 50 mm Tris (pH 7.4). After pelleting at 200 × g for 5 min, the beads were resuspended in 300 μl of freshly prepared 8 m urea in 50 mm Tris. The eluted proteins were then reduced with 5 mm Tris(2-carboxyethyl)phosphine for 30 min at room temperature and alkylated with 10 mm iodoacetamide for 30 min at room temperature in the dark. Next, the proteins were digested with 0.4% trypsin (sequencing grade) in 50 mm Tris supplemented with 10.5 mm CaCl2 at 37 °C for 12 h. Samples were spun at 10,000 × g for 4 min, the peptide-containing supernatant was removed, and formic acid was added to a final concentration of 5%.
FIGURE 1.
Cell surface protein labeling and enrichment for identification by LC-MS/MS. Cell cultures were biotinylated or incubated in PBS to serve as the nonbiotinylated control. Following washes, cells were lysed in octyl-β-d-glucopyranoside buffer, and biotinylated molecules were precipitated with avidin beads. Stringent washes, including a wash in 4 m urea, were performed to reduce nonspecific protein binding. Precipitated proteins were eluted from the avidin beads with 8 m urea, reduced with Tris(2-carboxyethyl)phosphine (TCEP), alkylated with iodoacetamide, and digested with trypsin. Peptides were analyzed by LC-MS/MS (MudPIT) and identified by searching spectra against the SEQUEST data base. Proteins identified by LC-MS/MS in the nonlabeled control were excluded from further analysis, since they represent nonspecifically bound molecules. For this analysis, n = 2 HT-hi/diss biotinylated samples, n = 1 HT-lo/diss biotinylated sample, and n = 1 PBS control HT-hi/diss sample.
Identification of Cell Surface Proteins by MudPIT Analysis—To identify biotinylated cell surface proteins enriched by the avidin precipitation, the digested peptide mixtures were loaded onto a biphasic (strong cation exchange/reverse phase) capillary column and analyzed by two-dimensional liquid chromatography in combination with tandem mass spectrometry (MS) as described (15, 16). Briefly, a fused silica capillary (100-μm inner diameter) was pulled using a CO2-based laser puller to make a fritless nanospray column. This column was packed with 10 cm of 5-μm C18 reverse phase particles (Aqua 5 μ c18; Phenomenex) followed with 3 cm of 5-μm strong cation exchange particles (Partisphere 5 SCX; Whatman, Clifton, NJ). The peptide digest containing 100–500 μg of protein was loaded onto the SCX portion of the column, displaced with a stepwise salt gradient of KCl in 5% acetonitrile (pH 3), and reloaded onto the reverse phase portion of the column. The peptides were eluted from the reverse phase portion of the column after each salt step and analyzed in the mass spectrometer (Finnigan LTQ; Thermo Electron Corp., Waltham, MA) using a linear gradient of 80% acetonitrile in 0.5% acetic acid. The mass spectrometer was set in data-dependent acquisition mode, where one full MS scan is followed by MS/MS scans of the seven most abundant unique peptides. The MS/MS data were then searched against the most recent version of the human IPI data base using the SEQUEST search algorithm (20) to identify the proteins present in the sample. The relative abundance of the peptides was estimated by spectral counting, with the following criteria set for a peptide to be included in the analysis: cross-correlation score greater than 1.8 (+1), 2.5 (+2), 3.5 (+3), and δ correlation greater than 0.08 (21, 22). Additionally, to ensure accuracy, only those proteins that were identified by two or more independent peptides were included in the final comparison.
Silver Staining—Proteins were separated by SDS-PAGE on 4–20% Tris-glycine gels under reducing conditions. The gels were fixed for 2 h in a mixture of 50% methanol, 12% acetic acid, and 0.05% formaldehyde, washed three times in 35% ethanol for 20 min each, and sensitized in 0.02% sodium thiosulfate for 2 min. Gels were washed three times with water for 5 min each, stained for 20 min with 0.2% silver nitrate and 0.076% formaldehyde, followed by two washes in water (1 min each), and developed with a solution of 6% sodium carbonate, 0.05% formaldehyde, 0.0004% sodium thiosulfate. Once the gels were sufficiently stained, the developing reaction was stopped with a solution of 50% methanol and 12% acetic acid. Finally, the gels were imaged with an Alpha Imager 2000 (Alpha Innotech, San Leandro, CA).
Quantitative Chick Embryo Spontaneous Metastasis Assay—Fertilized White Leghorn eggs were incubated at 37 °C in a rotary incubator with 60% humidity. On day 10 of incubation, a portion of the eggshell was shaved off to expose the shell membrane. The CAM was separated from the shell membrane using mild suction through a hole in the air sac of the egg. A window was created in the eggshell, and 0.25–0.5 × 106 HT-hi/diss or HT-lo/diss cells were applied to the dropped CAM. The windows were sealed with tape, and the embryos were returned to a stationary incubator to allow for primary tumor development and dissemination of cells from the primary tumors. After 5 days, the embryos were sacrificed and primary tumors were excised, weighed, and frozen for biochemical analysis. Distal portions of the CAM were also harvested and frozen at -80 °C to determine the number of intravasated human cells by Alu-qPCR (described below).
Western and Avidin-Horseradish Peroxidase Blot Analysis—HT-hi/diss and HT-lo/diss cells were lysed in octyl-β-d-glucopyranoside buffer as described above. Primary CAM tumors were lysed by mechanical dissociation with scissors in modified radioimmune precipitation assay buffer containing protease inhibitors (aprotinin, leupeptin, and pepstatin, each at 10 μg/ml, and phenylmethylsulfonyl fluoride at 1 mm), followed by incubation under agitation for 30 min at 4 °C. The lysates were clarified by centrifugation at 6,000 × g for 15 min. Protein concentrations of the cell and tumor lysates were determined using the BCA™ (bicinchoninic acid) protein assay kit (Pierce). Equal amounts of HT-hi/diss or HT-lo/diss cell lysates, tumor lysates (10–40 μg), or cell surface enriched eluates were resolved on 4–20% SDS-polyacrylamide gels and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Billerca, MA). After transfer, the membranes were blocked with 5% nonfat milk in PBS plus 0.05% Tween 20 (PBS-T) for 1 h. Membranes were probed overnight at 4 °C with the following primary antibodies: murine anti-human TIMP-2 (Oncogene, Cambridge, MA), goat anti-human TF (isolated in the laboratory of Dr. W. Ruf), murine anti-human NCAM-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and goat anti-human JAM-C (Santa Cruz Biotechnology). To control for variability of human cell numbers within individual CAM tumors and to ensure that similar quantities of human protein were loaded, lysates were probed with murine mAb 29-7, which recognizes human CD44 on HT-hi/diss and HT-lo/diss but does not cross-react with chick tissue (7). After incubation with primary antibodies, the membranes were washed three times with PBS-T and then probed with horseradish peroxidase-conjugated secondary antibodies (anti-goat IgG from Vector Laboratories (Burlingame, CA) or anti-mouse IgG from Bio-Rad) in PBS-T/nonfat milk for 2 h. After washing three times in PBS-T, immunoreactive bands were visualized with SuperSignal West Pico Chemiluminescent Substrate (Pierce) and quantified with a Molecular Imager Gel Doc XR System (Bio-Rad).
Down-regulation of TF—Down-regulation of TF was achieved by siRNA silencing and by function-blocking antibody ligation. Two 19-mer siRNA sequences were generated against TF mRNA (Qiagen, Valencia, CA): TF-si167 (sense, GCGCUUCAGGCACUACAAA; antisense, UUUGUAGUGCCUGAAGCGC) (23) and TF-si1086, which recognizes the 3′-untranslated region (sense, GGAAACGCAAAUGAGUAUU; antisense, AAUACUCAUUUGCGUUUCC). The nonsilencing scrambled sequence of TF-si167 (i.e. scr-si167) was used as a control (sense, GCGUCUAGACGUCCACAAA; antisense, UUUGUGGACGUCUAGACGC). A BLAST-n search was performed to confirm that siRNA sequences did not cross-react with other known mRNA sequences. For siRNA transfections, 1.75 × 106 HT-hi/diss cells were plated in 10-cm dishes in D-10 without antibiotics. After 24 h, the 70–85% confluent cultures were transfected with 20 nm TF-si167, TF-si1086, scr-si167, or no siRNA (mock) with 9 μl of Lipofectamine 2000 (Invitrogen). Following overnight incubation, the cells were detached with trypsin/EDTA, washed in D-10, and resuspended in serum-free Dulbecco's modified Eagle's medium. A total of 0.25–0.4 × 106 cells were grafted onto the CAM of day 10 chick embryos. Down-regulation of TF was confirmed by Western blotting in cell lysates 24 h after siRNA transfection and in primary tumors harvested 5 days after grafting of tumor cells.
The TF function-blocking antibody 5G9 (24) was introduced either mixed with the cells prior to CAM grafting or by daily topical application to developing tumors at 25 or 50 μg in 0.1 ml of PBS plus 5% DMSO/embryo. Five days after cell grafting, the embryos were sacrificed, primary tumors were weighed, and distal portions of CAM were frozen for genomic DNA extraction and Alu-qPCR analysis.
Quantitative Analysis of Tumor Cell Intravasation by Alu-qPCR—Genomic DNA was extracted from distal CAM samples using the Gentra PureGene system (Qiagen). To determine actual numbers of human cells contained in individual samples, 60 ng of genomic DNA was used for quantitative PCR using primers recognizing primate-specific Alu repeat sequences and SYBR green double-stranded DNA binding dye for quantification, as previously described (19, 25). The qPCR was carried out in a Bio-Rad MyIQ light cycler. The Ct values were converted into numbers of human cells using a standard curve generated by spiking constant numbers of chicken cells with serial dilutions of HT-1080 cells.
RESULTS
Cell Surface Protein Enrichment and Identification by Mass Spectrometry—To compare the cell surface protein profiles of the highly disseminating (HT-hi/diss) and low disseminating (HT-lo/diss) variants of the parental HT-1080 cell line, an enrichment procedure was necessary, since cell surface proteins are present in relatively low abundance compared with the proteins in the intracellular pool. Enrichment was accomplished by biotinylation of cell monolayers using EZ-Link Sulfo-NHS-LC-Biotin and precipitation of the biotin-labeled proteins with avidin beads. Cell monolayers incubated with PBS instead of the biotinylation reagent underwent the same avidin precipitation procedure to serve as nonlabeled controls. Since nonspecific binding of intracellular proteins to avidin beads represents a frequent problem associated with surface protein enrichment, we increased the stringency of the washes after avidin precipitation by incubating the beads in urea. To control for stringency of the wash step, we compared three different conditions, employing 4 m urea, 6 m urea, or PBS. Western blotting with avidin-horseradish peroxidase was performed to estimate amounts of biotinylated material eluted after each wash condition. The resulting blot showed no decrease in the yield or distribution of biotinylated proteins after washing the beads with 4 or 6 m urea as compared with PBS, thus indicating that the urea wash did not cause significant displacement of biotinylated proteins from the avidin beads. However, the stringent washes with urea substantially reduced the total amount of proteins pulled down in the nonbiotinylated control (data not shown). Since washing with 4 and 6 m urea resulted in a similar decrease of nonspecific protein binding, the stringent wash step with 4 m urea was used during sample preparation for mass spectrometry.
To identify the most abundant proteins and compare the surface protein expression profiles of the intravasation variants, the cell surface enriched protein samples from HT-lo/diss and HT-hi/diss were analyzed by MudPIT. To subtract highly abundant intracellular proteins precipitated nonspecifically, we included a sample containing HT-hi/diss proteins that had undergone the same enrichment procedures but were not biotinylated. Since this sample included only those proteins that bound nonspecifically to the avidin beads, these molecules were excluded from analysis, further narrowing the pool of proteins identified as cell surface molecules. A list of the proteins identified in the biotinylated samples in either one or both of the HT-1080 variants is presented in supplemental Table 1.
Ultimately, by using this proteomic array, a total of 168 proteins were identified as differentially expressed between the HT-1080 intravasation variants. Despite our efforts to increase stringency and to exclude nonspecifically bound proteins from analysis, approximately two-thirds of the resulting proteins were intracellular or secreted molecules. However, among the proteins identified as true cell surface molecules, 26 and 21 were more abundant in HT-hi/diss and HT-lo/diss, respectively. A list of these proteins and their UniProt accession numbers is presented in Table 1. Known functions of some of these proteins include cell-cell or cell-matrix adhesion, signaling in response to cytokines or growth factors, induction of coagulation, and proteolysis (i.e. all processes linked to metastasis).
TABLE 1.
Cell surface proteins identified by LC-MS/MS with abundance differences between HT-hi/diss and HT-lo/diss Percent coverage, UniProt accession numbers, and summarized known functions are as indicated. Four molecules (underlined) were selected to verify their abundance difference in biochemical assays and their potential role in intravasation.
| Coverage | Accession number | Protein identified | Function |
|---|---|---|---|
| Cell surface proteins enriched in HT-lo/diss | |||
| 35.3 | P05556 | Integrin β-1 precursor (splice isoform 1) | Extracellular matrix protein interactions |
| 29.9 | P30450 | HLA class I histocompatibility antigen, A-26 α chain | Antigen presentation |
| 27.1 | P30447 | HLA class I histocompatibility antigen, A-23 α chain | Antigen presentation |
| 26 | P30459 | HLA class I histocompatibility antigen, A-74 α chain | Antigen presentation |
| 19.4 | P84095 | Rho-related GTP-binding protein RhoG | Cell motility/cytoskeletal rearrangement |
| 17.9 | O14828 | Secretory carrier-associated membrane protein 3 | Recycling |
| 15.5 | P16035 | Metalloproteinase inhibitor 2 precursor (TIMP-2) | Inhibition of active MMPs; activation of proMMP-2 |
| 11.6 | P14923 | JUP protein | Junctional plaque formation |
| 10.2 | Q96S97 | Myeloid-associated differentiation marker | Hematopoetic differentiation |
| 7.2 | P36897 | Transforming growth factor-β receptor type I precursor | Signaling in response to transforming growth factor-β |
| 6.8 | P11234 | Ras-related protein Ral-B | Signal transduction |
| 5.7 | P55287 | Cadherin-11 precursor | Homophilic cell adhesion |
| 5.1 | P49757 | Numb protein homolog | Cell fate during development |
| 4.9 | Q92542 | Nicastrin precursor | γ-Secretase complex |
| 4.1 | P23469 | Receptor-type tyrosine-protein phosphatase ε | Dephosphorylation |
| 4 | Q86UN3 | Nogo receptor-like 3 | Neuronal plasticity |
| 3.4 | Q14114 | Low density lipoprotein receptor-related protein 8 | Endocytosis of reelin/apolipoprotein |
| 1.8 | Q13443 | ADAM 9 precursor | Proteolysis, cell-cell, and cell-matrix interactions |
| 1.6 | Q8WWQ8 | Stabilin 2 precursor | Hyaluronic acid endocytosis |
| 0.9 | P98161 | Polycystin 1 precursor | Ion channel regulation |
|
0.2
|
Q8WX17
|
Ovarian cancer-related tumor marker CA125
|
Mucin barrier maintenance, heterotypic cell adhesion
|
| Cell surface proteins enriched in HT-hi/diss | |||
| 32.6 | P32970 | Tumor necrosis factor ligand superfamily member 7 | Cytokine signaling (CD27 ligand) |
| 33.6 | P13592 | Neural cell adhesion molecule-1 (NCAM-1) | Cell adhesion; neurite outgrowth |
| 19.1 | Q9BY32 | Inosine triphosphate pyrophosphatase | Hydrolyze ITP and dITP, possible oncogene |
| 26.5 | Q99536 | Synaptic vesicle membrane protein VAT-1 homolog | Oxidoreductase |
| 15.8 | Q658P3 | TSAP6 protein | Iron homeostasis, cell cycle regulation |
| 13.5 | Q12891 | Hyaluronidase 2 precursor | Hyaluronic acid hydrolysis |
| 22.3 | P18206 | Vinculin isoform VCL | Cell Adhesion |
| 15.1 | P13726 | Tissue Factor (TF) | Initiation of coagulation cascade |
| 16.4 | Q14118 | Dystroglycan precursor | Linkage of cytoskeleton to ECM |
| 10.9 | Q9HD45 | Transmembrane 9 superfamily protein member 3 | Nonaspanin protein, potential role in transport |
| 9.7 | Q01650 | Large neutral amino acids transporter small subunit 1 | Amino acid transport/uptake |
| 10.1 | Q9BX67 | Junctional adhesion molecule 3 precursor (JAM-C) | Cell-cell adhesion; migration |
| 12.3 | P37173-1 | Transforming growth factor-β receptor type II precursor | Signaling in response to transforming growth factor-β |
| 12.9 | Q9NZ53-1 | Podocalyxin-like protein | Leukocyte membrane protein |
| 13.3 | Q96JA1-1 | Leucine-rich repeats and Ig-like domains protein 1 | Negative feedback regulation of receptor tyrosine kinase signaling |
| 7.6 | Q9Y6N7-1 | Roundabout homolog 1 precursor | Migration (axonal guidance) |
| 10.2 | P22413 | Ectonucleotide pyrophospatase/phosphodiesterase 1 | ATP hydrolysis |
| 10.7 | P10586 | Receptor-type tyrosine-protein phosphatase F precursor | Possible adhesion receptor, PTPase |
| 10.4 | Q9ULF5 | KIAA1265 protein | Zinc transport, associated with invasive breast cancer |
| 4.8 | P05067 | Amyloid β A4 protein precursor | Adhesion, migration, neuronal outgrowth, signaling |
| 5.5 | P27544 | LAG1 longevity assurance homolog 2 | Lipid synthesis |
| 6.1 | P08069 | Insulin-like growth factor I receptor precursor | Signaling in response to insulin |
| 4.4 | P28827 | Receptor-type tyrosine-protein phosphatase μ | Cell to cell adhesion, growth, signaling |
| 6.2 | P54760 | Receptor protein-tyrosine kinase variant (EphB4v1) | Possible role in differentiation/development |
| 4 | Q04721 | Neurogenic locus notch homolog protein 2 precursor | Transcriptional regulation in response to ligand binding |
| 5 | Q8N5L2 | AXL receptor tyrosine kinase, isoform 1 | Protein phosphorylation |
Confirmation of Abundance Differences of Cell Surface Proteins in Vitro—To validate the findings of the proteomic array, we analyzed by Western blotting the abundance differences of selected cell surface proteins between HT-lo/diss and HT-hi/diss cells cultured in vitro. TIMP-2, one of the tissue inhibitors of matrix metalloproteinases, was identified by LC-MS/MS as more abundant in HT-lo/diss (Table 1) and was selected for biochemical validation because of its known role in tumor cell invasion and dissemination (26). Three proteins that were demonstrated to be more abundant in HT-hi/diss (Table 1) were also chosen for further analysis: NCAM-1 (neural cell adhesion molecule-1), which modulates cell to cell adhesion (27); JAM-C (junctional adhesion molecule-C), known to be involved in leukocyte and potentially tumor cell transendothelial migration (28–30); and TF, a surface receptor that initiates the coagulation cascade by binding the secreted coagulation factor VII at the cell surface (31–34). The levels of these molecules in cell surface protein enriched eluates and in whole cell lysates were further assayed by comparative Western blot analysis.
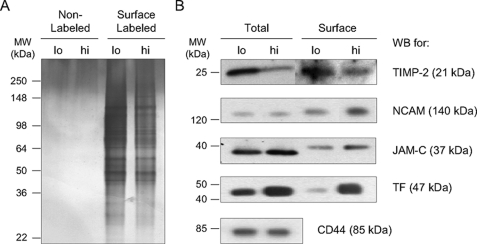
To compare the relative total protein content between the surface enriched eluates from HT-hi/diss and HT-lo/diss and to analyze the protein content of the nonbiotinylated control samples pulled down nonspecifically with avidin beads, an equal volume of each fraction was resolved on an SDS-polyacrylamide gel and silver-stained to determine the relative protein content of these fractions (Fig. 2A). The resulting gel allowed for visualization of surface enriched proteins from HT-hi/diss and HT-lo/diss cells and indicated that the HT-lo/diss fraction had a slightly higher protein content. The nonlabeled controls exposed to the same enrichment procedure did not contain detectable levels by silver staining of avidin-precipitated proteins, indicating high efficiency of the biotinylation, avidin precipitation, and 4 m urea stripping procedures. Equal amounts of the HT-hi/diss and HT-lo/diss biotinylated cell surface enriched fractions, along with whole cell lysates from HT-lo/diss and HT-hi/diss, were resolved on SDS-polyacrylamide gels for probing with antibodies to the selected proteins (Fig. 2B).
FIGURE 2.
Validation of LC-MS/MS data for selected proteins by Western blot analysis. HT-lo/diss (lo) and HT-hi/diss (hi) cells were labeled with biotin or left nonlabeled as a control. After avidin precipitation, eluates containing the biotinylated surface proteins or nonspecifically bound proteins from the control were resolved by SDS-PAGE and visualized by silver stain (A). The proteins eluted in the nonlabeled control samples are not detectable, indicating efficiency of the stringent washes after the enrichment procedure (A, left lanes). The overall protein content in the surface-biotinylated HT-lo/diss eluate is slightly higher than the HT-hi/diss eluate (A, right lanes). This silver-stained gel was used to determine relative protein content in the corresponding surface eluates and ensure equal loading in subsequent Western blot analyses. B, comparative Western blot (WB) analyses of cell lysates (Total) and cell membrane protein enriched fractions (Surface) of HT-hi/diss (hi) and HT-lo/diss (lo) were performed using primary antibodies specific to TIMP-2, NCAM-1, JAM-C, or TF, as indicated on the right. Anti-human CD44 (B, bottom) was included as a loading control. Since CD44 is not biotinylated efficiently and therefore is not precipitated by avidin beads, it was used as a loading control for total lysates only. Positions of the molecular weight standards in kDa (MW) are indicated on the left.
We observed that TIMP-2 levels were increased in both the surface fraction and total cell lysates of HT-lo/diss (Fig. 2B), the latter being in agreement with our previous findings (25).
Consistent with the observed LC-MS/MS differential of NCAM-1 between the HT-1080 variants (Table 1), Western blot analysis of the cell surface protein pool demonstrated that NCAM-1 levels were ∼2-fold greater on the surface of HT-hi/diss compared with HT-lo/diss cells, although the total cell content of NCAM-1 was similar between the two variants (Fig. 2B).
Surface enriched protein fractions also manifested 1.7-fold higher levels of JAM-C in HT-hi/diss than in HT-lo/diss (Fig. 2B), thus confirming the LC-MS/MS data (Table 1). Levels of JAM-C in the total cell lysates also differed (1.3-fold) between the two cell lines, albeit not as dramatically as in the surface fraction. There was a slight difference in the apparent molecular weight of this protein between the cell surface pool and the whole cell lysate, possibly reflecting a different glycosolation pattern in cell surface JAM-C, as this protein has two potential N-linked glycosolation sites (35).
Among the selected proteins, TF represented a molecule exhibiting the highest protein expression differential between the HT-1080 intravasation variants. In the cell surface fractions, TF protein levels were dramatically, 10.8-fold, higher in HT-hi/diss as compared with HT-lo/diss and also 2-fold higher in the HT-hi/diss total cell lysates over HT-lo/diss (Fig. 2B).
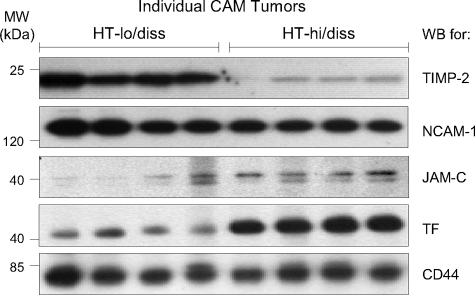
Confirmation of Protein Abundance Differences in Primary Tumors—Relative in vivo protein levels of TIMP-2, NCAM-1, JAM-C, and TF were next analyzed in primary tumor xenografts. HT-hi/diss and HT-lo/diss cells were grafted on the CAM of day 10 embryos and allowed to form primary tumors for 5 days, at which time the tumors were harvested and lysed. Lysates from individual CAM tumors were resolved by SDS-PAGE and transblotted, and membranes were immunoprobed for TIMP-2, NCAM-1, JAM-C, and TF. TIMP-2 levels were higher in the HT-lo/diss tumors as compared with the HT-hi/diss tumors (Fig. 3), thus confirming the expression differential of this molecule demonstrated in the cells cultured in vitro. The HT-hi/diss and HT-lo/diss primary tumor lysates contained comparable amounts of NCAM-1 protein (Fig. 3), which was in agreement with the similar levels of NCAM-1 between the two variants detected in total cell lysates of in vitro cultures. JAM-C protein levels were higher in HT-hi/diss CAM primary tumors compared with the HT-lo/diss counterparts (Fig. 3), consistent with the differential indicated by the proteomic array and Western blot analyses of both whole cell lysates and cell surface enriched fractions (Fig. 2B). The most dramatic in vivo observation, however, was the average 4.2-fold difference in TF protein levels between HT-hi/diss and HT-lo/diss primary tumors (Fig. 3), reaffirming its pronounced differential determined by the proteomic approach in cultured cells (10.8-fold differential between the cell surface eluates). This finding, along with the documented role of TF in tumor progression (reviewed in Ref. 34) prompted us to modulate the function of this protein in vivo to verify its contribution to tumor cell intravasation.
FIGURE 3.
Confirmation of protein abundance differences in primary tumors. HT-hi/diss or HT-lo/diss cells were grafted onto the CAM of day 10 embryos. After 5 days of development, individual primary tumors were harvested and lysed in modified radioimmune precipitation assay (mRIPA) buffer, and equal amounts of proteins were resolved by SDS-PAGE. After transblotting, membranes were analyzed by Western blotting (WB) with antibodies against TIMP-2, NCAM-1, JAM-C, TF, or CD44, as indicated on the right. The CD44 antibody does not cross-react with chick tissues and serves as a human protein loading control, since primary tumor samples contain a mixture of chick and human proteins. Positions of the molecular weight standards in kDa (MW) are indicated at the left.
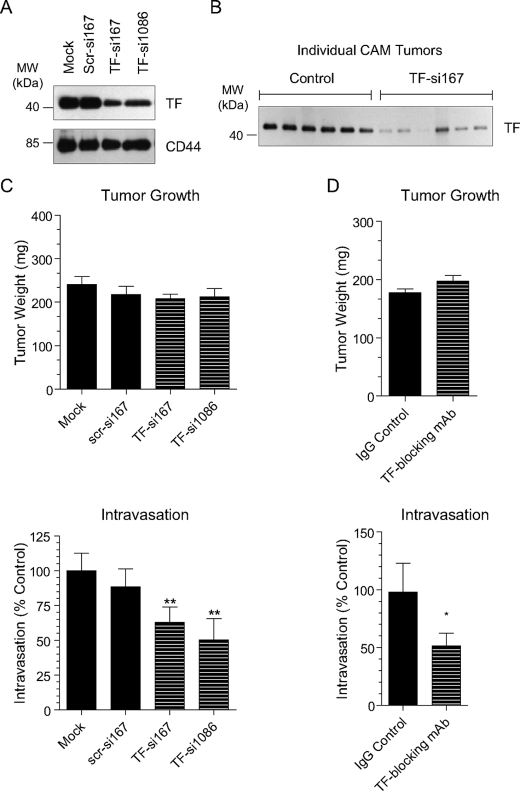
Down-regulation of Tissue Factor Protein Decreases Intravasation—In order to functionally link the increased expression of TF to enhanced intravasation and dissemination of HT-hi/diss, we took two approaches to decrease TF function in vivo:(a) down-regulation of TF expression by siRNA interference and (b) inhibition of TF with a function-blocking antibody.
One of the advantages of the chick embryo metastasis assay is that intravasation can be quantified as early as 4–5 days after tumor cell grafting (i.e. when transient siRNA transfections still continue to repress gene expression). TF expression was down-regulated in HT-hi/diss using two independent siRNA constructs, TF-si167 and TF-si1086. Control cells were mock-transfected (Lipofectamine alone) or transfected with a nonsilencing scrambled construct (scr-si167). Both TF-specific siRNA constructs decreased TF protein expression to 36–53% of control levels 24 h after transfection (Fig. 4A), at which time the TF-si167-, TF-si1086-, scr-si167-, or mock-transfected cells were inoculated onto the CAMs of day 10 chick embryos. To confirm that down-regulation of TF persisted until the time of tumor harvest, individual primary tumors were lysed to analyze in vivo TF expression by Western blotting. As can be seen in Fig. 4B, protein levels of TF were still reduced 5 days after cell grafting on the CAM in TF-siRNA primary tumors compared with control tumors. Overall, the siRNA treatment did not significantly affect primary tumor growth (Fig. 4C, top).
FIGURE 4.
In vivo down-regulation of TF by siRNA interference and function-blocking antibody decreases HT-hi/diss intravasation. Tissue factor expression was down-regulated in HT-hi/diss by two different siRNA constructs (TF-si167 or TF-si1086). Control cells were mock-transfected or transfected with a scrambled siRNA control (scr-si167). TF silencing by the siRNA constructs was confirmed by Western blot analysis of cell lysates 24 h after transfection (A, top). Membranes were probed with the CD44-specific mAb to confirm equal protein loading (A, bottom). Twenty-four hours after transfection, the cells were grafted onto the CAM of day 10 embryos. Individual primary tumors were harvested and lysed after 5 days and verified by Western blot for sustained TF down-regulation (B). Positions of the molecular weight standards in kDa (MW) are indicated on the left. Tumor weights were not affected by the siRNA treatment (C, top). Numbers of intravasated cells were quantified by Alu-qPCR analysis of genomic DNA (as described under “Experimental Procedures”). Levels of intravasation of siRNA-treated cells are presented as the percentage of mock-transfected control (C, bottom). Bars are means ± S.E. calculated from pooling percentage changes from 2–6 individual experiments, each involving 7–10 embryos (n) per variable (mock, n = 45; scr-si167, n = 18; TF-si167, n = 52; TF-si1086, n = 15). In a second set of experiments, developing HT-hi/diss tumors were treated with 25 or 50 μg of TF function-blocking antibody mAb 5G9 or control mouse IgG. The mAb 5G9 treatment did not affect primary tumor growth (D, top) but significantly reduced intravasation to 51% of control (mouse IgG) levels (D, bottom). Bars, means ± S.E. from three independent experiments, each involving 9–12 embryos (n) per variable (control, n = 30; TF mAb, n = 33). ** and *, p < 0.05 and p < 0.05 in two- and one-tailed t test, respectively.
Whether intravasation of HT-hi/diss was affected by down-regulation of TF was determined by analysis of distal portions of the CAM using Alu-qPCR to detect and quantify intravasated tumor cells. The numbers of human cells in the CAM vasculature was significantly reduced in TF-si167 and TF-si1086 transfectants to 63 and 50% of control levels, respectively, whereas nonsilencing scr-si167 did not have a significant effect on intravasation (Fig. 4C, bottom). Thus, it appears that the increase in TF protein expression in HT-hi/diss facilitates tumor cell intravasation.
To further analyze the involvement of TF in tumor cell dissemination and to control for any potential off-target effects of the siRNA transfection, we turned to another method of blocking protein function, namely the inhibition by a specific function-blocking antibody. We employed mAb 5G9, which efficiently prevents TF from initiating the coagulation cascade at the cell surface interface (24). This function-blocking antibody was applied topically to developing CAM tumors daily or introduced with the cells at the time of grafting onto the CAM. Since both antibody treatment strategies produced similar inhibitory effects, the data from these experiments were pooled to calculate fold changes over a mouse IgG control. The TF function-blocking mAb 5G9 decreased HT-hi/diss intravasation to 51% of control levels (Fig. 4D, bottom) without significantly affecting primary tumor growth (Fig. 4D, top). Thus, both methods of down-regulating TF (i.e. by decreasing expression at the message level or blocking function by specific antibody ligation) reduced HT-hi/diss intravasation.
DISCUSSION
To study tumor cell intravasation and dissemination, our laboratory has generated a pair of congenic variants of the human fibrosarcoma HT-1080 cell line (i.e. HT-hi/diss and HT-lo/diss), which exhibit a 50–100-fold differential in their ability to spontaneously metastasize. Importantly, both cell types display similar abilities to form primary tumors in spontaneous metastasis and to colonize secondary organs after intravenous injection in experimental metastasis, suggesting that their substantial difference in tumor dissemination must lie in their ability to successfully complete early steps in the metastatic cascade (7). We have taken multiple approaches to investigate the nature of this metastasis differential, including activity-based protein profiling (9), MMP expression profiling (25), cDNA and cytokine arrays (data not shown), and the cell surface proteomic approach described herein. Alterations in expression and function of cell surface proteins have long been associated with cancer progression which is not surprising, since many growth factor and cytokine receptors, adhesion molecules, and proteases or protease receptors are functionally active at the cell membrane (36). We therefore focused this study on identifying in vitro surface proteins differentially expressed between the HT-1080 dissemination variants and validating in vivo the functional impact of selected protein abundance differences.
In this study, several modifications were introduced to standard cell surface protein enrichment procedures in order to increase specificity and decrease the contamination with nonsurface proteins (Fig. 1). Analysis of the resulting surface proteins from HT-lo/diss and HT-hi/diss by LC-MS/MS provided means to compare their surface proteomes and identify proteins with potential abundance differences between the intravasation variants. Ultimately, we identified 26 known cell surface proteins with higher abundance in HT-hi/diss and 21 known cell surface-associated proteins with higher abundance in HT-lo/diss. It should be kept in mind that the identification by this analysis of a particular protein in only one cell variant does not indicate the absence of that protein in the other variant but points to a difference in surface expression levels of the protein between the two tumor cell counterparts.
Cell surface protein enrichment combined with MudPIT provided a valuable tool for identifying proteins with differential expression between the cell dissemination variants and, therefore, with potential roles in metastasis. Specifically, we focused on one protein that was identified by LC-MS/MS as more abundant in HT-lo/diss (i.e. TIMP-2) and three proteins identified as more abundant in HT-hi/diss (i.e. JAM-C, NCAM-1, and TF) and confirmed their differentials by Western blot analyses in both whole cell lysates and surface protein enriched fractions. It has been documented that tumor cells cultured in the presence of fetal calf serum, which contains an abundance of growth factors and cytokines, often have protein expression profiles different from those of primary tumors in vivo (37). Therefore, we sought to confirm that the differentials in surface protein expression identified by LC-MS/MS in cultured cells were also manifested within the tumor microenvironment and would have functional significance during actual intravasation and dissemination of the tumor cells in vivo. To this end, we next validated in vivo that our method of in vitro labeling and surface protein enrichment can be exploited to identify protein differentials with functional relevance for metastasis. This was accomplished by probing primary tumors for the selected proteins and by in vivo modulating the expression and function of TF, our most attractive candidate protein.
By Western blot analysis, we confirmed that the MMP inhibitor TIMP-2, identified by LC-MS/MS as more abundant in HT-lo/diss was present at higher levels by Western blot analysis in HT-lo/diss surface fractions, whole cell lysates, and primary tumors as compared with HT-hi/diss. Previously, high levels of TIMP-2 in HT-lo/diss were linked to their reduced ability to intravasate (25), affirming the functional relevance of this protein identified by our cell surface proteomic approach. Although TIMP-2 is a secreted protein, it can be detected at the cell surface due to its binding with MT1-MMP (38, 39). Since both HT-lo/diss and HT-hi/diss express MT1-MMP (7, 25), the presence and detection of TIMP-2 was not unexpected and probably represented the MT1-MMP-bound pool of TIMP-2. In accordance with this, MT1-MMP was detected by the proteomic array in the surface fraction of both cell variants, but since it was also detected in the nonbiotinylated control, MT1-MMP was not listed in supplemental Table 1. The confirmatory finding that TIMP-2 was more abundant in HT-lo/diss by Western blotting provided a validation of our approach to cell surface proteomics by LC-MS/MS. However, for further investigation, we chose to focus on proteins identified as more abundant in HT-hi/diss, since these proteins would represent targets for in vivo down-regulation to directly assess their functional involvement in intravasation.
Western blot analysis confirmed the proteomic array data demonstrating that JAM-C, NCAM-1, and TF were significantly more abundant in HT-hi/diss than in HT-lo/diss. In each case, these proteins were immunologically detected in greater quantities in the cell surface protein fractions of HT-hi/diss as compared with HT-lo/diss. Interestingly, in the case of NCAM-1, protein levels in the cell surface enriched eluate differed 2-fold between the two variants, whereas total lysates of cells cultured in vitro and primary tumors grown in vivo had similar levels of this protein. A difference specifically in surface NCAM-1 might represent a difference in cellular trafficking or turnover of the surface pool (e.g. by degradation or shedding) between the variants. This result indicates that the described cell surface proteomic approach can be used to identify differentials in cell surface pools even when total cellular contents of a specific protein are similar and also highlights the importance of identifying aberrant or differential subcellular localization and not just total protein expression of potentially important targets. Thus, the specific difference in membrane-associated NCAM-1 identified by cell surface proteomics in this study, for example, would be missed by the more global arrays analyzing total cellular proteins.
Another protein that has been identified by our array as enriched in HT-hi/diss is a transmembrane adhesion molecule, JAM-C, known to interact with the endothelium during leukocyte transendothelial migration (28, 29). JAM-C is an intriguing target, since several parallels can be drawn between tumor cell intravasation and leukocyte transendothelial migration in terms of interactions with the endothelium. In this regard, a recent study has reported that down-regulation of JAM-C by siRNA inhibited dissemination of HT-1080 fibrosarcoma cells in a mouse experimental metastasis model (40), implicating JAM-C in the late stages of metastasis. We observed a difference in JAM-C protein expression between the HT-lo/diss and HT-hi/diss intravasation variants in primary tumor samples as well as in cell surface enriched fractions, suggesting that JAM-C might also have a functional role in the early steps of metastasis. Importantly, the difference in JAM-C levels between the HT-lo/diss and HT-hi/diss variants was most pronounced in the cell surface enriched fraction. Since JAM-C functions at the cell surface where ligand binding occurs, our findings highlight an important difference in the specific functional pool of this cell surface protein, which again might be overlooked if only the total cell lysates are analyzed. A direct role of JAM-C in the early steps of tumor dissemination is currently under investigation.
The most dramatic difference between HT-lo/diss and HT-hi/diss observed in this study was the up to 10.8-fold differential in TF levels in the surface enriched protein fractions, whole cell lysates, and primary tumors. Since TF is the major initiator of the coagulation cascade, increased TF expression has long been associated with a hypercoagulable state frequently observed in advanced, metastatic cancer (41). This association has proved to be functionally relevant both for primary tumor growth and late stages of metastasis as cells overexpressing TF become more angiogenic and metastatic through TF-mediated thrombin generation, fibrin deposition, and platelet activation. Additionally, TF can induce signaling events mediated by protease-activated receptor-2 and, thus, may contribute to cancer progression independently of coagulation (34, 42, 43). Tumor growth appears to be facilitated by TF expression (34) via mechanisms that may involve PAR-mediated signaling events (42). In addition, recent evidence has implicated TF-induced signaling in cell migration (50, 51), a process critical for cancer cell dissemination. In experimental metastasis models, which bypass intravasation and recapitulate late stages of the metastatic cascade, overexpression of TF in tumor cells enhanced colonization, whereas inhibition of TF function correspondingly decreased experimental metastasis (24, 44–49).
Despite the documented role of TF in tumor growth, angiogenesis, and late stages of metastasis, its role in the early process of tumor cell intravasation has not yet been elucidated. Therefore, we investigated the functional role of TF in intravasation using an in vivo spontaneous metastasis model. To this end, TF function was modulated in HT-hi/diss by down-regulating TF expression with TF-specific siRNA and by inhibiting protein function with a TF function-blocking antibody. Both approaches to down-regulate TF function substantially reduced HT-hi/diss intravasation without affecting primary tumor development, thus implicating TF as a possible critical factor in early steps of tumor cell dissemination. These findings are consistent with previous reports linking TF expression to tumor progression and also validate our proteomic method for identifying functionally relevant, metastasis-associated protein targets.
In summary, our cell surface proteomic array generated a list of 47 surface proteins with abundance differences between the two congenic HT-1080 intravasation variants. Confirmation of the LC-MS/MS data was accomplished by analyzing levels of selected proteins in the two variants by a second independent method, Western blotting, performed with cells cultured in vitro and primary tumors grown in vivo. Differential expression at the cell surface was demonstrated between the variants for all selected proteins, whereas differences in total protein contents were more restricted. Importantly, selected proteins indicated by cell surface proteomics have been implicated in vivo in spontaneous metastasis, since TIMP-2, JAM-C, and TF were each differentially expressed between the two phenotypically distinct variants in primary tumors. Functional modulation of one of the selected proteins (i.e. TF) substantially decreased intravasation of the HT-hi/diss variant. Overall, our approach to enrich and purify cell surface proteins allowed us to identify by LC-MS/MS several specific molecules that are related to tumor cell intravasation and represent attractive targets for inhibition of tumor cell dissemination and metastasis.
Supplementary Material
Acknowledgments
We thank Chenxing Li, Lauren Hayden, and David Balser for excellent technical assistance and Sherry Niessen for assistance with the MudPIT analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants CA55852 and CA105412 (to J. P. Q.) and National Institutes of Health, NCI, Grant 2 T32CA77109-08 (to E. M. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
Footnotes
The abbreviations used are: MudPIT, multidimensional protein identification technology; MMP, matrix metalloproteinase; MT1-MMP, membrane-type 1 MMP; TIMP, tissue inhibitor of metalloproteinase; TF, tissue factor; MS, mass spectrometry; LC, liquid chromatography; CAM, chorioallantoic membrane; qPCR, quantitative real-time PCR; Alu-qPCR, qPCR for Alu DNA repeat sequences; siRNA, small interfering RNA; PBS, phosphate-buffered saline; TBS, Tris-buffered saline; mAb, monoclonal antibody.
References
- 1.Chambers, A. F., Groom, A. C., and MacDonald, I. C. (2002) Nat. Rev. Cancer 2 563-572 [DOI] [PubMed] [Google Scholar]
- 2.Fidler, I. J. (2003) Nat. Rev. Cancer 3 453-458 [DOI] [PubMed] [Google Scholar]
- 3.Pantel, K., and Brakenhoff, R. H. (2004) Nat. Rev. Cancer 4 448-456 [DOI] [PubMed] [Google Scholar]
- 4.Wang, W., Goswami, S., Sahai, E., Wyckoff, J. B., Segall, J. E., and Condeelis, J. S. (2005) Trends Cell Biol. 15 138-145 [DOI] [PubMed] [Google Scholar]
- 5.Gupta, G. P., and Massague, J. (2006) Cell 127 679-695 [DOI] [PubMed] [Google Scholar]
- 6.Zijlstra, A., Lewis, J., Degryse, B., Stuhlmann, H., and Quigley, J. P. (2008) Cancer Cell 13 221-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deryugina, E. I., Zijlstra, A., Partridge, J. J., Kupriyanova, T. A., Madsen, M. A., Papagiannakopoulos, T., and Quigley, J. P. (2005) Cancer Res. 65 10959-10969 [DOI] [PubMed] [Google Scholar]
- 8.Liu, Y., Patricelli, M. P., and Cravatt, B. F. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 14694-14699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madsen, M. A., Deryugina, E. I., Niessen, S., Cravatt, B. F., and Quigley, J. P. (2006) J. Biol. Chem. 281 15997-16005 [DOI] [PubMed] [Google Scholar]
- 10.Hastie, C., Saxton, M., Akpan, A., Cramer, R., Masters, J. R., and Naaby-Hansen, S. (2005) Oncogene 24 5905-5913 [DOI] [PubMed] [Google Scholar]
- 11.Scheurer, S. B., Roesli, C., Neri, D., and Elia, G. (2005) Proteomics 5 3035-3039 [DOI] [PubMed] [Google Scholar]
- 12.Jang, J. H., and Hanash, S. (2003) Proteomics 3 1947-1954 [DOI] [PubMed] [Google Scholar]
- 13.Shin, B. K., Wang, H., Yim, A. M., Le Naour, F., Brichory, F., Jang, J. H., Zhao, R., Puravs, E., Tra, J., Michael, C. W., Misek, D. E., and Hanash, S. M. (2003) J. Biol. Chem. 278 7607-7616 [DOI] [PubMed] [Google Scholar]
- 14.Zhang, W., Zhou, G., Zhao, Y., and White, M. A. (2003) Electrophoresis 24 2855-2863 [DOI] [PubMed] [Google Scholar]
- 15.Link, A. J., Eng, J., Schieltz, D. M., Carmack, E., Mize, G. J., Morris, D. R., Garvik, B. M., and Yates, J. R., III (1999) Nat. Biotechnol. 17 676-682 [DOI] [PubMed] [Google Scholar]
- 16.Washburn, M. P., Wolters, D., and Yates, J. R., III (2001) Nat. Biotechnol. 19 242-247 [DOI] [PubMed] [Google Scholar]
- 17.Liu, H., Lin, D., and Yates, J. R., III (2002) BioTechniques 32 898, 900, 902 passim [DOI] [PubMed] [Google Scholar]
- 18.Kim, J., Yu, W., Kovalski, K., and Ossowski, L. (1998) Cell 94 353-362 [DOI] [PubMed] [Google Scholar]
- 19.Zijlstra, A., Mellor, R., Panzarella, G., Aimes, R. T., Hooper, J. D., Marchenko, N. D., and Quigley, J. P. (2002) Cancer Res. 62 7083-7092 [PubMed] [Google Scholar]
- 20.Eng, J. K., McCormack, Ashley L., Yates, and John, R., III (1994) J. Am. Soc. Mass. Spectrom. 5 976-989 [DOI] [PubMed] [Google Scholar]
- 21.Pang, J. X., Ginanni, N., Dongre, A. R., Hefta, S. A., and Opitek, G. J. (2002) J. Proteome Res. 1 161-169 [DOI] [PubMed] [Google Scholar]
- 22.Liu, H., Sadygov, R. G., and Yates, J. R., III (2004) Anal. Chem. 76 4193-4201 [DOI] [PubMed] [Google Scholar]
- 23.Holen, T., Amarzguioui, M., Wiiger, M. T., Babaie, E., and Prydz, H. (2002) Nucleic Acids Res. 30 1757-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller, B. M., Reisfeld, R. A., Edgington, T. S., and Ruf, W. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 11832-11836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Partridge, J. J., Madsen, M. A., Ardi, V. C., Papagiannakopoulos, T., Kupriyanova, T. A., Quigley, J. P., and Deryugina, E. I. (2007) J. Biol. Chem. 282 35964-35977 [DOI] [PubMed] [Google Scholar]
- 26.Blavier, L., Henriet, P., Imren, S., and Declerck, Y. A. (1999) Ann. N. Y. Acad. Sci. 878 108-119 [DOI] [PubMed] [Google Scholar]
- 27.Cunningham, B. A., Hemperly, J. J., Murray, B. A., Prediger, E. A., Brackenbury, R., and Edelman, G. M. (1987) Science 236 799-806 [DOI] [PubMed] [Google Scholar]
- 28.Bradfield, P. F., Scheiermann, C., Nourshargh, S., Ody, C., Luscinskas, F. W., Rainger, G. E., Nash, G. B., Miljkovic-Licina, M., Aurrand-Lions, M., and Imhof, B. A. (2007) Blood 110 2545-2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chavakis, T., Keiper, T., Matz-Westphal, R., Hersemeyer, K., Sachs, U. J., Nawroth, P. P., Preissner, K. T., and Santoso, S. (2004) J. Biol. Chem. 279 55602-55608 [DOI] [PubMed] [Google Scholar]
- 30.Santoso, S., Orlova, V. V., Song, K., Sachs, U. J., Andrei-Selmer, C. L., and Chavakis, T. (2005) J. Biol. Chem. 280 36326-36333 [DOI] [PubMed] [Google Scholar]
- 31.Mackman, N., Morrissey, J. H., Fowler, B., and Edgington, T. S. (1989) Biochemistry 28 1755-1762 [DOI] [PubMed] [Google Scholar]
- 32.Davie, E. W., Fujikawa, K., and Kisiel, W. (1991) Biochemistry 30 10363-10370 [DOI] [PubMed] [Google Scholar]
- 33.Ruf, W., and Edgington, T. S. (1994) FASEB J. 8 385-390 [PubMed] [Google Scholar]
- 34.Belting, M., Ahamed, J., and Ruf, W. (2005) Arterioscler. Thromb. Vasc. Biol. 25 1545-1550 [DOI] [PubMed] [Google Scholar]
- 35.Cunningham, S. A., Arrate, M. P., Rodriguez, J. M., Bjercke, R. J., Vanderslice, P., Morris, A. P., and Brock, T. A. (2000) J. Biol. Chem. 275 34750-34756 [DOI] [PubMed] [Google Scholar]
- 36.Bogenrieder, T., and Herlyn, M. (2003) Oncogene 22 6524-6536 [DOI] [PubMed] [Google Scholar]
- 37.Lee, J., Kotliarova, S., Kotliarov, Y., Li, A., Su, Q., Donin, N. M., Pastorino, S., Purow, B. W., Christopher, N., Zhang, W., Park, J. K., and Fine, H. A. (2006) Cancer Cell 9 391-403 [DOI] [PubMed] [Google Scholar]
- 38.Strongin, A. Y., Collier, I., Bannikov, G., Marmer, B. L., Grant, G. A., and Goldberg, G. I. (1995) J. Biol. Chem. 270 5331-5338 [DOI] [PubMed] [Google Scholar]
- 39.Zucker, S., Drews, M., Conner, C., Foda, H. D., DeClerck, Y. A., Langley, K. E., Bahou, W. F., Docherty, A. J., and Cao, J. (1998) J. Biol. Chem. 273 1216-1222 [DOI] [PubMed] [Google Scholar]
- 40.Fuse, C., Ishida, Y., Hikita, T., Asai, T., and Oku, N. (2007) J. Biol. Chem. 282 8276-8283 [DOI] [PubMed] [Google Scholar]
- 41.Rao, L. V. (1992) Cancer Metastasis Rev. 11 249-266 [DOI] [PubMed] [Google Scholar]
- 42.Versteeg, H. H., Schaffner, F., Kerver, M., Petersen, H. H., Ahamed, J., Felding-Habermann, B., Takada, Y., Mueller, B. M., and Ruf, W. (2008) Blood 111 190-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Versteeg, H. H., Spek, C. A., Peppelenbosch, M. P., and Richel, D. J. (2004) Mol. Med. 10 6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller, B. M., and Ruf, W. (1998) J. Clin. Invest. 101 1372-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bromberg, M. E., Bailly, M. A., and Konigsberg, W. H. (2001) Thromb. Haemost. 86 1210-1214 [PubMed] [Google Scholar]
- 46.Wang, X., Wang, M., Amarzguioui, M., Liu, F., Fodstad, O., and Prydz, H. (2004) Int. J. Cancer 112 994-1002 [DOI] [PubMed] [Google Scholar]
- 47.Voigtlander, C., Rand, A., Liu, S. L., Wilson, T. J., Pittelkow, M. R., Getz, M. J., and Kelm, R. J., Jr. (2002) J. Cell. Biochem. 85 54-71 [DOI] [PubMed] [Google Scholar]
- 48.Esumi, N., Fan, D., and Fidler, I. J. (1991) Cancer Res. 51 4549-4556 [PubMed] [Google Scholar]
- 49.Ruf, W., and Mueller, B. M. (2006) Semin. Thromb. Hemost. 32 Suppl. 1, 61-68 [DOI] [PubMed] [Google Scholar]
- 50.Jiang, X., Bailly, M. A., Panetti, T. S., Cappello, M., Konigsberg, W. H., and Bromberg, M. E. (2004) J. Thromb. Haemost. 2 93-101 [DOI] [PubMed] [Google Scholar]
- 51.Koizume, S., Jin, M. S., Miyagi, E., Hirahara, F., Nakamura, Y., Piao, J. H., Asai, A., Yoshida, A., Tsuchiya, E., Ruf, W., and Miyagi, Y. (2006) Cancer Res. 66 9453-9460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.