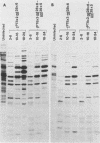
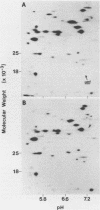
Abstract
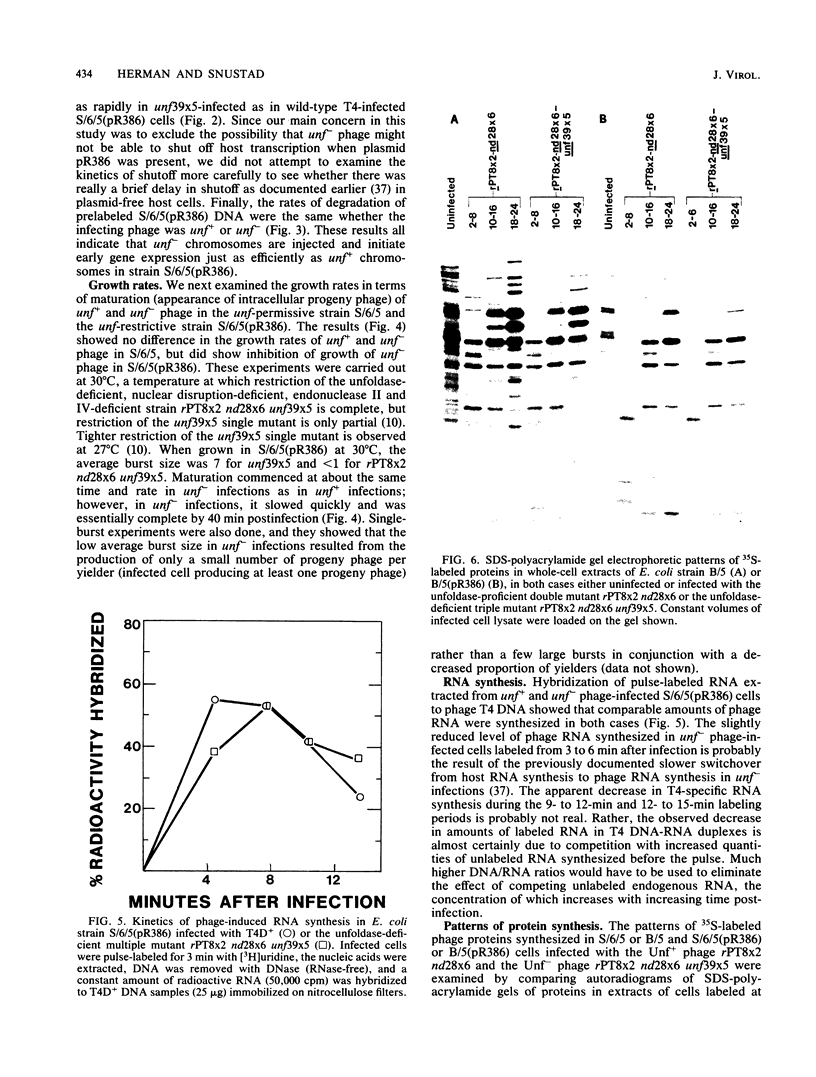
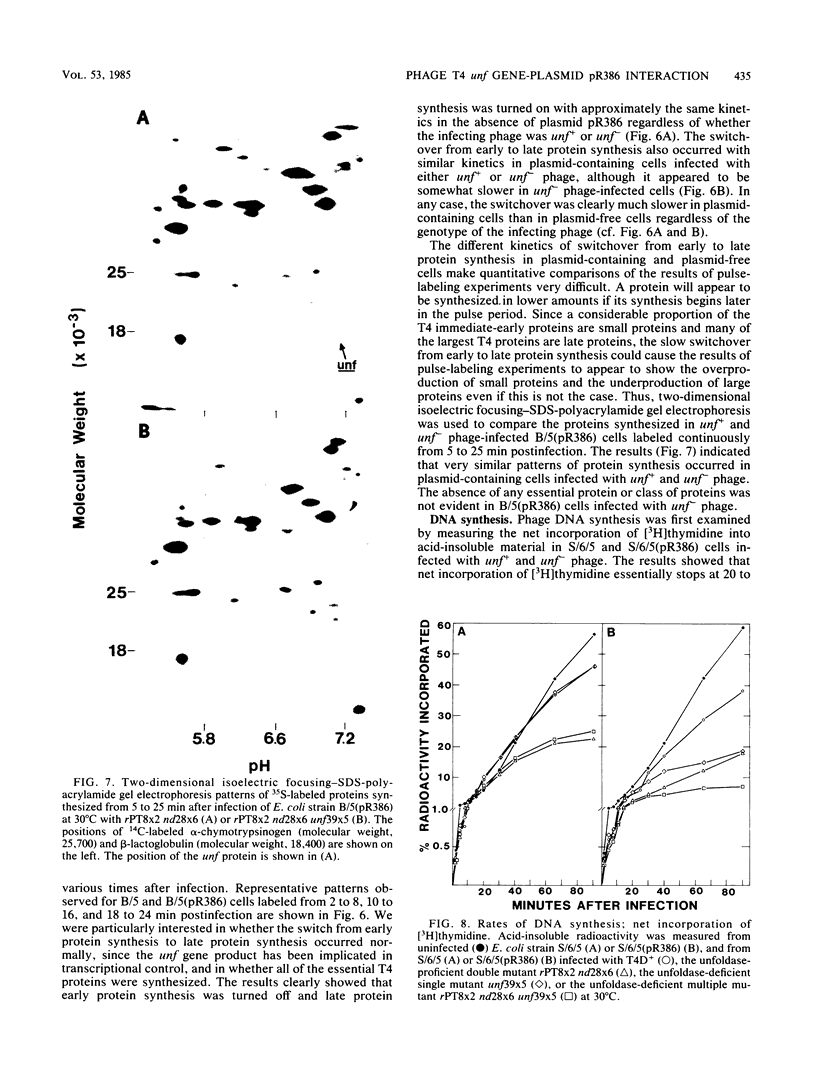
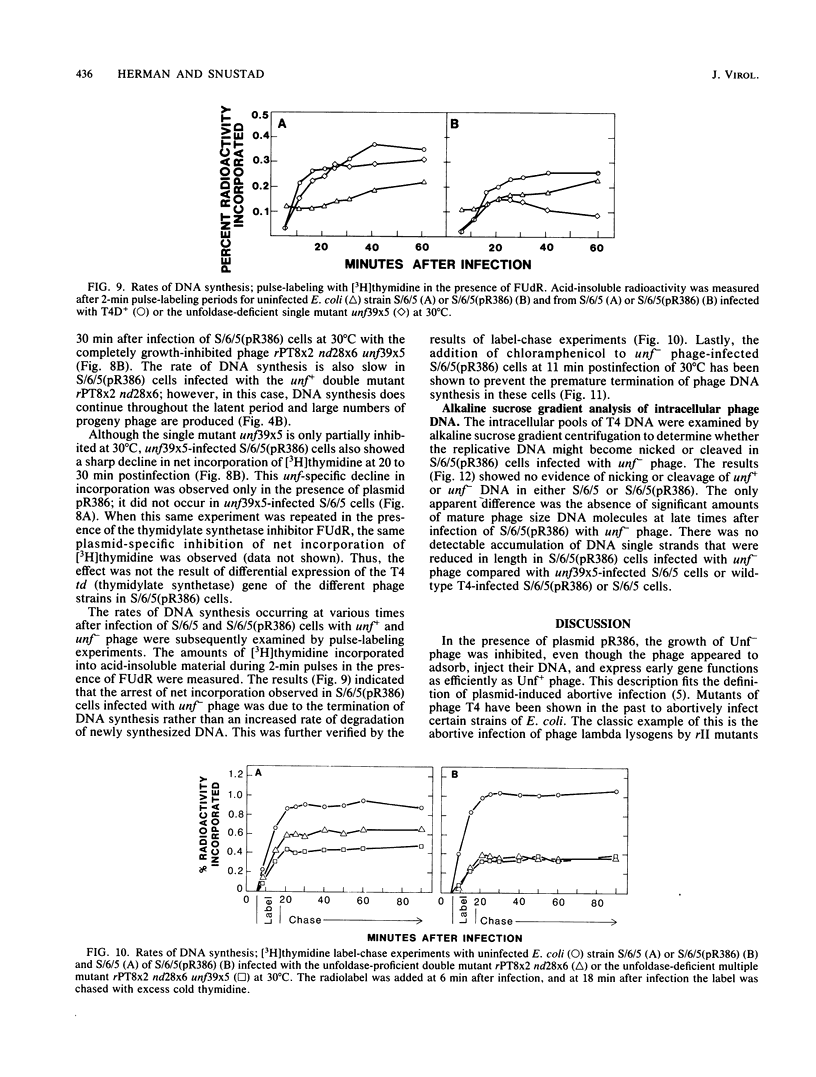
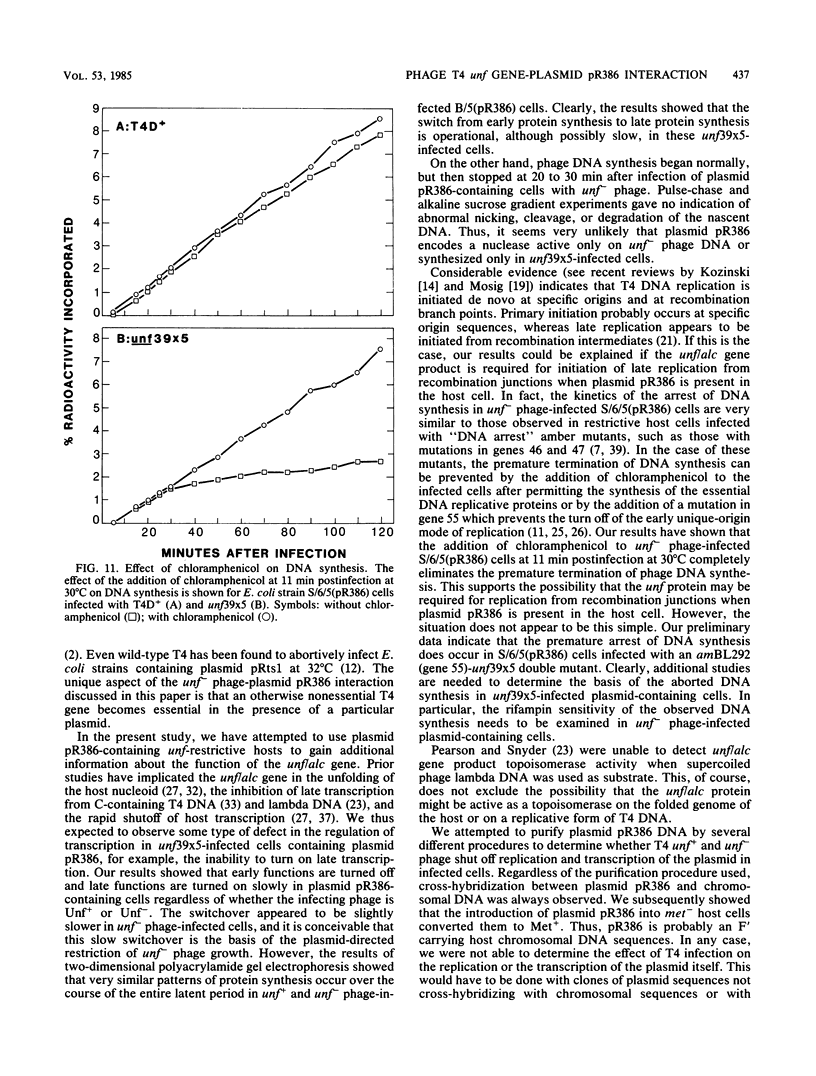
The bacteriophage T4 unf gene, known to be involved in the arrest of transcription from cytosine-containing DNA, is unessential except in Escherichia coli strains containing plasmid pR386. Comparative genetic and biochemical analyses of parameters of unf+ and unf- phage growth in host cells isogenic except for the presence or absence of plasmid pR386 have shown that unf gene function is required for late phage DNA synthesis in the presence of the plasmid. Shutoff of host DNA, RNA, and protein syntheses, degradation of host DNA, adsorption, injection, and early phage DNA, RNA, and protein syntheses all occurred with normal or near-normal kinetics in unf- infections, even in the presence of the plasmid. The switch from early to late protein synthesis occurred in plasmid pR386-containing cells infected with unf+ or unf- phage. However, this switchover was slow in both cases and may be slower in unf- infections than in unf+ infections. Net incorporation of [3H]thymidine terminated at about 30 min after infection of pR386-containing cells with unf- phage at 30 degrees C. Alkaline sucrose gradient studies of the intracellular pools of replicative DNA in unf-infected plasmid pR386-containing cells indicated that this DNA is not detectably nickel or cleaved at the time that DNA synthesis aborts. The addition of chloramphenicol subsequent to early enzyme synthesis prevented the arrest of DNA synthesis in plasmid-containing cells infected with unf-phage.
Full text
PDF

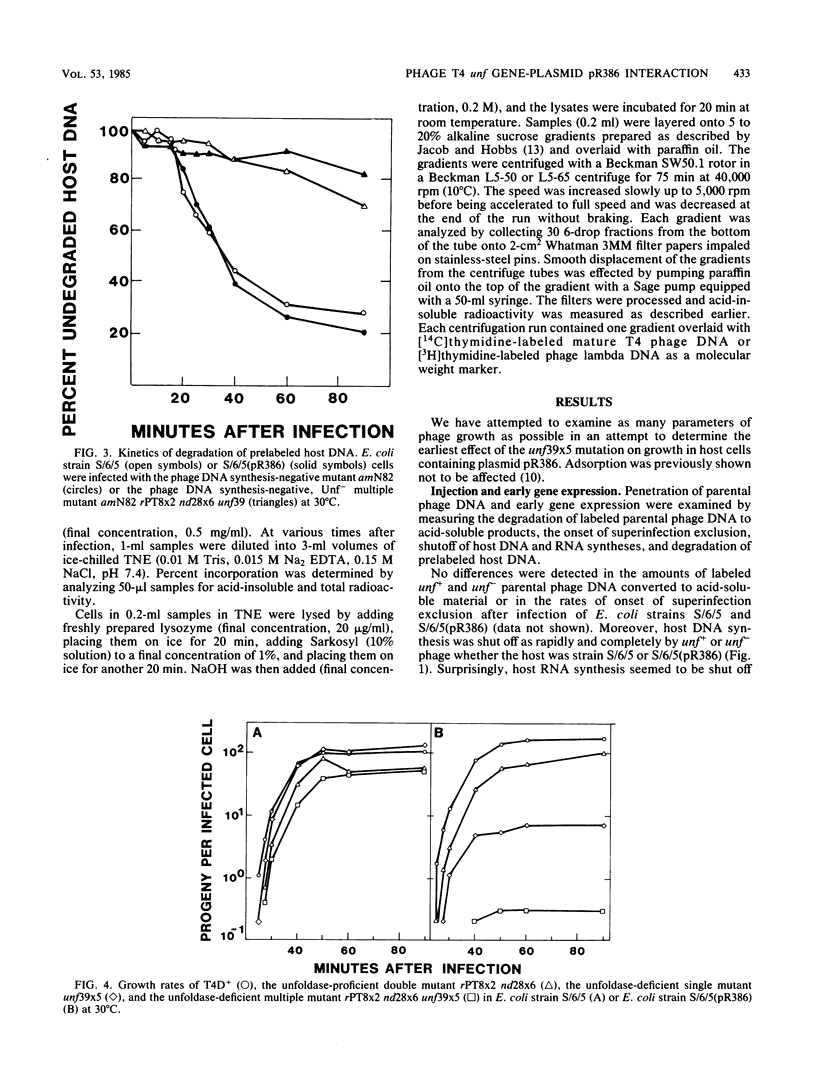

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget P. B., Warner H. R. Identification of P48 and P54 as components of bacteriophage T4 baseplates. J Virol. 1975 Dec;16(6):1669–1677. doi: 10.1128/jvi.16.6.1669-1677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Glenn J., McCorquodale D. J. Inhibition of bacteriophage replication by extrachromosomal genetic elements. Microbiol Rev. 1981 Mar;45(1):52–71. doi: 10.1128/mr.45.1.52-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Goff C. G., Setzer J. ADP ribosylation of Escherichia coli RNA polymerase is nonessential for bacteriophage T4 development. J Virol. 1980 Jan;33(1):547–549. doi: 10.1128/jvi.33.1.547-549.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. E., Haas N., Snustad D. P. Identification of the bacteriophage T4 unf ( = alc) gene product, a protein involved in the shutoff of host transcription. Genetics. 1984 Oct;108(2):305–317. doi: 10.1093/genetics/108.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. E., Snustad D. P. Plasmid pR386 renders Escherichia coli cells restrictive to the growth of bacteriophage T4 unf mutants. J Virol. 1982 Jan;41(1):330–333. doi: 10.1128/jvi.41.1.330-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Mathews E., Jansen B. Role of genes 46 and 47 in bacteriophage T4 reproduction. I. In vivo deoxyribonucleic acid replication. J Virol. 1971 Oct;8(4):372–387. doi: 10.1128/jvi.8.4.372-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq M., Kaji A. Mechanism of T4 phage restriction by plasmid Rts 1. Cleavage of T4 phage DNA by Rts 1-specific enzyme. J Biol Chem. 1980 May 10;255(9):4040–4047. [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E., Drivdahl R., Rand K. Identification and characterization of the alc gene product of bacteriophage T4. Genetics. 1984 Oct;108(2):291–304. doi: 10.1093/genetics/108.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M., Miller R. C., Jr T7 exonuclease (gene 6) is necessary for molecular recombination of bacteriophage T7. J Virol. 1974 Nov;14(5):1040–1048. doi: 10.1128/jvi.14.5.1040-1048.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhammer R., Yang H. L., Reiness G., Zubay G. Effects of bacteriophage T4-induced modification of Escherichia coli RNA polymerase on gene expression in vitro. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4928–4932. doi: 10.1073/pnas.72.12.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearson R. E., Snyder L. Shutoff of lambda gene expression by bacteriophage T4: role of the T4 alc gene. J Virol. 1980 Jul;35(1):194–202. doi: 10.1128/jvi.35.1.194-202.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. B., Berger H. Replication of gene 46-47 amber mutants of bacteriophage T4D. J Mol Biol. 1971 Apr 14;57(1):17–34. doi: 10.1016/0022-2836(71)90117-3. [DOI] [PubMed] [Google Scholar]
- Shalitin C., Naot Y. Role of gene 46 in bacteriophage T4 deoxyribonucleic acid synthesis. J Virol. 1971 Aug;8(2):142–153. doi: 10.1128/jvi.8.2.142-153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Wei J., Snyder L. T4 Bacteriophage-coded RNA polymerase subunit blocks host transcription and unfolds the host chromosome. Nature. 1977 Jan 6;265(5589):28–32. doi: 10.1038/265028a0. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Bursch C. J., Parson K. A., Hefeneider S. H. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption: shutoff of host DNA and protein synthesis gene dosage experiments, identification of a restrictive host, and possible biological significance. J Virol. 1976 Apr;18(1):268–288. doi: 10.1128/jvi.18.1.268-288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
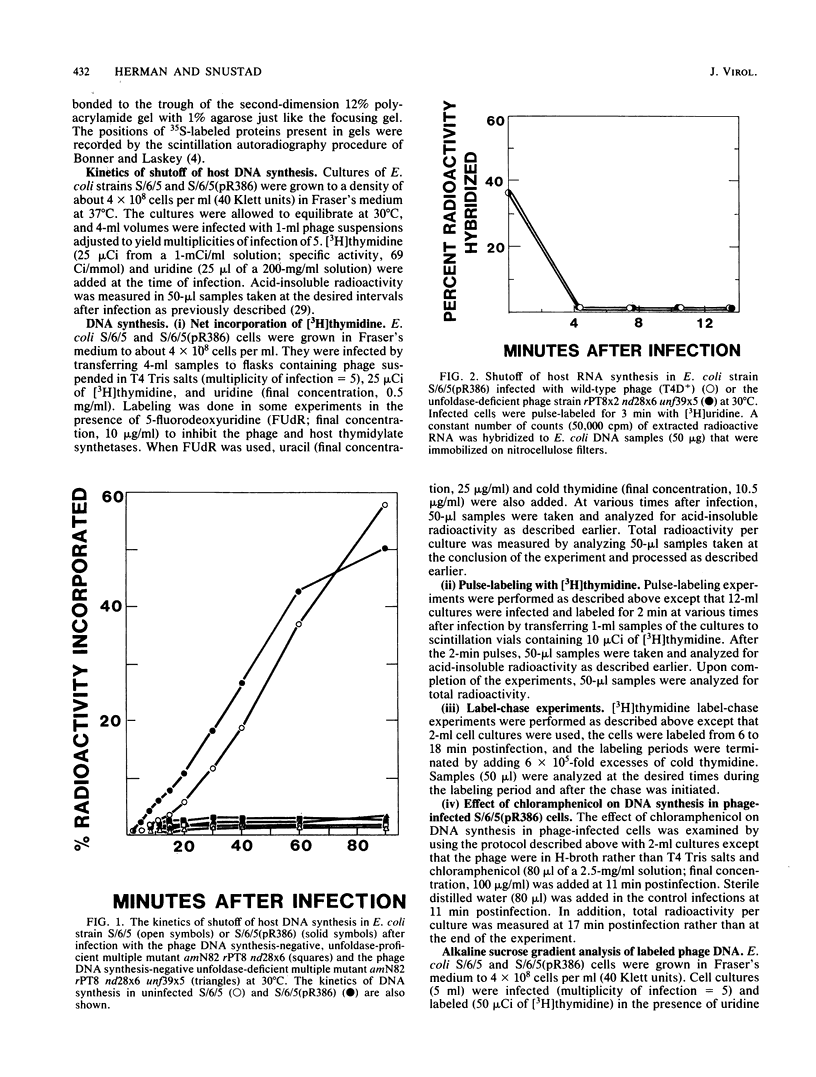
- Snustad D. P., Conroy L. M. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption. I. Isolation and genetic characterization. J Mol Biol. 1974 Nov 15;89(4):663–673. doi: 10.1016/0022-2836(74)90043-6. [DOI] [PubMed] [Google Scholar]
- Snustad D. P. Differential transmission of amber and wild-type alleles of bacteriophage T4 in mixedly infected cells of Escherichia coli. Virology. 1970 May;41(1):52–65. doi: 10.1016/0042-6822(70)90053-x. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Tigges M. A., Parson K. A., Bursch C. J., Caron F. M., Koerner J. F., Tutas D. J. Identification and preliminary characterization of a mutant defective in the bacteriophage T4-induced unfolding of the Escherichia coli nucleoid. J Virol. 1976 Feb;17(2):622–641. doi: 10.1128/jvi.17.2.622-641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tigges M. A., Bursch C. J., Snustad D. P. Slow switchover from host RNA synthesis to bacteriophage RNA synthesis after infection of Escherichia coli with a T4 mutant defective in the bacteriophage T4-induced unfolding of the host nucleoid. J Virol. 1977 Dec;24(3):775–785. doi: 10.1128/jvi.24.3.775-785.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutas D. J., Wehner J. M., Koerner J. F. Unfolding of the host genome after infection of Escherichia coli with bacteriophage T4. J Virol. 1974 Feb;13(2):548–550. doi: 10.1128/jvi.13.2.548-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]