Abstract
The requirement for phosphatidylinositol 3-kinase (PI3K) in the establishment of cell polarity and motility in a number of cell types has recently come into question. In this study, we demonstrate that inhibition of PI3K by wortmannin in neutrophil-like differentiated HL60 cells expressing CXCR2 resulted in reduced cell motility but normal chemotaxis in response to a gradient of CXCL8. However, wortmannin inhibition of PI3K did impair the ability of cells to re-orient their polarity and respond quickly to a change in the direction of the CXCL8 gradient. We hypothesized that Src-regulated ELMO-Dock2-Rac2 activation mediates chemotaxis in the absence of PI3K activity. Inhibition of Src with the small molecule inhibitor, PP2, or inhibition of Dock2 by shRNA knockdown confirmed the functional role of Src and Dock2 in regulating chemotaxis when PI3K was inhibited. Moreover, neutrophils isolated from bone marrow of hck-/-fgr-/-lyn-/- mice exhibited much more severe inhibition of chemotaxis when PI3K was blocked with wortmannin as compared with neutrophils isolated from bone marrow of wild-type mice. Thus, PI3K and Src-ELMO-Dock2 pathways work in parallel to activate Rac2 and modulate chemotaxis in response to a CXCL8 gradient in neutrophils.
Chemotaxis is the process by which cells migrate toward a chemical gradient. It has been reported that phosphatidylinositol 3-kinases (PI3K)2 and their product, phosphatidylinositol 3,4,5-trisphosphate (PIP3), play an important role in sensing and polarizing the cell toward a chemoattractant gradient(1–4). PI3Kγ, the dominant class I PI3K in neutrophils, catalyzes the 3′-phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate PIP3. In PI3Kγ knockout mice, recruitment of neutrophils to sites of inflammation was significantly reduced, though not completely inhibited. Additionally, in vitro chemotaxis assays showed incomplete inhibition of chemotaxis for neutrophils derived from these mice (5–7). Based on these data, it has been hypothesized that a PI3K-independent pathway exists in neutrophils that allows these cells to move toward a gradient of chemokine. Src family kinases (SFK) are a group of tyrosine kinases that play fundamental roles in mediating cell proliferation, survival, and focal adhesion regulation in response to a variety of environmental stimuli. In certain G protein-coupled receptor-mediated signal transduction pathways, SFK can bind and be activated by Gαi and Gαs subunits of the heterotrimeric G-protein (8). This PI3K-independent signal transduction pathway activated directly by heterotrimeric G proteins may lead to a different cascade of signal activation.
Members of the Rho family of small GTPases are essential for regulation of cell cytoskeleton reorganization during motility and thus have great impact on cell morphology (polarized versus non-polarized). Activation of Rac has been shown to be required for extension of pseudopodia at the leading edge of moving cells. Rac2, as a hematopoietic-specific member of Rac, plays critical roles in regulating chemotaxis, endothelial cell rolling, superoxide production, and kinase activation in neutrophils (9–12). Recently, it has been reported that a random cell movement consists of bifurcation of the pseudopod at several locations and in diverse directions. In contrast, for persistent directional cell movement induced by a gradient of chemoattractant, there is domination and persistence of the pseudopod closest to the gradient (13). In this circumstance, the level of activated Rho GTPases, especially Rac, is key to the directional cell movement. Small Rho family GTPases are bound and activated directly by a group of proteins called guanine exchange factors (GEFs). It has been reported that PI3K up-regulates several members of these GEFs (14, 15). More recently, an evolutionarily conserved family of proteins, CED-5 in Caenorhabditis elegans, Dock 180 in mammals and Mycoplast city in Drosophila (CDM) were identified to function as novel GEFs for Rac and other small Rho family GTPases (16–18). Unlike traditional GEFs, Dock180 proteins do not contain Db1 homology or pleckstrin homology (DH-PH) domains. Instead, they catalyze guanine exchange through the Dock homology region-2 (DHR2) or Docker domain (19). Dock180 proteins fold in such a way that the catalytic domain (Docker) is blocked, resulting in auto-inhibition. Only when the partner protein ELMO binds to a Dock180 family protein is the Docker domain exposed and allowed to bind and activate Rac (18). The binding of ELMO to the Dock180 family protein may also help target the complex to the plasma membrane (20). In addition, Dock180 proteins contain a DHR-1 domain that interacts with PIP3 and elimination of this domain blocks chemotaxis (21).
Dock2 is a leukocyte-specific Dock180 family member that plays a critical role in leukocyte polarization and migration by stimulating Rac activity (22). The binding of ELMO1 to Dock2, and often with another adaptor protein, CrkL, forms a multi-component complex that binds and activates the inactive form of Rac (23, 24). ELMO1 and CrkL were both found to be phosphorylated in lymphocytes by the Src family kinases, Hck and Lyn, respectively (24, 25). Although phosphorylation of ELMO1 is not required for binding of ELMO1 to Dock2, it is necessary for the function of this complex (25). In this report, we demonstrate that both PI3K- and Src-dependent pathways independently lead to activation of Rac2 in neutrophils, which is responsible for the directional cell movement in response to a gradient of CXCL8.
EXPERIMENTAL PROCEDURES
Materials—The triple knockout (hck-/-fgr-/-lyn-/-) mice were generated by Clifford Lowell as previously described (26). Animals were raised in the facilities at UCSF and used according to AALAC-recommended procedures. The HL60 cell line was kindly provided by Henry Bourne, UCSF. HL60 cells stably expressing CXCR2 were transfected and selected in our laboratory as previously described (27). HL60 cells were cultured and differentiated as previously described (27).
Chemicals and Antibodies—RPMI 1640 was purchased from Invitrogen (Carlsbad, CA). Inhibitors wortmannin, LY294002, and PP2 were purchased from Calbiochem. Antibodies used in this study were purchased from following companies: Rabbit polyclonal antibodies to Erk (K23), Akt1/2 (H136), c-Src (SRC2), CrkL(C-20), and mouse monoclonal antibody to phospho-Erk (E4) were from Santa Cruz Biotechnology (Santa Cruz, CA); Polyclonal rabbit antibody to phospho-Akt (Thr-308) was from Cell Signaling Technology (Beverly, MA); Polyclonal rabbit antibody to phospho-Src (pY418) was from BIOSOURCE, Invitrogen (Carlsbad, CA); Goat polyclonal antibody to human ELMO1 was from Abcam Inc. (Cambridge, MA); Mouse monoclonal antibody to phosphotyrosine (4G10) and rabbit polyclonal antibody to Rac2 were from Upstate Biotechnology (Lake Placid, NY); Normal goat IgG was from Jackson Biotechnology (Bar Harbor, ME). Anti-human Dock2 antibody was kindly provided by Michiyuki Matsuda (Kyoto University, Japan). Horseradish peroxidase-conjugated secondary antibodies to mouse or rabbit were from Chemicon International (Temecula, CA); infra-red conjugated secondary antibodies, IRdye 800, was from Rockland Immunochemicals, Inc. (Gilbertsville, PA) and Alexa 680 was from Molecular Probes, Inc. (Eugene, OR). ECL reagents were purchased from Amersham Biosciences (Piscataway, NJ), and x-ray films were from Kodak Company (Rochester, NY). CXC ligand 8 (CXCL8) was kindly provided by Krishna Rajarathnam, University of Texas, Galveston or was purchased from PeproTech (Rocky Hill, NJ). Rhodamine-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR). Human fibronectin was purchased from BD Biosciences (Bedford, MA). Proteinase inhibitor cocktails, puromycin, and NAG (4-nitrophenyl 2-acetamido-2-deoxy-β-d-glucopyranoside) were purchased from Sigma-Aldrich, Inc.
shRNA Knockdown—Two Dock2 shRNA clones were selected from the GIPZ Lentiviral shRNAmir library from Open Biosystems (Huntsville, AL), ID V2LHS 100893 and V2LHS 100889. A non-silencing sequence in the same vector was chosen as the non-silencing control (NS). The lentiviruses containing shRNA or non-silencing sequence were packaged in the 293-FT cell line (Invitrogen, Carlsbad, CA). The medium containing the viruses was collected and used to infect HL60-CXCR2 cells after concentration through an Amicon Ultra filter (Millipore, Billerica, MA). Polyclonal stable cell lines were selected in 0.5 μg/ml puromycin.
Western Blot and Co-immunoprecipitation Assay—Differentiated HL60-CXCR2 cells were washed and resuspended in serum-free medium. After stimulation of 5–10 × 107 cells/ml with CXCL8 at varying concentrations, cells were lysed by mixing with an equal volume of ice-cold 2× lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, and 1% Triton X-100) supplemented with protease inhibitor mixture, and phosphatase inhibitor cocktails I and II. Cell lysates were clarified by centrifugation at 16,000 rpm for 5 min, analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as previously described (27). Co-immunoprecipitation assays were performed with protein A/G-agarose beads (Santa Cruz Biotechnology) as the manufacturer recommended. Quantification was performed using the Image J software (NIH, Bethesda, MD).
Rac Activity Assay—GST pull-down assay for Rac activation was performed as previously described by Benard et al. (28).
Cell Polarization in Zigmond Chamber and Immunostaining—Differentiated HL60-CXCR2 cells were washed, resuspended in serum-free RPMI 1640 medium, and seeded on a fibronectin (100 μg/ml)-precoated coverslip for 10 min at 37 °C, 5% CO2. The coverslip was placed on a Zigmond chamber (Neuroprobe Inc., Gaithersburg, MD) with cells facing down. One groove of the chamber was filled with serum-free medium and the other with 25 ng/ml CXCL8. The CXCL8 gradient was generated in the interface of these two solutions on the bridge of Zigmond chamber. After incubation for 15 min at 37 °C, 5% CO2, cells on the coverslip were fixed in 4% paraformaldehyde for 10 min at room temperature. The orientation of the polarized cells on the coverslip was observed under the inverted microscope and counted manually. The cells polarized toward the direction of gradient were counted as P+, and the cells polarized in the opposite direction of gradient were counted as P-. If the percentage of P+ over total number of cells counted (P+ + P-) is 50%, there is no biased cell polarization toward the chemokine gradient or no chemotaxis. If [P+/(P+ + P-)] × 100% is significantly greater than 50%, chemotaxis occurs. Immunostaining was performed on these polarized cells based on the procedures previously described in Ref. 29, and confocal images were taken with LSM510 Zeiss inverted microscope (Carl Zeiss Microimage, Germany).
Preparation of Neutrophils (PMN) from Mouse Bone Marrow—Mouse neutrophils were isolated from long bones (femurs and tibias) of hck-/-fgr-/-lyn-/- C57BL/6 mice or wild-type C57BL/6 and purified as described in Ref. 30. Briefly, HBSS was forced through mouse bones with a syringe. Total bone marrow cells were collected and red blood cells were removed by hypotonic buffer. Neutrophils were further purified in 3-layers of percoll gradient (78, 69, and 52%) by centrifugation at 1500 × g, 30 min without the brake. Cells at the interface between 78 and 69%, as well as the band of cells separated in the 79% percoll layer were collected and tested by flow cytometry with a specific neutrophil marker (Cedarlane Lab, Burlington, NC). Cells were resuspended in RPMI medium containing 10% fetal bovine serum and maintained at 37 °C, 5% CO2 until use.
Flow Cytometry—Purified neutrophils (5 × 105 cells) were washed with 0.1% BSA in HBSS (pH 7.2) and resuspended in 100 μl of BSA/HBSS. FITC-conjugated neutrophil marker (1:50 dilution) (Cedarlane Lab, Burlington, NC) was added, and cells were allowed to sit on ice for 1 h. Cells were subsequently washed three times with ice-cold 0.1% BSA/HBSS and resuspended in 500 μl of 0.1% BSA/phosphate-buffered saline containing 7AAD (Molecular Probes, Eugene, OR) for labeling live cells. Flow cytometry was performed using FACScan (Becton-Dickinson, San Jose, CA).
Chemotaxis Assays—The chemotaxis assay in a microfluidic chamber was performed as previously described (27). The chemotaxis assay for mouse bone marrow neutrophils was performed in a modified Boyden chamber MBA96 (Neuroprobe, Gaithersburg, MD) as follows. Different concentrations of CXCL8 chemokine were prepared in chemotaxis buffer (1% BSA/RPMI 1640 serum-free and phenol red-free medium). The bottom wells of the chamber were filled with medium containing chemokine at each concentration in triplicate. A polycarbonate filter (3-μm pore size) was placed on the top of this plate, and the chamber was assembled according to the manufacturer's suggestion. Mouse neutrophils were pretreated for 30 min at 37 °C with either wortmannin or DMSO (carrier control) in RPMI 1640 media containing 10% fetal bovine serum. Cells were washed twice with chemotaxis buffer and resuspended in the same buffer at 106 cells/ml. Two hundred microliters of neutrophils were loaded into top wells of the chamber and incubated for 1 h at 37 °C, 5% CO2. The chamber was dissembled carefully and the 96-well plate containing the transmigrated cells was placed in a centrifuge to spin at 2,000 rpm for 2 min to bring down the cells to the bottom wells. Cells were washed three times and resuspended in 100 μl of HBSS. Sixty microliters of NAG solution (4 mm NAG, 25 mm sodium citrate, 25 mm citric acid, and 0.25% Triton X-100, pH 5.0) were added to each well and incubated overnight at room temperature in the dark. One hundred microliters of stop solution (50 mm glycine and 5 mm EDTA, pH 10.4) were added to each well, and the color was read at 405 nm. The number of migrated cells was determined based upon the standard curve calculated from OD readings of 0, 2,500, 5,000, 10,000, 20,000, and 40,000 cells.
Statistical Analysis—ANOVA with Bonferoni post-tests were used for statistical analysis.
RESULTS
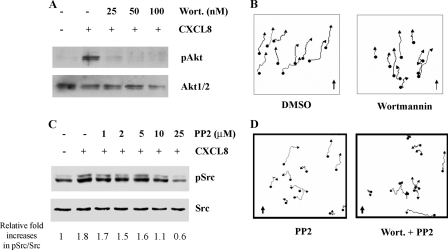
PI3K-independent Chemotaxis—Differentiated HL60 (dHL60) cells share many of the same characteristics of primary neutrophils and are therefore widely used for studying neutrophil chemotaxis. However, the expression of the chemokine receptor CXCR2 is too low to mediate effective chemotaxis toward CXCL8 (27). In this study, a polyclonal HL60 cell line stably expressing CXCR2 (HL60-CXCR2) with similar numbers of receptors per cell in comparison with human neutrophils (27) was developed and used to study the functional role of PI3K in CXCR2-mediated signal transduction pathways. To assess PI3K activity in response to CXCL8, we examined the phosphorylation status of Akt (Thr-308), an indirect downstream target of the class I PI3Ks, in the presence or absence of wortmannin, an inhibitor of the class I PI3Ks. The phosphorylation of Akt (Thr-308) was completely inhibited by pretreatment of dHL60-CXCR2 cells with 50 nm wortmannin for 30 min at 37 °C (Fig. 1A). When chemotaxis of dHL60-CXCR2 cells was tested in microfluidic chambers, cells migrated toward the CXCL8 gradient (0–25 ng/ml) at an average speed 6.54 μm/min (Fig. 1B and supplemental Movie S1). However, if cells were pretreated with wortmannin (50 nm, 30 min at 37 °C), the speed of cell migration was significantly slowed (average speed for wortmannin-treated cells, 5.37 μm/min versus control cells, 6.54 μm/min, p < 0.05). Surprisingly, the directionality of cell migration was not changed (Fig. 1B and supplemental Movie S2). In addition, when these cells were provided with universal stimulus of CXCL8 (25 ng/ml) in chemokinesis assays conducted in microfluidic chambers, the cells pretreated with wortmannin also showed slower movement (5.45 μm/min), compared with the control cells (6.65 μm/min, p < 0.01). These results suggest that PI3K plays a role in facilitating the speed of cell migration, but is dispensable for chemotaxis in response to a CXCL8 gradient in HL60-CXCR2. In testing the response of these cells to a directional change in the CXCL8 gradient using microfluidic gradient-reversing devices, we observed that cells changed the polarity instantly and migrated in the direction of the new gradient. However, the cells pretreated with wortmannin showed slower turning and a reduced number of cells changed the direction of migration in response to the gradient shift (31) These data suggest that PI3K plays an important role in changing cell polarity as the direction of chemokine gradient is altered. Because wortmannin is a pan-PI3K inhibitor, the effect of PI3Kγ on CXCR2-mediated chemotaxis in dHL60 cells was tested by using the PI3Kγ-specific inhibitor, AS605240 (32, 33). The experiment confirmed that inhibition of PI3Kγ alone with AS605240 pretreatment (10 μm, 30 min) did not inhibit chemotaxis of dHL60-CXCR2 cells in response to a CXCL8 gradient. However, if cells were pretreated with both AS605240 and PP2, this chemotaxis was inhibited, and cells moved only along the direction of flow due to force from flow (supplemental Table S2 and Fig. S1).
FIGURE 1.
Inhibition of PI3K and/or SFK activity and their effects on chemotaxis. A, inhibition of PI3K activity by wortmannin. dHL60-CXCR2 cells were pretreated with wortmannin at the concentrations indicated for 30 min at 37 °C, stimulated or not stimulated with CXCL8 (1 μg/ml), and then cells were lysed and subjected to Western blot. The phosphorylation of Akt and Erk in response to 1 μg/ml CXCL8 stimulation is shown and 50 nm wortmannin pretreatment completely inhibited phosphorylation of Akt. B, chemotaxis assays in microfluidic gradient devices (see supplemental Movies S1 and S2) of cells pretreated without (control, left panel and supplemental Movie S1) or with 50 nm wortmannin (right panel and supplemental Movie S2). Cell movements were tracked and analyzed with the Metamorph software from a minimum of 25 individual cell tracks from each movie (10 tracks were shown in the figure). The direction of the CXCL8 gradient (0–25 ng/ml) is indicated by the arrow. C, inhibition of SFK by PP2. Cells were pretreated with PP2 at the concentration indicated for 30 min at 37 °C. Western blot analysis shows the phosphorylation of Src in response to 1 μg/ml CXCL8 stimulation. The fold increases relative to the non-stimulated sample in pSrc/Src were quantified using the Image J software. D, chemotaxis assays in microfluidic gradient devices of cells pretreated with either PP2 (10 mm) or the combination of wortmannin and PP2 (see supplemental Movies S3 and S4, respectively). Cell tracking and analysis were performed as described in B. The direction of CXCL8 gradient (0–25 ng/ml) is indicated by the arrow.
Activation of Src and Chemotaxis in dHL60 Cells—The intricate relationship between cell adhesion and cell movement led us to investigate the functional role of SFK in mediating dHL60-CXCR2 chemotaxis in response to a CXCL8 gradient. The phosphorylation of c-Src at pY418 is required for Src activity. Therefore a specific pY418 antibody was used for testing its activation. Because Src family kinases share a conserved sequence in this region, this antibody reacts with all members of this kinase family. In dHL60-CXCR2 cells, SFKs were activated immediately upon stimulation with CXCL8, reached a peak between 1 and 5 min, and were reduced to the basal level at 10 min. The activation of SFK was completely inhibited by pretreatment with pertussis toxin (100 ng/ml for 4 h), suggesting CXCL8 activation of SFK is Gαi-mediated (supplemental Fig. S2). PP2, a specific inhibitor for Src family kinases, is widely used to study the function of SFK. A range of 1–25 μm PP2 were used to evaluate the optimal concentration to inhibit SFK in dHL60-CXCR2 after stimulation with CXCL8. Results from these experiments indicated that 10 μm PP2 pretreatment for 30 min at 37 °C completely inhibited activation of Src in response to CXCL8 stimulation, while 25 μm PP2 produced a level of phospho-Src even lower than the basal level in nonstimulated cells (Fig. 1C). Therefore, to avoid loss of Src kinase specificity or the toxicity of inhibitor, 10 μm PP2 was used in our experiments described here. Chemotaxis assay in the microfluidic chamber showed partial inhibition of chemotaxis in response to a CXCL8 gradient subsequent to PP2 treatment (Fig. 1D and supplemental Movie S3). The speed of migration was slower than with control pretreatment (4.88 μm/min versus 6.54 μm/min, p < 0.01). More importantly, when these cells were pretreated with a combination of wortmannin (50 nm) and PP2 (10 μm) for 30 min at 37 °C, they completely lost chemotaxis in response to a CXCL8 gradient in microfluidic chambers, though they still exhibited random movement with average speed at 5.07 μm/min, which was also significant slower than DMSO control cells (p < 0.01) (Fig. 1D and supplemental Movie S4). These results suggest that the PI3K and SFK function in parallel in the sensing of the chemokine gradient and consequent directional movement.
Cell Polarization in PI3K or SFK Inhibited dHL60 Cells—To evaluate the effect of inhibitors of PI3K or SFK on cell polarization in response to a chemokine gradient, experiments with the Zigmond chamber were performed, allowing for confocal analysis of polarized cells. In two independent experiments, 89% (99 of 110 cells analyzed) of the control cells pretreated with DMSO were polarized in the direction of the CXCL8 gradient (0–25 ng/ml). In cells pretreated with wortmannin (50 nm) or PP2 (10 μm) alone, the percentages of cells polarized in the direction of the CXCL8 gradient were 83% (54/65) and 86% (148/168), respectively. When cells were pretreated simultaneously with the combination of wortmannin and PP2, only 48% (33 of 68 cells) of the cells polarized toward the CXCL8 gradient, a number similar to the unbiased polarization of 50%. The percentage of non-polarized cells was increased when cells were pretreated with these inhibitors, 10.4% (wortmannin), 12% (PP2), and 23.2% (wortmannin + PP2) compared with vehicle-treated control (5.4%) (Fig. 2).
FIGURE 2.
Inhibition of cell polarization toward a CXCL8 gradient in the Zigmond chamber. Cells pretreated with different inhibitors for 30 min at 37 °C were induced to polarize toward a CXCL8 gradient in the Zigmond chamber for 15 min at 37 °C. The vertical line in each figure panel indicates the interface of CXCL8-containing medium (left) and RPMI medium alone (right). A higher magnification image of the boxed section was shown below each panel of images for a clearer view of cell polarization. The percentage of cells polarized along the direction of the gradient was quantified as described under “Experimental Procedures.” The scale bar is 20 μm. The direction of the CXCL8 gradient (0–25 ng/ml) is indicated by the arrow.
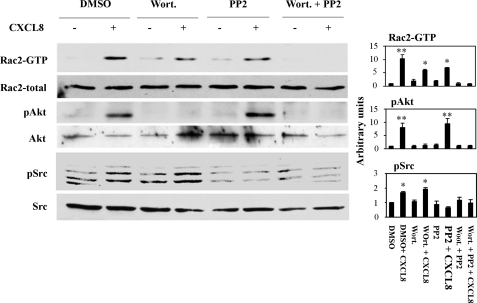
Rac2 Activation in PI3K or SFK Inhibited dHL60 Cells—To further investigate the functional roles of PI3K and SFK signal transduction pathways in chemotaxis, we tested CXCL8 activation of Rac by the pull-down assay with or without the inhibition of PI3K and SFK. When dHL60-CXCR2 cells were pretreated with either wortmannin or PP2, the ligand-triggered Rac2 activation was only partially inhibited. However, when cells were pretreated with a combination of wortmannin and PP2A, the ligand-triggered Rac2 activation was completely inhibited. To confirm the efficiency and specificity of inhibition with wortmannin and PP2 for PI3K and SFK, respectively, Western blots of phospho-Akt, phospho-Src, together with total Akt and Src were performed (Fig. 3). Because the antibody for phospho-Src detects all the kinase members in the Src family of kinases, multiple bands were seen in the blot, while the antibody for total Src was c-Src only, and a single band was detected in the Western blot. These results suggest the existence of PI3K-dependent and -independent signal transduction pathways leading to activation of Rac2 in response to CXCL8 in dHL60 cells. Additionally, these results suggest that high levels of activated Rac2 (resulting from activation of both PI3K and SFK) allow cells to move more efficiently, but activation of both PI3K and SFKs is not necessary for cells to exhibit directed movement along a chemokine gradient (supplemental Movies S1–S4). A similar finding was reported in Dictyostelium, where PI3K plays an important role in cell motility, but is not required for directed movement in a cAMP gradient (13, 34, 35).
FIGURE 3.
Inhibition of Rac activation with wortmannin and PP2. Cells were pretreated with either wortmannin or PP2 or the combination of wortmannin and PP2 for 30 min at 37 °C, and cells were activated with CXCL8 (1 μg/ml) for 1 min at room temperature. Rac2 activation was detected by the GST-PBD pull-down assay. The phosphorylation of Akt and Src are shown to indicate the effectiveness of the inhibitors. Quantification of Rac2, Akt, and Src activation from three independent experiments are shown in the right panel.*, p < 0.05 and **, p < 0.01 (one-way ANOVA with Bonferoni post-tests).
Recruitment of Dock2 and ELMO1 to the Leading Edge of Chemotaxing dHL60 Cells—In investigating the involvement of Dock2 and ELMO1 in neutrophil chemotaxis, we examined the localization of these proteins in dHL60-CXCR2 cells placed in a CXCL8 gradient in the Zigmond chamber. The resulting confocal images show a concentration of Dock2 and ELMO1 at the leading edge of the polarized cells, where they both colocalize with F-actin (Fig. 4A). Co-immunoprecipitation experiments confirm the association of ELMO1 with Dock2, but not CrkL (Fig. 4B). It is not clear at this time whether CrkL is required for the Dock2-ELMO1 complex to function as a Rac2-GEF. In experiments described herein, Dock2 and ELMO1 co-associate in the absence of ligand stimulation, and upon ligand stimulation (1 μg/ml CXCL8 for 30 s or 1 min), the levels of this association do not significantly change (Fig. 4B). It has been reported the phosphorylation status of ELMO1 plays a fundamental role in the function of the Dock2-ELMO1 complex (25). We postulated that the up-regulation of the Rac-GEF activity of Dock2-ELMO1 by ligand stimulation is through the ligand-induced tyrosine phosphorylation of ELMO1. We show in Fig. 4C that tyrosine phosphorylation of ELMO1 was increased upon CXCL8 stimulation (1 μg/ml for 1 min), and this increase in phosphorylation was ablated by the pretreatment of cells with PP2 (10 μm for 30 min), suggesting it is the SFK-involved tyrosine phosphorylation.
FIGURE 4.
Involvement of the Dock2 and ELMO1 complex in the chemotaxis of neutrophil-like cells. A, confocal images showing recruitment of Dock2 and ELMO1 to the leading edge of chemotaxing cells. The dHL60-CXCR2 cells were induced to polarize in a gradient of CXCL8 (0–25 ng/ml) (from top to bottom) in a Zigmond chamber. Immunostaining was performed on fixed cells with antibodies against human Dock2 and ELMO1, and F-actin was stained with rhodamine-conjugated phalloidin to indicate the leading edge of the cell. The scale bar is 10 μm. The direction of the CXCL8 gradient (0–25 ng/ml) is indicated by the arrow. Among the total 45 cells analyzed from three individual experiments, 41 cells showed the colocalization of Dock2 and ELMO1 with F-actin. B, co-immunoprecipitation showing association of Dock2 and ELMO1. Co-immunoprecipitation was performed with goat polyclonal anti-ELMO1 antibody or normal goat IgG (M: mock control) (right panel). Cells were stimulated with CXCL8 (1 μg/ml) for 0, 30 s and 1 min, respectively, lysed, and a portion of the cell lysates were included in each Western blot as a loading control (left panel). C, phosphorylation of ELMO1 and its inhibition by PP2 pretreatment of cells. ELMO1 was immunoprecipitated by goat polyclonal anti-ELMO1 antibody, and the level of tyrosine phosphorylation was detected with anti-phosphorylated tyrosine antibody (4G10). The membrane was stripped and re-blotted with anti-ELMO1 antibody.
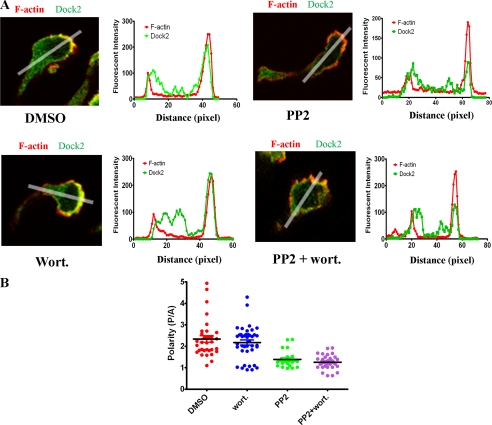
Inhibition of Dock2 Recruitment in the Leading Edge of Cells by PP2 Pretreatment—Because inhibition of ELMO1 phosphorylation by PP2 treatment did not affect the association of Dock2 and ELMO1, we tested the effect of ELMO1 phosphorylation on the localized accumulation of Dock2 at the leading edge of the cells. Immunostaining of cells polarized toward a CXCL8 gradient in a Zigmond chamber showed that Dock2 and F-actin accumulated at the leading edge of the cells when they were pretreated with DMSO or wortmannin alone, while the cells pretreated with PP2 alone or the combination of PP2 and wortmannin showed much weaker accumulation of Dock2 at the leading edge (Fig. 5A and supplemental Figs. S3A–2D). To quantify the polarization of Dock2 and F-actin within a cell, a line-scanned profile of fluorescence intensity was generated and the ratio of the peak value of fluorescence intensity from posterior over the peak value of fluorescence intensity from anterior (P/A) was calculated. The pooled data for Dock2 from 33 cells in the DMSO group, 40 cells in wortmannin-treated groups, 24 cells in PP2-treated groups, and 30 cells in the combination of PP2 and wortmannin-pretreated cells from three independent experiments were plotted in Fig. 5B. In DMSO control and wortmannin-treated groups, most of the cells (31 out of 33 in DMSO and 36 out of 40 in wortmannin groups) had a Dock2 polarity ratio (P/A ratio) larger than 1.5, while only 4 out of 24 and 6 out of 30 cells from PP2 alone and PP2 + wortmannin-pretreated cells, respectively, showed P/A ratio larger than 1.5. The mean P/A ratios for different groups of cells were 2.34 ± 0.15 (DMSO), 2.18 ± 0.12 (wortmannin), 1.39 ± 0.08 (PP2), and 1.26 ± 0.06 (PP2 + wortmannin). One-way ANOVA with Bonferoni post-tests indicated a significant difference between DMSO and PP2-treated groups (p < 0.01, and between DMSO and the combined PP2 + wortmannin-treated groups (p < 0.01).
FIGURE 5.
Inhibition of Dock2 accumulation at the leading edge of the polarized cells by PP2 treatment. A, confocal images and line scanned profiles of fluorescence intensities showing the polarized localization of F-actin (red) and Dock2 (green). The abscissa is the pixels from rear to front of the cell. Cells were pretreated with DMSO, PP2, wortmannin, or the combination of PP2 and wortmannin for 30 min at 37 °C before they were seeded in a Zigmond chamber. After cells were induced to polarize by a CXCL8 gradient in the Zigmond chamber, cells were fixed and immunostained. Images of one representative cell from each treatment are shown in the figure, and the images showing more cells from each treatment are available in the supplemental materials online (supplemental Fig. S2). B, quantification of polarized localization of Dock2 inside the cells. The ratio of the strongest fluorescence intensity at the front of the cell over the strongest fluorescence intensity at the rear was taken to demonstrate the polarization of Dock2 and a pooled data from three individual experiments with a minimum of 24 cells for each group were plotted. One-way ANOVA with Bonferoni post-tests were performed and showed the significant differences between DMSO and PP2 treatment, and between DMSO and the combination of PP2 and wortmannin treatment.
Compromised Rac2 Activation and Chemotaxis in Dock2 shRNA Cell Lines—To more specifically determine whether Dock2 plays a role in Rac2 activation and chemotaxis, small interference RNA (siRNA) was used to knockdown expression of Dock2 in HL60-CXCR2 cells. Two shRNAs targeting human Dock2 and one non-silencing shRNA control sequence were selected from the siRNAmir library. The expression of Dock2 shRNA significantly reduced the protein levels of Dock2, and the polyclonal HL-60 cell line expressing shRNA 893 most efficiently knocked down Dock2 expression based upon Western blot (Fig. 6A). A Rac2 activity assay showed that CXCL8-stimulated Rac2 activation was significantly reduced in the 893 shRNA polyclonal line (Fig. 6B). As observed with pretreatment with both PP2 and wortmannin (Fig. 3B), Rac2 activation stimulated by CXCL8 was completely inhibited in the 893 shRNA polyclonal line by wortmannin pretreatment, while only partial inhibition of Rac2 activation was observed in the non-silencing shRNA polyclonal line with the same pretreatment (Fig. 6B). Chemotaxis assays performed in microfluidic devices revealed no obvious inhibition of chemotaxis in the Dock2 knockdown cell line (893). Wortmannin pretreatment caused only mild inhibition in the non-silencing control cell line. However, wortmannin pretreatment resulted in greater inhibition of chemotaxis in Dock2 knockdown cells (Fig. 6C).
FIGURE 6.
Compromised Rac2 activation and chemotaxis in Dock2 shRNA cell lines. Polyclonal HL60-CXCR2 cells stably expressing Dock2 shRNA were constructed by lentiviral transfection followed by selection with 0.5 μg/ml puromycin. A, protein expression of Dock2 in dHL60 cells was detected with Western blot. Tubulin was used as loading control. NS is non-silencing shRNA control. 889 and 893 are two individual Dock2 shRNAs. B, Rac2 activation was detected by GST-PBD pull-down assay in DMSO (control) or wortmannin (50 nm, 30 min)-pretreated dHL60 cells. Quantification (right panel) from three independent experiments is shown in the right panel.*, p < 0.05 and **, p < 0.01 (one-way ANOVA with Bonferoni post-tests). C, chemotaxis assay performed in microfluidic devices. The movement of 15–20 randomly picked cells was tracked with the Metamorph program. The direction of the CXCL8 gradient is shown by the arrow in each panel.
Differential Inhibition of Neutrophil Chemotaxis by Wortmannin Pretreatment in hck-/-fgr-/-lyn-/- and WT Mice—To test the role of multiple pathways regulating chemotaxis in fresh mouse neutrophil isolates, neutrophils were isolated from mouse bone marrow from hck-/-fgr-/-lyn-/- as well as wild-type mice. The percentage of neutrophils in bone marrow cell isolates was examined by flow cytometry using a fluorescence tagged neutrophil marker. Results showed 60 and 74% neutrophil content for WT and hck-/-fgr-/-lyn-/- bone marrow isolates, respectively. The higher percentage of neutrophils in leukocyte isolates from knockout mice has been routinely observed and could be an attempt to restore function in a population of neutrophils with reduced activity.3 Similar to the effect on HL60 cells, 50 nm wortmannin pretreatment for 30 min at 37 °C in mouse neutrophils efficiently inhibited PI3K activity in response to CXCL8 stimulation based upon phospho-Akt levels (Fig. 7A). Chemotaxis assays performed in modified Boyden chambers showed that there was no significant difference in chemotaxis for DMSO-pretreated WT and hck-/-fgr-/-lyn-/- cells. In contrast to our results with HL60 cells, wortmannin alone inhibited chemotaxis in both WT and hck-/-fgr-/-lyn-/- neutrophils. However, more severe inhibition of chemotaxis was observed in wortmannin-pretreated hck-/-fgr-/-lyn-/- neutrophils than wortmannin-pretreated WT neutrophils (Fig. 7B). The inhibition was 56.36% for WT and 78.01% for hck-/-fgr-/-lyn-/- in response to 300 nm CXCL8. Two-way ANOVA with Bonferoni post-tests indicate there is a significant difference between the WT wortmannin-treated group and hck-/-fgr-/-lyn-/- wortmannin-treated group, but no difference between the WT DMSO-treated group and hck-/-fgr-/-lyn-/--treated group for chemotaxis in response to the 300 ng/ml CXCL8 gradient.
FIGURE 7.
Differential inhibition of neutrophil chemotaxis by wortmannin pretreatment in hck-/-fgr-/-lyn-/- knockout and WT mice. Neutrophils were isolated from mouse bone marrow as described under “Experimental Procedures.” The percentage of neutrophils in total bone marrow cells was tested by flow cytometry with the neutrophil marker. A, dosage effects of wortmannin pretreatment on phospho-Akt levels in response to CXCL8 stimulation (1 min at room temperature) in mouse bone marrow neutrophils. B, chemotaxis assay performed in a modified Boyden chamber. Cells were pretreated with DMSO or 10 nm or 50 nm wortmannin for 30 min at 37 °C. Chemotaxis was plotted as the percentage of cells that transmigrated through a 3-μm pore membrane. Data were pooled from two individual experiments with triplicates for each treatment. The error bars are S.E. Three mice for each genotype were used in each experiment. Numbers above bars indicate percentage reduction relative to each DMSO control group. **, p < 0.01 (two-way ANOVA with Bonferoni post-tests) indicates statistical significance between the WT wortmannin-treated group and hck-/-fgr-/-lyn-/- wortmannin-treated group at 300 ng/ml CXCL8.
DISCUSSION
Our studies show that dHL60 cells exhibit chemotaxis in response to a CXCL8 gradient even when PI3K activity is inhibited. However, the speed of cell migration in the PI3K-inhibited cells is reduced both in response to a gradient of chemokine (chemotaxis) and in response to a universal CXCL8 stimulation (chemokinesis). The essential role of PI3K in chemotaxis has been challenged in several recent reports in Dictyostelium (13, 34–36) and in mammalian cells (37, 38), where PI3K is believed to play a fundamental role in cell motility, but not chemotaxis. Through mutagenesis and combinational chemical inhibitor assays, PLA was shown to function in the chemotaxis pathway in Dictyostelium, where the PLA pathway is parallel to the PI3K pathway. Upon inhibition of both pathways, a severe defect of chemotaxis occurs in Dictyostelium in response to the cAMP gradient (39, 40). The co-existence of these redundant pathways allows cells greater flexibility to respond to different environmental circumstances, such as gradients of high or low steepness. In mammalian cells, it is also reported that PLA plays a role in neutrophil chemotaxis to fMLP, but not to CXCL8 (41). We show here that the SFK pathway functions in parallel to PI3K in determining the directional cell movement in response to a CXCL8 gradient. Depending on the cell type or the different environmental stimuli, some signal transduction pathways play a more important role than others in the regulation of chemotaxis. PI3K may not play a critical role in sensing and guiding cells to move in the direction of a chemoattractant gradient. However, when cells experience a change in the direction of the gradient, those cells without PI3K activity lost rapid directional responses to the new gradient (31). It is also interesting to observe that dHL60 cells reversed their polarity, rather than turn in response to the directional change of the CXCL8 gradient. It would be of great interest to know how PI3K facilitates this polarity reversion. We postulate that for cells to promptly reverse polarity in response to a directional change of gradient, massive cytoskeleton reorganization must occur. Rac activation assists in this rapid cytoskeleton reorganization. Thus, our data suggest PI3K is responsible for a portion of the Rac activation required for response to rapid change in the direction of the gradient, and this augmentation of Rac activation is not compensated for by other pathways.
Our experiments show that Dock2, along with its partner ELMO1, are recruited to the leading edge of dHL60 cells during chemotaxis toward CXCL8. It is well known that the binding partners of the Dock180 family of proteins function as Rac activators. The binding of ELMO relieves the inhibitory conformation of Dock 180 and exposes the binding domain for nucleotide-free Rac (42). The Armadillo repeats on the N terminus of ELMO work with another protein, RhoG, help to target the Dock180-ELMO complex to the membrane, where Rac is activated (43–45). Cote et al. (21) reported that Dock180 and possibly Dock2 contain a domain (DHR-1) that binds to PIP3, and this domain may also mediate the membrane translocation of the DOCK180 complex by the association with PIP3. In our study, Dock2, together with ELMO1, accumulated in the leading edge of dHL60 cells in response to the CXCL8 gradient, even after wortmannin pretreatment. These data suggest that the association of the DHR-1 domain with PIP3 may not be the only mediator for the membrane translocation of the Dock2 complex in dHL60 cells. Studies in lymphocyte migration also indicated that Dock2 was fully functional to mediate T and B cell chemotaxis in vitro and in vivo in cells lacking PI3K (46). However, Kunisaki et al. (22) reported that prolonged inhibition of PI3K with very high concentrations of LY294002 (400 μm) did inhibit Dock2 recruitment in mouse neutrophils in response to a fMLP gradient. Moreover, neutrophils isolated from Dock2 knockout mice exhibited inhibition of Rac activation and chemotaxis in response to fMLP stimulation (22). In contrast, our Dock2 knockdown HL60 cells showed compromised Rac2 activation, but little inhibition of chemotaxis in response to a CXCL8 gradient in the microfluidic gradient chamber assay. A complete inhibition of chemotaxis was observed when Dock2 knockdown cells were pretreated with the PI3K inhibitor, wortmannin. This suggests that the chemotaxis to a CXCL8 gradient in dHL60 cells can utilize either PI3K or Dock2. Experiments with mouse bone marrow neutrophils indicated that independent signal transduction pathways parallel to the PI3K regulate chemotaxis in primary mouse neutrophils to. While bone marrow isolates of neutrophils from WT mice did show reduced CXCL8-mediated chemotaxis when PI3K was inhibited, the inhibition of chemotaxis was significantly greater in hck-/-fgr-/-lyn-/- neutrophils treated with wortmannin. The PI3K-independent chemotaxis observed in dHL60 cells may reflect escape from PI3K dependence in this premalignant cell line.
In addition to relieving the auto-inhibition of Dock180, the binding of ELMO1 may also have other effects, such as inhibition of Dock180 ubiquitination and degradation. Moreover, Yokoyama et al. (25) reported that ELMO1 was phosphorylated by the Src family kinase Hck, and this phosphorylation was required for activation of Rac by the Dock180-ELMO1 complex. We observed that ELMO1 was phosphorylated by SFK, and the phosphorylation level increased upon stimulation with CXCL8 in dHL60-CXCR2 cells. The PP2-pretreated cells showed inhibition of Dock2 recruitment in the leading edge of chemotaxing cells, indicating the phosphorylation of ELMO1 is required for the membrane translocation of the Dock2-ELMO1 complex. In addition to the regulation of Rac activation through the regulation of ELMO phosphorylation, Src family kinases are also involved in the formation of integrin-mediated focal adhesions, where Crk I or II might also play a role to recruit Dock180 and ELMO1 to the focal adhesion sites (47). Mouse neutrophils lacking major Src family kinases (hck/fgr/lyn knockout) showed compromised cell basal motility, but not compromised chemotaxis in response to a CXCL8 gradient. These data suggest that the involvement of SFK in focal adhesion assembly/disassembly is necessary for basal cell motility, but is not essential for neutrophil chemotaxis.
We have described parallel Src- and PI3K-dependent signal transduction pathways that activate Rac2 in dHL60 cells and in mouse neutrophils in response to CXCL8. We conclude that the level of activation of Rac2 determines the efficiency of detection of a shift in gradient direction and the speed of movement of dHL60-CXCR2 cells. If Rac activation is completely inhibited by the combined pretreatment with wortmannin and PP2, cells do not undergo chemotaxis. This information suggests a paradigm shift away from earlier models wherein PI3K-dependent activation of Rac was thought to be required for chemotaxis. Our data point toward parallel SFK-dependent and PI3K-dependent pathways which can produce Rac activation and chemotaxis. Therefore, therapies designed to reduce neutrophil recruitment sites of inflammation may need to include PI3K as well as SFK inhibitors.
Supplementary Material
Acknowledgments
We thank Henry Bourne at UCSF for kindly providing the HL60 cells used in this study. We thank Michiyuki Matsuda at Kyoto University, Japan, for the Dock2 antibody. We thank Clifford Lowell and Yongmei Hu at UCSF for kindly providing hck-/-fgr-/-lyn-/- and wild-type mouse bones. We thank Paige Baugher and the Vanderbilt Editors Club for careful reading of this manuscript.
This work was supported, in whole or in part by National Institutes of Health Grants CA34590 (to A. R.) and NCI U54CA113007 (to Vito Quaranta). This work was also supported by a Senior Research Career Scientist Award from the Department of Veterans Affairs (to A. R.), the Vanderbilt Institute for Integrative Biosystems Research and Education and the Vanderbilt Academic Venture Capital Fund (to J. W.), and the Vanderbilt Ingram-Cancer Center Grant CA68485 (to Jennifer Pietenpol). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Movies S1–S4, Tables S1 and S2, and Figs. S1–S3.
Footnotes
The abbreviations used are: PI3K, phosphatidylinositol 3-kinase; SFK, c-Src family of kinases; Crk, CT10-related kinase; ELMO, engulfment and cell motility; CXCL8, CXC ligand 8; CXCR2, CXC receptor 2; WT, wild type; ANOVA, analysis of variance; BSA, bovine serum albumin; GST, glutathione S-transferase; siRNA, small interference RNA; shRNA, short hairpin RNA.
Clifford Lowell, personal communication.
References
- 1.Parent, C. A., Blacklock, B. J., Froehlich, W. M., Murphy, D. B., and Devreotes, P. N. (1998) Cell 95 81-91 [DOI] [PubMed] [Google Scholar]
- 2.Servant, G., Weiner, O. D., Herzmark, P., Balla, T., Sedat, J. W., and Bourne, H. R. (2000) Science 287 1037-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner, O. D., Neilsen, P. O., Prestwich, G. D., Kirschner, M. W., Cantley, L. C., and Bourne, H. R. (2002) Nat. Cell Biol. 4 509-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, F., Herzmark, P., Weiner, O. D., Srinivasan, S., Servant, G., and Bourne, H. R. (2002) Nat. Cell Biol. 4 513-518 [DOI] [PubMed] [Google Scholar]
- 5.Sasaki, T., Irie-Sasaki, J., Jones, R. G., Oliveira-dos-Santos, A. J., Stanford, W. L., Bolon, B., Wakeham, A., Itie, A., Bouchard, D., Kozieradzki, I., Joza, N., Mak, T. W., Ohashi, P. S., Suzuki, A., and Penninger, J. M. (2000) Science 287 1040-1046 [DOI] [PubMed] [Google Scholar]
- 6.Li, Z., Jiang, H., Xie, W., Zhang, Z., Smrcka, A. V., and Wu, D. (2000) Science 287 1046-1049 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch, E., Katanaev, V. L., Garlanda, C., Azzolino, O., Pirola, L., Silengo, L., Sozzani, S., Mantovani, A., Altruda, F., and Wymann, M. P. (2000) Science 287 1049-1053 [DOI] [PubMed] [Google Scholar]
- 8.Ma, Y. C., Huang, J., Ali, S., Lowry, W., and Huang, X. Y. (2000) Cell 102 635-646 [DOI] [PubMed] [Google Scholar]
- 9.Roberts, A. W., Kim, C., Zhen, L., Lowe, J. B., Kapur, R., Petryniak, B., Spaetti, A., Pollock, J. D., Borneo, J. B., Bradford, G. B., Atkinson, S. J., Dinauer, M. C., and Williams, D. A. (1999) Immunity 10 183-196 [DOI] [PubMed] [Google Scholar]
- 10.Gu, Y., Jia, B., Yang, F. C., D'Souza, M., Harris, C. E., Derrow, C. W., Zheng, Y., and Williams, D. A. (2001) J. Biol. Chem. 276 15929-15938 [DOI] [PubMed] [Google Scholar]
- 11.Filippi, M. D., Harris, C. E., Meller, J., Gu, Y., Zheng, Y., and Williams, D. A. (2004) Nat. Immunol. 5 744-751 [DOI] [PubMed] [Google Scholar]
- 12.Li, S., Yamauchi, A., Marchal, C. C., Molitoris, J. K., Quilliam, L. A., and Dinauer, M. C. (2002) J. Immunol. 169 5043-5051 [DOI] [PubMed] [Google Scholar]
- 13.Andrew, N., and Insall, R. H. (2007) Nat. Cell Biol. 9 193-200 [DOI] [PubMed] [Google Scholar]
- 14.Welch, H. C., Coadwell, W. J., Ellson, C. D., Ferguson, G. J., Andrews, S. R., Erdjument-Bromage, H., Tempst, P., Hawkins, P. T., and Stephens, L. R. (2002) Cell 108 809-821 [DOI] [PubMed] [Google Scholar]
- 15.Shinohara, M., Terada, Y., Iwamatsu, A., Shinohara, A., Mochizuki, N., Higuchi, M., Gotoh, Y., Ihara, S., Nagata, S., Itoh, H., Fukui, Y., and Jessberger, R. (2002) Nature 416 759-763 [DOI] [PubMed] [Google Scholar]
- 16.Meller, N., Merlot, S., and Guda, C. (2005) J. Cell Sci. 118 4937-4946 [DOI] [PubMed] [Google Scholar]
- 17.Lu, M., and Ravichandran, K. S. (2006) Methods Enzymol. 406 388-402 [DOI] [PubMed] [Google Scholar]
- 18.Cote, J. F., and Vuori, K. (2006) Methods Enzymol. 406 41-57 [DOI] [PubMed] [Google Scholar]
- 19.Cote, J. F., and Vuori, K. (2002) J. Cell Sci. 115 4901-4913 [DOI] [PubMed] [Google Scholar]
- 20.Gumienny, T. L., Brugnera, E., Tosello-Trampont, A. C., Kinchen, J. M., Haney, L. B., Nishiwaki, K., Walk, S. F., Nemergut, M. E., Macara, I. G., Francis, R., Schedl, T., Qin, Y., Van Aelst, L., Hengartner, M. O., and Ravichandran, K. S. (2001) Cell 107 27-41 [DOI] [PubMed] [Google Scholar]
- 21.Cote, J. F., Motoyama, A. B., Bush, J. A., and Vuori, K. (2005) Nat. Cell Biol. 7 797-807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunisaki, Y., Nishikimi, A., Tanaka, Y., Takii, R., Noda, M., Inayoshi, A., Watanabe, K., Sanematsu, F., Sasazuki, T., Sasaki, T., and Fukui, Y. (2006) J. Cell Biol. 174 647-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanui, T., Inayoshi, A., Noda, M., Iwata, E., Stein, J. V., Sasazuki, T., and Fukui, Y. (2003) Blood 102 2948-2950 [DOI] [PubMed] [Google Scholar]
- 24.Nishihara, H., Maeda, M., Oda, A., Tsuda, M., Sawa, H., Nagashima, K., and Tanaka, S. (2002) Blood 100 3968-3974 [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama, N., deBakker, C. D., Zappacosta, F., Huddleston, M. J., Annan, R. S., Ravichandran, K. S., and Miller, W. T. (2005) Biochemistry 44 8841-8849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowell, C. A., Soriano, P., and Varmus, H. E. (1994) Genes Dev. 8 387-398 [DOI] [PubMed] [Google Scholar]
- 27.Sai, J., Walker, G., Wikswo, J., and Richmond, A. (2006) J. Biol. Chem. 281 35931-35941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benard, V., Bohl, B. P., and Bokoch, G. M. (1999) J. Biol. Chem. 274 13198-13204 [DOI] [PubMed] [Google Scholar]
- 29.Neel, N. F., Lapierre, L. A., Goldenring, J. R., and Richmond, A. (2007) J. Cell Sci. 120 1559-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boxio, R., Bossenmeyer-Pourie, C., Steinckwich, N., Dournon, C., and Nusse, O. (2004) J. Leukoc. Biol. 75 604-611 [DOI] [PubMed] [Google Scholar]
- 31.Liu, Y., Sai, J., Richmond, A., and Wikswo, J. P. (2008) Biomed. Microdevices, 10 499-507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camps, M., Ruckle, T., Ji, H., Ardissone, V., Rintelen, F., Shaw, J., Ferrandi, C., Chabert, C., Gillieron, C., Francon, B., Martin, T., Gretener, D., Perrin, D., Leroy, D., Vitte, P. A., Hirsch, E., Wymann, M. P., Cirillo, R., Schwarz, M. K., and Rommel, C. (2005) Nat. Med. 11 936-943 [DOI] [PubMed] [Google Scholar]
- 33.Smith, L. D., Hickman, E. S., Parry, R. V., Westwick, J., and Ward, S. G. (2007) Cell Signal 19 2528-2539 [DOI] [PubMed] [Google Scholar]
- 34.Loovers, H. M., Postma, M., Keizer-Gunnink, I., Huang, Y. E., Devreotes, P. N., and van Haastert, P. J. (2006) Mol. Biol. Cell 17 1503-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda, K., Sasaki, A. T., Ha, H., Seung, H. A., and Firtel, R. A. (2007) J. Biol. Chem. 282 11874-11884 [DOI] [PubMed] [Google Scholar]
- 36.Hoeller, O., and Kay, R. R. (2007) Curr. Biol. 17 813-817 [DOI] [PubMed] [Google Scholar]
- 37.Ferguson, G. J., Milne, L., Kulkarni, S., Sasaki, T., Walker, S., Andrews, S., Crabbe, T., Finan, P., Jones, G., Jackson, S., Camps, M., Rommel, C., Wymann, M., Hirsch, E., Hawkins, P., and Stephens, L. (2007) Nat. Cell Biol. 9 86-91 [DOI] [PubMed] [Google Scholar]
- 38.Heit, B., Liu, L., Colarusso, P., Puri, K. D., and Kubes, P. (2008) J. Cell Sci. 121 205-214 [DOI] [PubMed] [Google Scholar]
- 39.Chen, L., Iijima, M., Tang, M., Landree, M. A., Huang, Y. E., Xiong, Y., Iglesias, P. A., and Devreotes, P. N. (2007) Dev. Cell 12 603-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Haastert, P. J., Keizer-Gunnink, I., and Kortholt, A. (2007) J. Cell Biol. 177 809-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heit, B., Robbins, S. M., Downey, C. M., Guan, Z., Colarusso, P., Miller, B. J., Jirik, F. R., and Kubes, P. (2008) Nat. Immunol. 9 743-752 [DOI] [PubMed] [Google Scholar]
- 42.Lu, M., Kinchen, J. M., Rossman, K. L., Grimsley, C., Hall, M., Sondek, J., Hengartner, M. O., Yajnik, V., and Ravichandran, K. S. (2005) Curr. Biol. 15 371-377 [DOI] [PubMed] [Google Scholar]
- 43.Grimsley, C. M., Kinchen, J. M., Tosello-Trampont, A. C., Brugnera, E., Haney, L. B., Lu, M., Chen, Q., Klingele, D., Hengartner, M. O., and Ravichandran, K. S. (2004) J. Biol. Chem. 279 6087-6097 [DOI] [PubMed] [Google Scholar]
- 44.Katoh, H., and Negishi, M. (2003) Nature 424 461-464 [DOI] [PubMed] [Google Scholar]
- 45.deBakker, C. D., Haney, L. B., Kinchen, J. M., Grimsley, C., Lu, M., Klingele, D., Hsu, P. K., Chou, B. K., Cheng, L. C., Blangy, A., Sondek, J., Hengartner, M. O., Wu, Y. C., and Ravichandran, K. S. (2004) Curr. Biol. 14 2208-2216 [DOI] [PubMed] [Google Scholar]
- 46.Nombela-Arrieta, C., Lacalle, R. A., Montoya, M. C., Kunisaki, Y., Megias, D., Marques, M., Carrera, A. C., Manes, S., Fukui, Y., Martinez, A. C., and Stein, J. V. (2004) Immunity 21 429-441 [DOI] [PubMed] [Google Scholar]
- 47.Hasegawa, H., Kiyokawa, E., Tanaka, S., Nagashima, K., Gotoh, N., Shibuya, M., Kurata, T., and Matsuda, M. (1996) Mol. Cell. Biol. 16 1770-1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.