Abstract
Ubiquitin chain complexity in cells is likely regulated by a diverse set of deubiquitinating enzymes (DUBs) with distinct ubiquitin chain preferences. Here we show that the polyglutamine disease protein, ataxin-3, binds and cleaves ubiquitin chains in a manner suggesting that it functions as a mixed linkage, chain-editing enzyme. Ataxin-3 cleaves ubiquitin chains through its amino-terminal Josephin domain and binds ubiquitin chains through a carboxyl-terminal cluster of ubiquitin interaction motifs neighboring the pathogenic polyglutamine tract. Ataxin-3 binds both Lys48- or Lys63-linked chains yet preferentially cleaves Lys63 linkages. Ataxin-3 shows even greater activity toward mixed linkage polyubiquitin, cleaving Lys63 linkages in chains that contain both Lys48 and Lys63 linkages. The ubiquitin interaction motifs regulate the specificity of this activity by restricting what can be cleaved by the protease domain, demonstrating that linkage specificity can be determined by elements outside the catalytic domain of a DUB. These findings establish ataxin-3 as a novel DUB that edits topologically complex chains.
The conjugation of ubiquitin to proteins regulates diverse cellular processes ranging from protein degradation to DNA repair (1–6). Topologically and functionally distinct ubiquitin chains can be formed by covalent linkage through any of seven lysines in ubiquitin (7–13). Lys48-linked chains, the best studied type of chain, function in the ubiquitin-proteasome pathway of protein degradation (1–3, 6). In contrast, less is known about the functions of chains linked through other lysines, including Lys63. Chain complexity has recently emerged as critically important to the regulation of various cellular pathways (5). Mixed linkage and multiply branched chains, for which evidence is now emerging, likely add to this complexity (10, 14, 15). Mixed linkage chains, for example, may impede some cellular processes such as the efficient handling of substrates by the proteasome (15). However, little is known about how chain complexity is regulated, especially concerning how deubiquitinating enzymes (DUBs)2 act on specific types of chains.
Ubiquitin signaling is regulated by dozens of DUBs (16, 17). By removing ubiquitin from substrates, DUBs can facilitate substrate entry into the proteasome as well as terminate ubiquitin signals underlying various ubiquitin-dependent pathways. One such DUB is ataxin-3, the disease protein in the polyglutamine (polyQ) neurodegenerative disorder spinocerebellar ataxia type 3, also known as Machado-Joseph disease (18). Ataxin-3 possesses a catalytic amino-terminal Josephin domain and a carboxyl-terminal ubiquitin-binding domain that contains three ubiquitin interacting motifs (UIMs) (see Fig. 1A) (19, 20). Mutating the catalytic cysteine residue (Cys14) in ataxin-3 abolishes protease activity, whereas mutating conserved residues in the UIMs diminishes ubiquitin chain binding (21–24). The polyQ tract, which is expanded in persons afflicted with spinocerebellar ataxia type 3, resides between the second and third UIMs. The normal role of this polyQ tract and the consequences of its expansion on ataxin-3 activity are poorly understood.
FIGURE 1.
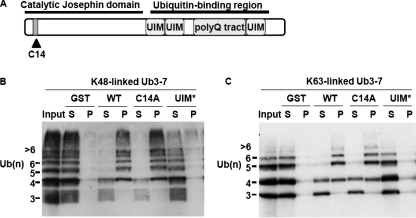
Ataxin-3 binds Lys48- or Lys63-linked ubiquitin chains. A, diagram of ataxin-3 depicting known functional domains. C14, catalytic cysteine residue 14; B and C, ataxin-3 binding to Lys48- and Lys63-linked ubiquitin chains. GST or GST-ataxin-3 (Gln22) (250 nm) prebound to glutathione-Sepharose beads was incubated with ubiquitin chains (Ub3–7, 250 nm) at 4 °C. Unbound supernatant (S) and bound pellet (P) fractions that had been extensively washed were immunoblotted with anti-ubiquitin antibody. WT, wild type; C14A, catalytically inactive; UIM*, UIM-mutated.
At least nine neurodegenerative disorders are caused by polyQ expansions in otherwise unrelated proteins (25, 26). PolyQ diseases are marked by perturbations in protein homeostasis, including disease protein and ubiquitin chain accumulation in the brain (27–30). Although protein misfolding contributes to disease pathogenesis, the events leading to neuronal dysfunction and cell death have not been fully elucidated for any polyQ disorder. It is nevertheless clear that the protein context in which expansion occurs plays a key role (31).
Growing evidence implicates ataxin-3 in protein quality control pathways. For example, ataxin-3 interacts with ubiquitin-proteasome pathway components (32–34), localizes to protein inclusions in various disease states (35, 36), regulates the formation of aggresomes (37), and assists in degrading substrates retrotranslocated from the endoplasmic reticulum (34, 38). In Drosophila, wild type ataxin-3 can suppress polyQ-mediated neurodegeneration through its ubiquitin-binding and cleavage activities, and expanded ataxin-3 retains this suppressor activity despite its pathogenic mutation (39). These unusual properties of ataxin-3 underscore both the importance of quality control pathways in polyQ diseases and the need to define the function of ataxin-3 in such pathways. Here we present evidence that ataxin-3 possesses novel ubiquitin chain binding and cleavage properties that suggest it functions as the first identified, mixed linkage ubiquitin chain-editing enzyme.
EXPERIMENTAL PROCEDURES
Plasmids—The ataxin-3 variant used in all assays is isoform 1, variant 1 (NM_004993), which contains all 11 exons and encodes the full protein with all three UIMs. Ataxin-3 plasmids pGEX6P1-ATX3-WT, -C14A, and -SA were generated by digesting the corresponding pcDNA3-Myc-ATX3 (23) plasmids with BamHI and NotI and inserting the released fragments into pGEX-6P1 (GE Healthcare) vector. For the truncation mutant containing only the Josephin domain, the wild type construct was mutated with forward primer 5′-GAC CAA CTC CTG CAG ATG ATT CGC TAA ATA AGA ATG CGG CCG CAT CG and reverse primer 5′-CG ATG CGG CCG CAT TCT TAT TTA GCG AAT CAT CTG CAG GAG TTG GTC to introduce a stop codon after residue 182.
SDS-PAGE and Immunoblotting—SDS-PAGE was performed with constant voltage on 10% (see Figs. 1, B and C, and 2A and supplemental Fig. S1), 15% (see Figs. 2, C, D, and G, 3B, 4B, and 5A and supplemental Fig. S2), 10–20% (see Fig. 2, B and F, 4A, and 5, B and C) or 4–15% (see Fig. 5, D–G, and supplemental Fig. S3) gels. Immunoblotting was performed as described previously (33). The following antibodies were used: anti-ataxin-3 polyclonal (Machado-Joseph disease, 1:20,000 (29)), anti-ataxin-3 monoclonal (1H9, kindly provided by Y. Trottier, 1:2,000), anti-HA polyclonal (Y11, Santa Cruz, 1:500), anti-ubiquitin monoclonal (P4D1, Santa Cruz, 1:10,000), anti-ubiquitin polyclonal (Dako, 1:1,000), anti-tubulin monoclonal (Sigma, 1:50,000), anti-FLAG polyclonal (Sigma, 1:1,000), anti-GAPDH monoclonal (Chemicon, 1:1,000), goat anti-mouse peroxidase-conjugated secondary (Jackson Laboratories, 1:15,000), and goat anti-rabbit peroxidase-conjugated secondary antibodies (Jackson Laboratories, 1:15,000).
FIGURE 2.
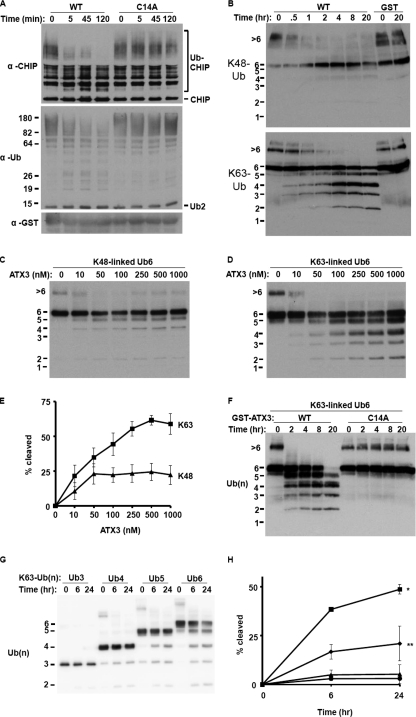
Ataxin-3 preferentially cleaves longer chains and Lys63 linkages. A, ataxin-3 (Gln22) cleavage of ubiquitinated CHIP generated in vitro with the E2/E3 pair, UbcH5c/CHIP. For the indicated times at 37 °C, GST-ataxin-3 was incubated with a quenched CHIP auto-ubiquitination reaction, and then analyzed by immunoblotting with anti-CHIP (top panel) and anti-ubiquitin (bottom panel) antibodies. B, time course of ataxin-3 (Gln22) cleavage of Lys48-linked (top panel) or Lys63-linked (bottom panel) Ub6. GST or GST-ataxin-3 (1 μm) was incubated with Ub6 chains (250 nm) for the indicated times and then analyzed by anti-ubiquitin immunoblotting. C and D, concentration curve of ataxin-3 (Gln22) cleavage of Lys48-linked Ub6 (C) or Lys63-linked Ub6 (D). Increasing concentrations of GST-ataxin-3 were incubated with Ub6 chains (250 nm) at 37 °C for 2 h and then analyzed by anti-ubiquitin immunoblotting. E, quantification of ataxin-3 cleavage of Lys48- and Lys63-linked Ub6. Shown is the percentage of the total ubiquitin signal in each lane that is lower molecular weight reaction product (<Ub6) (n = 2; means ± S.D.). F, requirement of catalytic cysteine for both Lys63-Ub6 cleavage and activity against HMW ubiquitin chain complexes (>6). Normal (WT) or catalytically inactive (C14A) ataxin-3 (Gln22) (1μm) was incubated with Lys63-linked Ub6 for the indicated times. G, chain length dependence of ataxin-3 activity. Performed as in B, except GST-ataxin-3 (100 nm) was incubated with the indicated lengths of ubiquitin chains. H, quantification of chain length dependant ataxin-3 activity from G. Shown is the percentage of the total ubiquitin signal in each lane that is lower molecular weight reaction product (n = 2; means ± S.D.). Cleavage at 24 h of Ub6 was greater than of Ub3, 4, and 5 (*, p < 0.01), and cleavage of Ub5 was greater than of Ub3 (**, p < 0.05).
FIGURE 3.
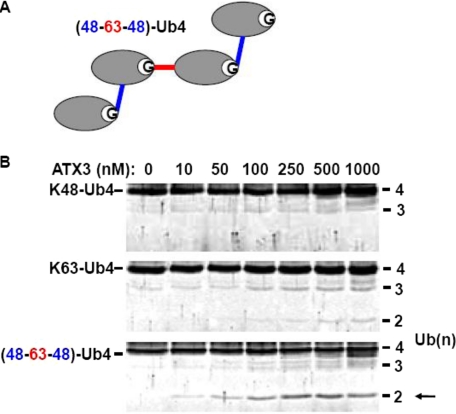
Ataxin-3 cleaves mixed linkage ubiquitin chains. A, diagram of mixed linkage Ub4 chain with Lys48- and Lys63-linkages used in this study. B, comparison of ataxin-3 (Gln22) cleavage of Ub4 chains with pure or mixed linkages. GST-ataxin-3 was incubated with the indicated Ub4 chains for 1 h at 37 °C before analysis by SDS-PAGE and Sypro Ruby staining. The arrow indicates Lys48-linked Ub2 reaction product.
FIGURE 4.
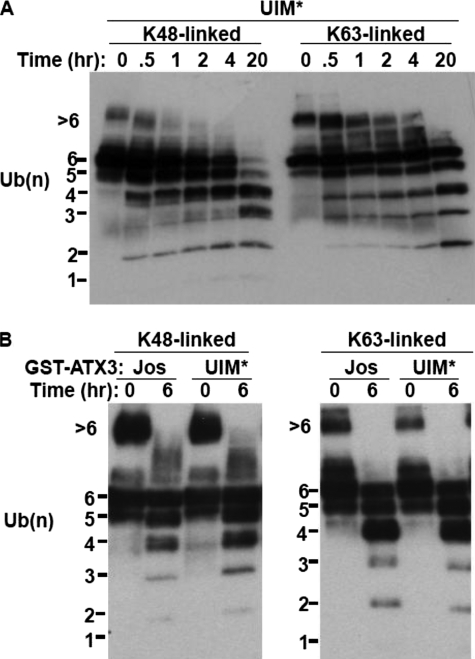
UIMs restrict Lys48-linked ubiquitin chain cleavage by ataxin-3. A, effect of UIM mutations on Lys48- and Lys63-linked ubiquitin proteolysis. Performed as in Fig. 2B, except that UIM-mutated (UIM*) ataxin-3 (Gln22) was incubated with Lys48- or Lys63-linked Ub6 for the indicated times. B, similar Ub6 cleavage by UIM-mutated ataxin-3 (Gln22) and Josephin domain alone. As in A, except Josephin domain-only (Jos) or UIM-mutated (UIM*) ataxin-3 were incubated with Lys48- or Lys63-linked Ub6.
FIGURE 5.
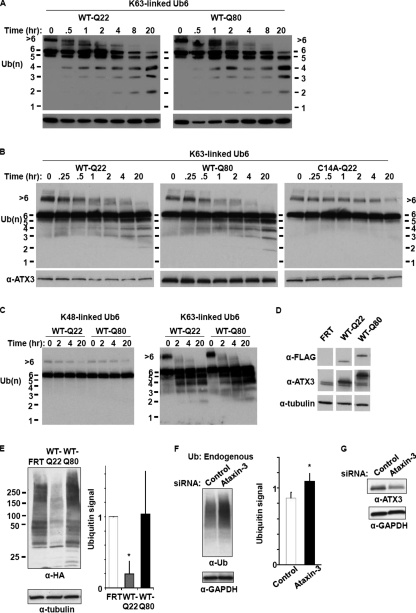
Effect of polyQ expansion on ataxin-3 activity. A, absence of significant effect of polyQ expansion on Lys63-linked ubiquitin chain proteolysis in vitro. GST-ataxin-3 (250 nm) with normal (Gln22) or expanded (Gln80) glutamine repeat was incubated with Lys63-linked Ub6 (250 nm) at 37 °C for the indicated times and then analyzed by anti-ubiquitin immunoblotting. B, absence of effect of polyQ expansion on Lys63 linkage proteolysis by ataxin-3 immunopurified from mammalian FLP-In 293 cells. As in A, except immunopurified FLAG-ataxin-3 (Gln22 or Gln80) was incubated with Lys63-linked Ub6. C, absence of effect of polyQ expansion on ataxin-3 chain cleavage preference. As in A, except immunopurified FLAG-ataxin-3 (Gln22 or Gln80) was incubated with Lys48- or Lys63-linked Ub6. D, ataxin-3 levels in stably transfected HEK293 cell lines. Lysates from vector control line expressing no exogenous ataxin-3 (FRT) and cell lines expressing normal (WT-Q22) or expanded (WT-Q80) FLAG-ataxin-3 were immunoblotted with the indicated antibodies. Anti-ATX3 recognizes all ataxin-3, whereas anti-FLAG recognizes only exogenous ataxin-3. E, effects of normal or expanded ataxin-3 expression on cellular protein ubiquitination. HEK293 cells stably expressing normal or expanded FLAG-ataxin-3 were transfected with HA-ubiquitin, lysed, and analyzed by immunoblotting. Densitometric quantification of ubiquitin smears (relative to tubulin and normalized to FRT control) shows significantly reduced ubiquitination in ataxin-3-Gln22-expressing cells compared with FRT control cells or ataxin-3-Gln80-expressing cells (p < 0.05; n = 3; mean ± S.D.). F, effect of ataxin-3 knockdown on endogenous cellular protein ubiquitination. The cells transfected with RNAi duplexes were lysed and immunoblotted with anti-ubiquitin and anti-GAPDH antibodies. Ataxin-3 knockdown resulted in increased ubiquitin levels relative to GAPDH when compared with control (p < 0.01; n = 6 for control duplexes and n = 12 for ataxin-3-targeting duplexes; mean ± S.D.). G, levels of ataxin-3 after RNAi knockdown. 293 cells were transfected with the indicated RNAi duplexes at 0 and 48 h, lysed at 72 h, and immunoblotted with ataxin-3 and GAPDH antibodies.
Recombinant Protein Purification—GST fusion proteins were purified as described previously (33), except that columns were washed two additional times with Buffer A (50 mm HEPES, 0.5 mm EDTA, 1 mm dithiothreitol, and 0.1 mg/ml bovine albumin at pH 7.5) or Buffer B (50 mm HEPES, 0.5 mm EDTA, 1 mm dithiothreitol, and 0.1 mg/ml ovalbumin at pH 7.5).
Binding Assays—GST fusion proteins (250 nm) were incubated with 250 nm (assuming average size of 40 kDa) mixed length (Ub3–7) ubiquitin chains (Boston Biochem) for 30 min. Incubations were performed at 4 °C to minimize proteolysis. Unbound supernatant fractions were removed and added to loading buffer. The beads were washed four times with Buffer A (see Fig. 1, B and C) or Buffer A with 0.1% Nonidet P-40 (supplemental Fig. S1).
Protease Assays—Ubiquitin conjugation reactions were performed as described previously (15) and were stopped by adding 10 mm EDTA. GST fusion proteins were incubated with ubiquitinated proteins or 250 nm ubiquitin chains (Boston Biochem) at 37 °C in conjugation buffer with EDTA (see Fig. 2A), Buffer A (see Figs. 2, B–F, 3B, and 4A), or Buffer B (see Figs. 4B and supplemental Fig. S2). Protein concentrations were determined by UV absorbance with a spectrophotometer (Nano-Drop, ND-1000) for Figs. 2 (C and D) and 3. FLAG-tagged proteins immunopurified from FLP-In 293 cells were incubated with 100 or 250 nm ubiquitin chains (Boston Biochem) at 37 °C in Buffer D (Buffer A with 1% Sigma Protease Inhibitor mixture P8340). Because equal numbers of cells from the different stable cell lines did not always yield the same amount of enzyme (see Fig. 5A), we standardized FLAG-ataxin-3 concentrations by comparison with known amounts of purified ataxin-3 (data not shown) for other experiments (Fig. 5C and data not shown). The reactions were stopped with loading buffer at room temperature. Quantification of immunoblots was performed with QuantityOne (Bio-Rad), using the rolling disk method of background subtraction. For Fig. 2H, significances were determined by single factor analysis of variance and Tukey's test.
Immunoaffinity Purification—FLP-In 293 cells (33) were lysed in ice-cold Buffer C (1% Nonidet P-40, 0.1% SDS, 0.5% deoxycholic acid, 50 mm Tris-HCl, 150 mm NaCl, and 10% Sigma Protease Inhibitor mixture P8340 at pH7.4). The lysates were incubated with 25 μl of anti-FLAG M2 affinity beads (Sigma) for 2 h at 4 °C. The beads were washed four times with Buffer C and twice with Buffer A. The proteins were eluted with 3× FLAG peptide in 50 μl of Buffer A. Immunopurified proteins were frozen in liquid nitrogen and stored at –80 °C.
Ubiquitin Accumulation Experiments—FLP-In cell lines were transiently transfected with HA-tagged ubiquitin, using Lipofectamine-PLUS per the manufacturer's instructions (Invitrogen). The cells were harvested with SDS sample buffer 48 h later. After immunoblotting with anti-HA antibody, the intensities of the HA-ubiquitin smears were quantified by densitometry (ImageJ) (40). Student's t test was used to evaluate significance (n = 5, p < 0.05).
RNAi Knockdown—Ataxin-3 knockdown was performed with IDT TriFECTA dicer substrate (DsiRNA) duplexes (HSC.RNAI.N001024631.3), including three DsiRNAs targeting ataxin-3 mRNA (accession number NM_001024631) and a scrambled negative control DsiRNA. 293 cells were transfected with 5–45 nm DsiRNA duplexes using Lipofectamine 2000 (Invitrogen) per manufacturer's instructions at 0 and 48 h. The cells were then lysed in Laemmli buffer at 72 h, and the lysates were analyzed by immunoblotting and quantification by densitometry with ImageJ (40). Two-tailed t tests were used to evaluate significance (n = 7, p < 0.01).
RESULTS
Ataxin-3 Binds Lys48- and Lys63-linked Ubiquitin Chains—We sought to determine the preferred ubiquitin chain substrates for ataxin-3, which is shown schematically in Fig. 1A. Unless otherwise noted, ataxin-3 with a nonexpanded polyglutamine length of 22 was used for these studies. We first performed pull-down binding assays of GST-ataxin-3 with ladders of Lys48- or Lys63-linked polyubiquitin employing extensive washes. As shown in Fig. 1 (B and C), wild type or catalytically inactive (C14A) ataxin-3 bound both Lys48- and Lys63-linked chains, provided the chain contained at least four ubiquitin moieties. Ataxin-3, in which critical conserved serine residues in each UIM had been mutated to alanine (UIM*) displayed negligible binding in this assay. Normal and expanded ataxin-3 bound similarly to both Lys48- and Lys63-linked chains (supplemental Fig. S1). Ataxin-3 bound nearly all Lys48- and Lys63-linked chains with five or more ubiquitins. In contrast, Ub4 was partially bound and Ub3 poorly bound, revealing a length dependence to binding. Therefore, ataxin-3 preferentially binds longer ubiquitin chains, and binding is not restricted to Lys48 linkages.
Ataxin-3 Preferentially Cleaves Longer Chains and Lys63 Linkages—Consistent with its binding of longer chains, ataxin-3 has been shown to cleave long ubiquitin chains (22, 37). To verify that ataxin-3 cleaves chains attached to a substrate, we incubated it with auto-ubiquitinated CHIP (an E3 ligase) generated in vitro under conditions where all ubiquitin chain linkage types can be formed (15). Ataxin-3 rapidly and efficiently cleaved highly ubiquitinated CHIP but failed to deubiquitinate CHIP conjugated to shorter chains (Fig. 2A). This selective cleavage only of heavily ubiquitinated substrate suggests that ataxin-3 is inefficient at, or restricted from, processing most chains containing fewer than approximately six ubiquitins.
The ability of ataxin-3 to bind both Lys48- and Lys63-linked chains suggested that it might also cleave both. Alternatively, given the distinct topologies of Lys48- and Lys63-linked chains (9, 11, 13), ataxin-3 could possess divergent catalytic activity toward the two chain types. To address this question, we compared the DUB activity of ataxin-3 in a controlled system containing chains of defined linkage and length (Lys48-Ub6 or Lys63-Ub6). These studies revealed that ataxin-3 preferentially cleaves Lys63-linked polyubiquitin (Fig. 2, B–E). Cleavage of Lys63-linked Ub6 led to the accumulation of smaller reaction products (Ub1 through Ub5), whereas little cleavage was observed with Lys48-linked Ub6. We could not determine a precise kcat for ataxin-3 because the total number of linkages cleaved by ataxin-3, in both reactant ubiquitin chains and lower molecular weight reaction products, is unclear. Mutating the catalytic cysteine residue (Cys14) abolished protease activity toward either chain type (Figs. 2F and 5B and data not shown). Ataxin-3 immunopurified from mammalian cells also cleaved Lys63-linked Ub6 more efficiently than Lys48-linked Ub6 (shown later in Fig. 5C), indicating that preferential Lys63 chain cleavage is an intrinsic property of ataxin-3, whether expressed in bacterial or mammalian cells.
We noticed that ataxin-3 possessed robust activity toward a subset of Ub6 chains that electrophorese on denaturing gels as much higher molecular weight (HMW) chains, whether derived from Lys48- or Lys63-linked chains (Fig. 2B). Mutating the catalytic cysteine abolished activity toward these HMW chains (Fig. 2F), indicating that ataxin-3 acts on HMW chains through its DUB activity rather than by simply dissociating HMW chain complexes. By quantitative mass spectrometry, HMW chains were nearly uniformly Lys48- or Lys63-linked (supplemental Table S1). These HMW chains are likely covalently linked complexes of Ub6 because they are resistant to various denaturants including heat, SDS, urea, and ethylene glycol (data not shown) and are present whether or not samples are boiled.
The pattern of Ub reaction products generated by ataxin-3 sheds light on the nature of chain cleavage. With Ub6 chains, ataxin-3 does not primarily produce Ub1 and thus is not exclusively a processive enzyme. In addition, Ub4 and Ub2 are more prevalent reaction products than Ub3, implying nonrandom cleavage (Fig. 2, B–D). These results suggest that ataxin-3 recognizes a four-ubiquitin patch and cleaves adjacent Lys63 linkages internally within the chain.
To define the chain length dependence of ataxin-3 activity, we explored cleavage of Lys48- and Lys63-linked chains containing three to six ubiquitin moieties (Fig. 2, G and H). Proteolysis of Lys63-linked chains did not occur with Ub3 but increased incrementally as chains were lengthened from four to six. In contrast, ataxin-3 possessed very little activity toward any tested length of Lys48-linked ubiquitin chains (data not shown).
Ataxin-3 Cleaves Mixed Linkage Ubiquitin Chains—The discrepancy between which linkages ataxin-3 can bind and which it can cleave led us to analyze its activity against ubiquitin chains of greater topological complexity. Ataxin-3 activity was tested against mixed linkage Ub4 chains with a central Lys63 linkage flanked by Lys48 linkages (Fig. 3A). Analysis by Sypro Ruby staining of parallel reactions with Lys48, Lys63, or mixed linkage Ub4 incubated with varying ataxin-3 concentrations revealed enhanced catalytic activity toward mixed linkage chains (Fig. 3B). Observable reaction products appeared at 10-fold lower ataxin-3 concentration from mixed linkage chains than from Lys63-linked chains (10 and 100 nm, respectively). Cleavage of heterotypic Ub4 containing a central Lys63 linkage flanked by Lys48 linkages yielded only a prominent Ub2 reaction product, indicating preferred cleavage at the central Lys63 linkage. Indeed, mass spectrometry analysis of the Ub2 reaction products derived from heterotypic chains confirmed that they were exclusively Lys48-linked (supplemental Fig. S2).
Ubiquitin Binding by UIMs Regulates Cleavage Specificity of Ataxin-3—Although Lys48- and Lys63-linked ubiquitin chains adopt different higher order structures, the structure of each individual ubiquitin moiety and its exposed surfaces is conserved. Linkage-specific DUBs like ataxin-3 must possess structural determinants that enforce this specificity. In ataxin-3, Lys63 linkage specificity could stem from the catalytic Josephin domain or from elements outside this region, such as the UIMs. Intriguingly, mutating all three UIMs eliminated linkage specificity; UIM-mutated ataxin-3 displayed increased activity toward Lys48-linked chains that was similar to activity toward Lys63-linked chains (Fig. 4A). Thus, the UIMs in ataxin-3 serve to prevent Lys48 linkage cleavage rather than merely promote Lys63 linkage cleavage. Consistent with this, the Josephin domain by itself cleaved both chain types nonselectively (Fig. 4B).
Effect of PolyQ Expansion on Ataxin-3 Activity—The polyQ tract in ataxin-3 resides between the second and third UIMs. Although polyQ expansion does not markedly alter chain binding by ataxin-3 in vitro (23), it could alter chain presentation to the catalytic domain and thereby affect protease activity. We thus compared the protease activity of normal versus expanded ataxin-3 purified from bacteria. As shown in Fig. 5A, we did not detect a difference in catalytic activity toward Lys63-linked Ub6. Ataxin-3 purified from bacteria was soluble and monomeric (supplemental Fig. S3); as previously reported, it separates by size exclusion chromatography larger than expected (160 kDa versus 42–48 kDa) (41) probably because of its elliptical shape (42).
The experiments described above were performed using ataxin-3 purified from bacteria. To determine whether ataxin-3 biosynthesis inside mammalian cells might lead to polyglutamine-dependent effects on protease activity, we tested the activity of ataxin-3 immunopurified from stably transfected cell lines. We generated a series of FLP-In 293 cell lines expressing epitope-tagged ataxin-3. These included lines that express normal or catalytically inactive forms of ataxin-3 with glutamine repeats of normal (Gln22) or expanded (Gln80) residues (WT-Gln22, C14A-Gln22, and WT-Gln80), and a negative control line that expresses no exogenous ataxin-3 (FRT). The low level expression of FLAG-ataxin-3 in these lines favors physiological folding of normal or expanded ataxin-3. Indeed, expanded ataxin-3 expressed in these lines does not form inclusions (data not shown) and remains soluble when purified, eluting on size exclusion chromatography in fractions centered at 160 kDa and not in the void volume (supplemental Fig. S3).
To compare the activity of normal and expanded ataxin-3, we incubated the various immunopurified forms of ataxin-3 with Lys63-linked Ub6. Normal (Gln22) and expanded (Gln80) ataxin-3 cleaved Ub6 similarly, yielding an identical pattern of lower molecular weight reaction products (Fig. 5B). The preferential cleavage of Lys63 over Lys48 linkages was not altered by polyQ expansion (Fig. 5C), and we did not detect polyQ length-dependent differences in activity toward mixed linkage chains (data not shown). Thus, polyQ expansion does not greatly alter ataxin-3 protease activity toward polyubiquitin test substrates in vitro.
Differences in activity caused by polyQ expansion, however, might not be revealed in our in vitro assays because the immunopurified ataxin-3 protein has been removed from its normal cellular context. As shown in Fig. 5E, expression of ataxin-3 with a nonpathogenic polyQ repeat (Gln22) leads to a reduction in total ubiquitinated protein levels in 293-FLP-In cells. In contrast, this reduction in ubiquitinated protein is not observed upon expression of expanded ataxin-3 (Gln80). This difference could reflect inefficient deubiquitination of cellular proteins by expanded ataxin-3 among other possibilities.
Finally, we sought to determine whether reducing endogenous ataxin-3 by RNA interference would affect the cellular state of protein ubiquitination. Knockdown of ataxin-3 in HEK293 cells (Fig. 5G) resulted in a significant increase in the levels of endogenous ubiquitinated proteins (Fig. 5F). The observed effect across a broad smear of ubiquitinated proteins (Fig. 5, E and F) suggests that ataxin-3 regulates the ubiquitination state of a large number of substrates rather than only a few.
DISCUSSION
Our results reveal unprecedented complexity to the ubiquitin binding and protease activities of ataxin-3. These properties suggest that ataxin-3 functions as a ubiquitin chain-editing enzyme with preference for non-Lys48 linkages in mixed linkage chains. Ataxin-3 binds similarly to longer Lys48- and Lys63-linked ubiquitin chains yet preferentially cleaves Lys63 linkages, especially in mixed linkage chains. The fact that some ubiquitin ligases can generate multiple types of chain linkage (15, 43), coupled with the emerging recognition that intracellular chain complexity is far greater than once anticipated (10, 27, 44), implies that cells also must possess DUBs to handle topologically complex ubiquitin chains. Ataxin-3 is the first reported DUB in this category.
Seven kinds of homotypic ubiquitin chains can exist in cells (10), and recent studies indicate that heterotypic and branched chains are also present (10, 14). Although in some cases mixed linkage chains may be unwanted byproducts of indiscriminant ligase activity, in other cases they may constitute signals that regulate specific cellular pathways. In either case ataxin-3 is ideally suited to act on mixed linkage chains and terminate their effects in the cell. It is also possible that ataxin-3, which interacts with ubiquitin ligases (34, 45), edits chains as they are generated.
Ataxin-3 Preferentially Acts on Long and Complex Ubiquitin Chains—Collectively, our binding and cleavage results demonstrate that ataxin-3 prefers to act on topologically complex or very long ubiquitin chains. Ataxin-3 showed robust activity toward the very long, possibly structurally aberrant, ubiquitin chains present in our reactions (Fig. 2, B–D and G). The preparations of defined length chains used in this study typically contain apparent chain dimers and sometimes even higher order complexes. These HMW chains are resistant to denaturants, suggesting that they are covalently linked. The precise nature of the linkages between chains in these complexes, however, remains unknown. Intriguingly, ataxin-3 effectively acted on these HMW chains regardless of the type of chain linkage, which is consistent with the observed preferential cleavage of the longest chains on a ubiquitinated substrate (Fig. 2, A–D and G). These results raise the possibility that ataxin-3 functions in the cell to prevent the accumulation of very large and possibly aberrantly structured ubiquitin chains.
With Lys63-linked chains we observed a clear length dependence for proteolysis by ataxin-3; whereas chains of six ubiquitins are cleaved, activity toward shorter (less than five ubiquitins) chains is reduced (Fig. 2, G and H). Lys48-linked chains of any length are inefficiently cleaved by ataxin-3. However, proteolysis of short mixed linkage chains containing only four ubiquitins is comparatively efficient (Fig. 3). The presence of an adjacent Lys48 linkage in mixed linkage chains increases cleavage of Lys63 linkages in short chains. This activity is consistent with a potential role for ataxin-3 in trimming non-Lys48 linkages from ubiquitinated substrates to ensure optimal signaling for proteasomal delivery.
UIMs Regulate Ubiquitin Binding and Proteolysis by Ataxin-3—Whereas ataxin-3 binds Lys48- or Lys63-linked ubiquitin chains, it has higher catalytic activity toward Lys63 linkages (Figs. 2, B–E, and 5C). The binding of ubiquitin chains by the UIMs of ataxin-3 determines this specificity. Mutating the UIMs reduces ubiquitin chain binding while unexpectedly expanding the range of linkages that can be cleaved. Thus, the UIMs of ataxin-3 constitute selectivity determinants that allow cleavage of Lys63-linked ubiquitin while preventing cleavage of Lys48-linked ubiquitin. Such specificity for Lys63-linked chains could be achieved through negative regulation of protein activity toward nonpreferred but potentially very similar substrates (46). For example, the Lys63-specific protease CYLD may maintain specificity in part by inhibiting endodeubiquitinase activity toward Lys48-linked chains (47). The regulatory elements of CYLD are located within the catalytic domain, however, rather than in distant motifs such as the UIMs of ataxin-3.
There are at least eight known DUBs that contain ubiquitin-binding regions, suggesting that DUBs other than ataxin-3 may use ubiquitin binding domains to restrict activity to certain types of chains. The UIM of DUBA (OTU domain containing protein 5) has been shown to be important for its protease activity (48), though its role in chain specificity is unknown. Interestingly, most DUBs do not have predicted ubiquitin-binding regions. The chain specificity of these DUBs may be determined, in some cases, by interactions with other ubiquitin-binding proteins.
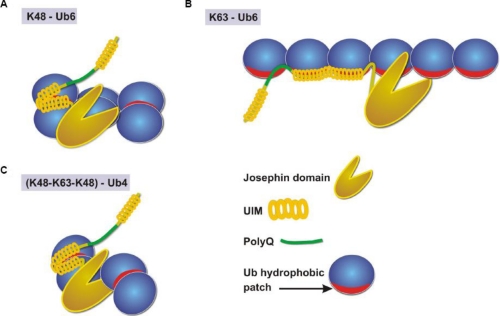
Model of Ataxin-3 Activity—We propose a model in which the precise topology of ubiquitin chains bound by ataxin-3 is critical for isopeptide bond cleavage (Fig. 6). According to this model, binding of purely Lys48-linked ubiquitin chains by the UIMs does not properly position the Lys48-Gly76 isopeptide bond for access to the catalytic pocket of the Josephin domain. In contrast, when ataxin-3 binds a chain containing adjacent Lys48- and Lys63 linkages, the Josephin domain is well positioned to attack the Lys63-Gly76 bond. Chain positioning determined by binding through the UIMs dictates the catalytic specificity of ataxin-3, as indicated by the loss of Lys63 linkage specificity when the UIMs are mutated. This model neither requires nor rules out allosteric activation of protease activity, which has been observed for other DUBs (49–51).
FIGURE 6.
Model of ataxin-3 ubiquitin chain binding and cleavage. Binding of a Lys48-linked ubiquitin chain (A) to the UIMs of ataxin-3 positions it such that cleavage by the catalytic Josephin domain is not favored. In contrast, ataxin-3 binding of Lys63-linked ubiquitin (B) permits proteolysis of long chains. Ataxin-3 binding of mixed linkage chains (C) favorably orients the catalytic region for proteolysis of Lys63 linkages.
A precise biophysical model of ataxin-3 catalytic activity must await structural studies of ataxin-3 bound to specific types of ubiquitin chains. Although the solution structure of the Josephin domain has been solved (24, 52, 53), the structures of the carboxyl-terminal ubiquitin-binding region and the full protein have not.
Insights into the Role of Ataxin-3 in PolyQ Disease—The role played by the polyQ domain in each of the nine identified polyQ disease proteins remains elusive. In ataxin-3, the polyQ tract may function as a flexible spacer between the first two UIMs and the third UIM. Although we did not observe polyQ length-dependent differences in ubiquitin binding or chain cleavage by ataxin-3, subtle effects of expansion will require further analysis in the physiological context of the cell. In contrast to normal ataxin-3, expanded ataxin-3 lacked the capacity to reduce the amount of ubiquitinated protein in cells (Fig. 5E), reminiscent of the effect of ataxin-3 knockdown in cells (Fig. 5F) and Atxn3 knock-out in mice (54).
Normal ataxin-3 suppresses polyQ disease pathogenesis in Drosophila through a mechanism requiring the ubiquitin-related properties of ataxin-3 (39). Expanded ataxin-3 retains this suppressor activity in the fly despite its pathogenic properties, which is consistent with our observation that expanded ataxin-3 retains enzymatic activity. Ataxin-3 may suppress neurotoxicity by facilitating ubiquitin-dependent clearance of abnormal proteins through its chain editing function.
Conclusion—A quality control mechanism that handles complex ubiquitin chains would be advantageous to the cell. In this light, the preference of ataxin-3 to cleave mixed linkage ubiquitin chains is intriguing. Ataxin-3 binds Lys48-linked ubiquitin chains, and then cleaves Lys63 linkages and possibly other non-Lys48 linkages. This activity would help ensure efficient proteasomal degradation of ubiquitinated substrates and/or facilitate the formation of specific chain linkages by ubiquitin ligases with which ataxin-3 interacts, such as Hrd1 (34) and E4B (43). We further propose that ataxin-3 editing activity underlies the observed disease suppression by ataxin-3 (39). Ataxin-3 may fill a previously unrecognized niche among DUBs as a regulator of ubiquitin chain quality and topology.
Supplementary Material
Acknowledgments
We thank members of the Paulson laboratory for helpful discussions and Ted Dawson for generously providing HA-ubiquitin constructs.
This work was supported, in whole or in part, by National Institutes of Health Grants NS038712 (to H. L. P.) and AG025688 (to J. P.). This work was also supported by a National Ataxia Foundation grant (to S. M. T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Table S1.
Footnotes
The abbreviations used are: polyQ, polyglutamine; UIM, ubiquitin-interacting motif; DUB, deubiquitinating enzyme; CHIP, carboxyl terminus of HSC70-interacting protein; Ub, ubiquitin; HMW, high molecular weight; HA, hemagglutinin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GST, glutathione S-transferase; RNAi, RNA interference; FRT, FLP recombinase target.
References
- 1.Hershko, A., and Ciechanover, A. (1998) Annu. Rev. Biochem. 67425 –479 [DOI] [PubMed] [Google Scholar]
- 2.Johnson, E. S., Ma, P. C. M., Ota, I. M., and Varshavsky, A. (1995) J. Biol. Chem. 27017442 –17456 [DOI] [PubMed] [Google Scholar]
- 3.Pickart, C. (1997) FASEB J. 111055 –1066 [DOI] [PubMed] [Google Scholar]
- 4.Pickart, C. M., and Fushman, D. (2004) Curr. Opin. Chem. Biol. 8610 –616 [DOI] [PubMed] [Google Scholar]
- 5.Sun, L., and Chen, Z. J. (2004) Curr. Opin. Cell Biol. 16119 –126 [DOI] [PubMed] [Google Scholar]
- 6.Varshavsky, A. (2005) Trends Biochem. Sci. 30283 –286 [DOI] [PubMed] [Google Scholar]
- 7.Cook, W., Jeffrey, L., Carson, M., Chen, Z., and Pickart, C. (1992) J. Biol. Chem. 26716467 –16471 [DOI] [PubMed] [Google Scholar]
- 8.Cook, W. J., Jeffrey, L. C., Kasperek, E., and Pickart, C. M. (1994) J. Mol. Biol. 236601 –609 [DOI] [PubMed] [Google Scholar]
- 9.Eddins, M. J., Varadan, R., Fushman, D., Pickart, C. M., and Wolberger, C. (2007) J. Mol. Biol. 367204 –211 [DOI] [PubMed] [Google Scholar]
- 10.Peng, J. S. D., Elias, J. E., Thoreen, C. C., Cheng, D., Marsischky, G., Roelofs, J., Finley, D., and Gygi, S. P. (2003) Nat. Biotechnol. 21921 –926 [DOI] [PubMed] [Google Scholar]
- 11.Tenno, T., Fujiwara, K., Tochio, H., Iwai, K., Morita, E. H., Hayashi, H., Murata, S., Hiroaki, H., Sato, M., Tanaka, K., and Shirakawa, M. (2004) Genes Cells 9865 –875 [DOI] [PubMed] [Google Scholar]
- 12.Varadan, R., Walker, O., Pickart, C., and Fushman, D. (2002) J. Mol. Biol. 324637 –647 [DOI] [PubMed] [Google Scholar]
- 13.Varadan, R., Assfalg, M., Haririnia, A., Raasi, S., Pickart, C., and Fushman, D. (2004) J. Biol. Chem. 2797055 –7063 [DOI] [PubMed] [Google Scholar]
- 14.Ben-Saadon, R., Zaaroor, D., Ziv, T., and Ciechanover, A. (2006) Mol. Cell 24701 –711 [DOI] [PubMed] [Google Scholar]
- 15.Kim, H. T., Kim, K. P., Lledias, F., Kisselev, A. F., Scaglione, K. M., Skowyra, D., Gygi, S. P., and Goldberg, A. L. (2007) J. Biol. Chem. 28217375 –17386 [DOI] [PubMed] [Google Scholar]
- 16.Amerik, A. Y., and Hochstrasser, M. (2004) Biochim. Biophys. Acta 1695189 –207 [DOI] [PubMed] [Google Scholar]
- 17.Nijman, S. M. B., Luna-Vargas, M. P. A., Velds, A., Brummelkamp, T. R., Dirac, A. M. G., Sixma, T. K., and Bernards, R. (2005) Cell 123773 –786 [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi, Y., Okamoto, T., Taniwaki, M., Aizawa, M., Inoue, M., Katayama, S., Kawakami, H., Nakamura, S., Nishimura, M., Akiguchi, I., Kimura, J., Narumiya, S., and Kakizuka, A. (1994) Nat. Genet. 8221 –228 [DOI] [PubMed] [Google Scholar]
- 19.Hofmann, K., and Falquet, L. (2001) Trends Biochem. Sci. 26347 –350 [DOI] [PubMed] [Google Scholar]
- 20.Scheel, H., Tomiuk, S., and Hofmann, K. (2003) Hum. Mol. Genet. 122845 –2852 [DOI] [PubMed] [Google Scholar]
- 21.Berke, S. J. S., Chai, Y., Marrs, G. L., Wen, H., and Paulson, H. L. (2005) J. Biol. Chem. 28032026 –32034 [DOI] [PubMed] [Google Scholar]
- 22.Burnett, B., Li, F., and Pittman, R. N. (2003) Hum. Mol. Genet. 123195 –3205 [DOI] [PubMed] [Google Scholar]
- 23.Chai, Y., Berke, S. S., Cohen, R. E., and Paulson, H. L. (2004) J. Biol. Chem. 2793605 –3611 [DOI] [PubMed] [Google Scholar]
- 24.Mao, Y., Senic-Matuglia, F., Di Fiore, P. P., Polo, S., Hodsdon, M. E., and De Camilli, P. (2005) Proc. Natl. Acad. Sci. U. S. A. 10212700 –12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orr, H. T., and Zoghbi, H. Y. (2007) Annu. Rev. Neurosci. 30575 –621 [DOI] [PubMed] [Google Scholar]
- 26.Perutz, M. F. (1999) Trends Biochem. Sci. 2458 –63 [DOI] [PubMed] [Google Scholar]
- 27.Bennett, E. J., Shaler, T. A., Woodman, B., Ryu, K. Y., Zaitseva, T. S., Becker, C. H., Bates, G. P., Schulman, H., and Kopito, R. R. (2007) Nature 448704 –708 [DOI] [PubMed] [Google Scholar]
- 28.Michalik, A., and Van Broeckhoven, C. (2003) Hum. Mol. Genet. 12R173 –R186 [DOI] [PubMed] [Google Scholar]
- 29.Paulson, H. L., Perez, M. K., Trottier, Y., Trojanowski, J. Q., Subramony, S. H., Das, S. S., Vig, P., Mandel, J.-L., Fischbeck, K. H., and Pittman, R. N. (1997) Neuron 19333 –344 [DOI] [PubMed] [Google Scholar]
- 30.Taylor, J. P., Hardy, J., and Fischbeck, K. H. (2002) Science 2961991 –1995 [DOI] [PubMed] [Google Scholar]
- 31.La Spada, A. R., and Taylor, J. P. (2003) Neuron 38681 –684 [DOI] [PubMed] [Google Scholar]
- 32.Doss-Pepe, E. W., Stenroos, E. S., Johnson, W. G., and Madura, K. (2003) Mol. Cell Biol. 236469 –6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todi, S. V., Laco, M. N., Winborn, B. J., Travis, S. M., Wen, H. M., and Paulson, H. L. (2007) J. Biol. Chem. 28229348 –29358 [DOI] [PubMed] [Google Scholar]
- 34.Wang, Q., Li, L., and Ye, Y. (2006) J. Cell Biol. 174963 –971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi, J., Tanaka, J., Arai, K., Funata, N., Hattori, T., Fukuda, T., Fujigasaki, H., and Uchihara, T. (2001) J. Neuropathol. Exp. Neurol. 6036 –376 [DOI] [PubMed] [Google Scholar]
- 36.Uchihara, T. F. H., Koyano, S., Nakamura, A., Yagishita, S., and Iwabuchi, K. (2001) Acta Neuropathol. 102149 –152 [DOI] [PubMed] [Google Scholar]
- 37.Burnett, B. G., and Pittman, R. N. (2005) Proc. Natl. Acad. Sci. U. S. A. 1024330 –4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong, X., and Pittman, R. N. (2006) Hum. Mol. Genet. 152409 –2420 [DOI] [PubMed] [Google Scholar]
- 39.Warrick, J. M., Morabito, L. M., Bilen, J., Gordesky-Gold, B., Faust, L. Z., Paulson, H. L., and Bonini, N. M. (2005) Mol. Cell 1837 –48 [DOI] [PubMed] [Google Scholar]
- 40.Abramoff, M., Magelhaes, P., and Ram, S. (2004) Biophotonics Int. 1136 –42 [Google Scholar]
- 41.Chow, M. K. M., Ellisdon, A. M., Cabrita, L. D., and Bottomley, S. P. (2006) Methods Enzymol. 4131 –19 [DOI] [PubMed] [Google Scholar]
- 42.Bevivino, A. E., and Loll, P. J. (2001) Proc. Natl. Acad. Sci. U. S. A. 9811955 –11960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, M., Windheim, M., Roe, S. M., Peggie, M., Cohen, P., Prodromou, C., and Pearl, L. H. (2005) Mol. Cell 20525 –538 [DOI] [PubMed] [Google Scholar]
- 44.Xu, P., Cheng, D., Duong, D. M., Rush, J., Roelofs, J., Finley, D., and Peng, J. (2006) Israel J. Chem. 46171 –182 [Google Scholar]
- 45.Matsumoto, M., Yada, M., Hatakeyama, S., Ishimoto, H., TTanimura, Tsuji, S., Kakizuka, A., Kitagawa, M., and Nakayama, K. (2003) EMBO J. 23659 –669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolon, D. N., Grant, R. A., Baker, T. A., and Sauer, R. T. (2005) Proc. Natl. Acad. Sci. U. S. A. 10212724 –12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komander, D., Lord, C. J., Scheel, H., Swift, S., Hofmann, K., Ashworth, A., and Barford, D. (2008) Mol. Cell 29451 –464 [DOI] [PubMed] [Google Scholar]
- 48.Kayagaki, N., Phung, Q., Chan, S., Chaudhari, R., Quan, C., O'Rourke, K. M., Eby, M., Pietras, E., Cheng, G., Bazan, J. F., Zhang, Z., Arnott, D., and Dixit, V. M. (2007) Science 3181628 –1632 [DOI] [PubMed] [Google Scholar]
- 49.Cohn, M. A., Kowal, P., Yang, K., Haas, W., Huang, T. T., Gygi, S. P., and D'Andrea, A. D. (2007) Mol. Cell 28786 –797 [DOI] [PubMed] [Google Scholar]
- 50.Reyes-Turcu, F. E., Horton, J. R., Mullally, J. E., Heroux, A., Cheng, X., and Wilkinson, K. D. (2006) Cell 1241197 –1208 [DOI] [PubMed] [Google Scholar]
- 51.Yao, T., Song, L., Xu, W., DeMartino, G. N., Florens, L., Swanson, S. K., Washburn, M. P., Conaway, R. C., Conaway, J. W., and Cohen, R. E. (2006) Nat. Cell Biol. 8994 –1002 [DOI] [PubMed] [Google Scholar]
- 52.Nicastro, G., Menon, R. P., Masino, L., Knowles, P. P., McDonald, N. Q., and Pastore, A. (2005) Proc. Natl. Acad. Sci. U. S. A. 10210493 –10498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicastro, G., Habeck, M., Masino, L., Svergun, D. I., and Pastore, A. (2006) J. Biomol. NMR 36267 –277 [DOI] [PubMed] [Google Scholar]
- 54.Schmitt, I., Linden, M., Khazneh, H., Evert, B. O., Breuer, P., Klockgether, T., and Wuellner, U. (2007) Biochem. Biophys. Res. Commun. 362734 –739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.