Abstract
Energized mouse liver mitochondria displayed the same calcium retention capacity (a sensitive measure of the propensity of the permeability transition pore (PTP) to open) irrespective of whether phosphate, arsenate, or vanadate was the permeating anion. Unexpectedly, however, phosphate was specifically required for PTP desensitization by cyclosporin A (CsA) or by genetic inactivation of cyclophilin D (CyP-D). Indeed, when phosphate was replaced by arsenate, vanadate, or bicarbonate, the inhibitory effects of CsA and of CyP-D ablation on the PTP disappeared. After loading with the same amount of Ca2+ in the presence of arsenate or vanadate but in the absence of phosphate, the sensitivity of the PTP to a variety of inducers was identical in mitochondria from wild-type mice, CyP-D-null mice, and wild-type mice treated with CsA. These findings call for a reassessment of conclusions on the role of the PTP in cell death that are based on the effects of CsA or of CyP-D ablation.
An increased permeability of the mitochondrial inner membrane, the permeability transition, is a key event in cell death (1). The permeability transition is due to opening of the permeability transition pore (PTP),2 a high conductance channel of unknown molecular structure that is modulated by cyclophilin D (CyP-D) (2). The PTP can be desensitized by the CyP inhibitor cyclosporin A (CsA) (3–6) or by ablation of CyP-D (7–10), and CyP-D-null mice are strikingly resistant to ischemic heart (7, 9) and brain (10) damage and to experimental autoimmune encephalomyelitis (11). Treatment with CsA cured a mouse model of collagen VI muscular dystrophy through PTP inhibition (12) and normalized mitochondrial function and apoptosis in patients with collagen VI muscular dystrophies (13, 14). CyP-D ablation led to recovery from muscle pathology in other mouse models of muscular dystrophy, suggesting that PTP opening may play a role in more than one form of myopathy (15). The mechanism through which treatment with CsA and lack of CyP-D affects the PTP remains unsolved, however; and the extent to which it is possible to apply results obtained from in vitro studies to the status of the PTP in situ remains an open question (2). Here, we show that ablation of CyP-D or treatment with CsA does not directly cause PTP inhibition, but rather unmasks an inhibitory site for Pi. Indeed, we found that the inhibitory effects of CsA and of CyP-D ablation disappeared when Pi was replaced by vanadate (Vi), arsenate (Asi), or bicarbonate and that, in the absence of Pi, the PTP sensitivity to Ca2+ and oxidative stress was identical in wild-type and CyP-D-null mitochondria. Our results indicate that the PTP is not sensitive to CsA or to CyP-D ablation unless Pi is present.
EXPERIMENTAL PROCEDURES
Mitochondria were isolated from the livers of C57BL/6J (wild-type) and Ppif–/– C57BL/6J (CyP-D-null) mice by standard differential centrifugation. The properties of the CyP-D-null mitochondria have been described elsewhere (8). Protein concentration was determined using the biuret method, and the mitochondrial suspension was kept on ice and used within 4 h of preparation.
The incubation medium contained 120 mm KCl, 10 mm Tris/MOPS, 5 mm Tris glutamate, 2.5 mm Tris malate, 20 μm Tris/EGTA, and Pi, Vi, or Asi (as specified in the figure legends) at pH 7.4. For all measurements, the concentration of mitochondrial protein was 0.5 mg/ml at 25 °C. Oxygen consumption was measured with a Clark-type oxygen electrode in a closed 2-ml vessel equipped with magnetic stirring. Membrane potential was estimated based on fluorescence quenching of rhodamine 123 (16) in 2-ml stirred cuvettes with a PerkinElmer Life Sciences LS50B spectrofluorometer (0.3 μm rhodamine 123; excitation and emission wavelengths of 503 and 525 nm, respectively). The mitochondrial calcium retention capacity (CRC) (17) was determined in medium supplemented with 1 μm Calcium Green-5N (Molecular Probes) either with the PerkinElmer Life Sciences spectrofluorometer (excitation and emission wavelengths of 505 and 535 nm, respectively) or with a Fluoroskan Ascent FL (Thermo Electron Corp.) equipped with a plate shaker (excitation and emission wavelengths of 485 and 538 nm, respectively, with a 10-nm band-pass filter) in a final volume of 0.2 ml. Ten micromolar CaCl2 pulses were added at 1-min intervals until onset of the permeability transition, which is marked by a precipitous release of the accumulated Ca2+. All chemicals were of the highest purity commercially available. Reported results are the means of at least three experiments for each condition, and error bars refer to S.E.
RESULTS
Preliminary experiments were carried out to test whether substitution of Pi with Asi or Vi affects basic mitochondrial properties. Resting membrane potential was identical irrespective of the anion, and cycles of depolarization-repolarization were observed during the train of Ca2+ pulses, with the precipitous depolarization expected of PTP opening occurring at lower Ca2+ loads with Asi than with Pi and Vi (Fig. 1). As expected, (i) in the presence of Vi, ADP did not stimulate respiration; and (ii) because of the lability of ADP-Asi, which immediately regenerates ADP, in the presence of Asi, basal respiration was higher, and ADP-stimulated respiration did not return to state 4 levels unless oligomycin was added (data not shown). Thus, replacement of Pi with Asi or Vi does not impair the ability of mitochondria to develop and maintain the membrane potential, which is a prerequisite for energy-dependent Ca2+ uptake.
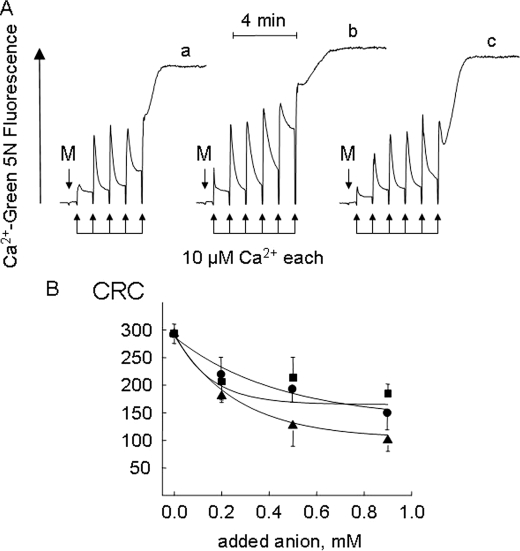
FIGURE 1.
Effect of anions on the mitochondrial permeability transition. A, the incubation medium was supplemented with 1 mm Asi (trace a), 1 mm Vi (trace b), or 1 mm Pi (trace c). The conditions were as follows: 2-ml final volume, pH 7.4, and 25 °C. Where indicated, 1 mg of mouse liver mitochondria (M) was added, followed by a train of Ca2+ pulses of 10 μm each (arrows). B, the experimental conditions were as described for A, except that the medium contained 0.1 mm Pi plus the indicated concentrations of Asi (▴), Vi (▪), and Pi (•). The CRC is expressed in nanomoles of Ca2+/mg of protein.
We then investigated whether the PTP would open when Pi was replaced by Asi or Vi, which both support uptake of Ca2+ by energized mitochondria (Fig. 1A). Mitochondria incubated in the presence of 1 mm Asi (trace a), Vi (trace b), or Pi (trace c) readily accumulated a train of Ca2+ pulses until a threshold matrix Ca2+ was reached, causing opening of the PTP, which was detectable as the precipitous release of the accumulated Ca2+. Asi was slightly more potent than Pi or Vi, but no major differences among the three anions were observed when the CRC (a sensitive measure of the propensity of the PTP to open (17)) was studied as a function of the anion concentration (Fig. 1B), indicating that Vi and Asi can replace Pi as PTP inducers.
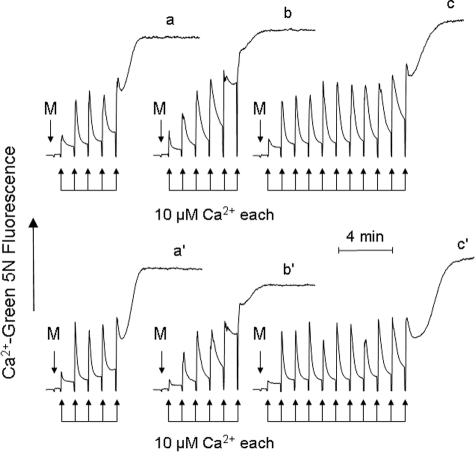
Treatment with CsA or ablation of CyP-D desensitizes the PTP to Ca2+ in vitro, as shown by experiments that are routinely performed in the presence of Pi (7–10). Surprisingly, when Ca2+ uptake was studied in the presence of Asi (Fig. 2, traces a and a′) or Vi (traces b and b′), the CRC of mitochondria was unaffected by treatment with CsA (traces a and b) or by ablation of CyP-D (traces a′ and b′), whereas the expected increase of the CRC (i.e. PTP desensitization to Ca2+) was readily observed in the presence of Pi (traces c and c′). Results similar to those seen with Vi and Asi were also obtained with bicarbonate (data not shown). These experiments suggest that Pi is the actual inhibitor of the PTP when CsA is present or CyP-D is absent.
FIGURE 2.
Desensitization of the PTP by CsA or by CyP-D ablation depends on Pi. Where indicated (M), 1 mg of wild-type mitochondria treated with 0.8 μm CsA (traces a–c) or of CyP-D-null mitochondria (traces a′–c′) was added to medium containing 1 mm Asi (traces a and a′), 1 mm Vi (traces b and b′), or 1 mm Pi (traces c and c′) under conditions otherwise identical to those described in the legend to Fig. 1A.
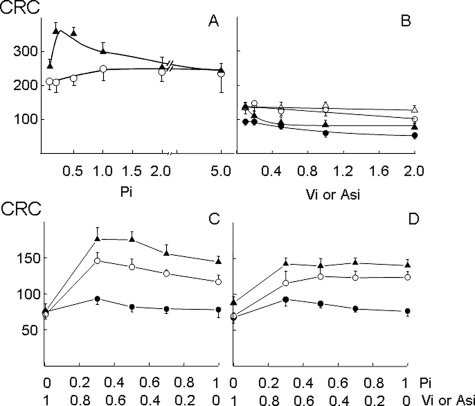
We determined the concentration dependence of PTP inhibition by Pi. In the absence of CyP-D, the peak CRC increase was observed between 0.2 and 0.5 mm Pi (Fig. 3A, closed symbols). The desensitizing effect of CsA in wild-type mitochondria was lower and reached its maximal value at 1 mm Pi (Fig. 3A, open symbols). Neither Vi (Fig. 3B, open symbols) nor Asi (closed symbols) increased the CRC in wild-type (circles) or CyP-D-null (triangles) mitochondria.
FIGURE 3.
Desensitization of the PTP by CsA or by CyP-D ablation depends on Pi. A, wild-type mitochondria treated with 0.8 μm CsA (○) or CyP-D-null mitochondria (▴) were incubated in the presence of the indicated millimolar concentrations of Pi. B, wild-type (circles) or CyP-D-null (triangles) mitochondria were incubated in the presence of the indicated millimolar concentrations of Vi (open symbols) or Asi (closed symbols). C and D, wild-type mitochondria (•), CyP-D-null mitochondria (▴), or wild-type mitochondria treated with 0.8 μm CsA (○) were incubated with the indicated millimolar concentrations of Pi and Vi (C) or Pi and Asi (D). The incubation conditions were otherwise identical to those described in the legend to Fig. 1A, and the CRC is expressed in nanomoles of Ca2+/mg of protein.
Because accumulation of anions decreases matrix free [Mg2+] and [Ca2+] (18), we also studied the CRC of mitochondria incubated in media containing increasing concentrations of Pi and decreasing concentrations of Vi or Asi so as to maintain the total anion concentration at 1 mm. These experiments confirmed that only Pi desensitized the PTP to Ca2+ in CsA-treated wild-type mitochondria and in CyP-D-null mitochondria and that neither Vi (Fig. 3C) nor Asi (Fig. 3D) could substitute for Pi in PTP inhibition. These findings demonstrate that the inhibitory site unmasked by pharmacological inhibition or by genetic ablation of CyP-D is strikingly selective for Pi over its analogs Asi and Vi.
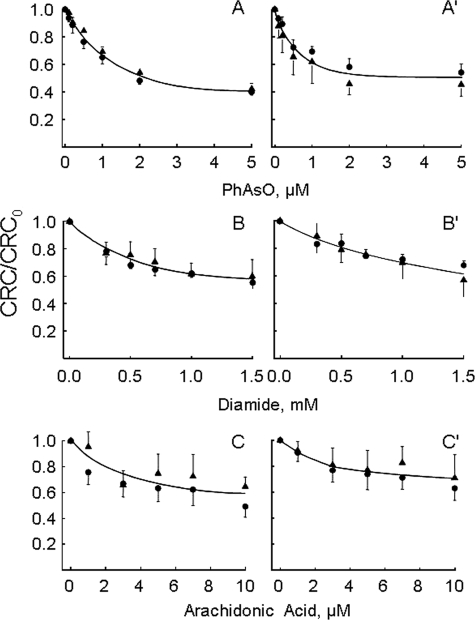
Because the CRC of wild-type and CyP-D-null mitochondria is the same when Pi is replaced by Vi or Asi, we could test the sensitivity to PTP inducers under exactly the same Ca2+-loading conditions. Strikingly, the response of the PTP to phenylarsine oxide, diamide, and arachidonic acid was indistinguishable for the two genotypes (Fig. 4). This finding is remarkable because it indicates that the basal sensitivity of the PTP to effectors of pathophysiological relevance is not affected by the lack of CyP-D unless the concentration of Pi is high enough for PTP inhibition.
FIGURE 4.
Sensitivity of PTP to inducers is unaffected by CyP-D ablation in the absence of Pi. Wild-type (•) or CyP-D-null (▴) mitochondria were incubated in the presence of 1 mm Vi (A–C) or 1 mm Asi (A′–C′) in the presence of the indicated concentrations of phenylarsine oxide (PhAsO), diamide, or arachidonic acid. Experimental conditions were otherwise identical to those described in the legend to Fig. 1A, and the CRC is normalized to the value measured in the absence of PTP inducers (CRC0).
DISCUSSION
Ca2+ and Pi Uptake in
Energized Mitochondria—Mitochondria isolated from a wide variety of
tissues have a remarkable capacity to accumulate Ca2+
(19–22).
Ca2+ uptake is an electrophoretic process driven by the
Ca2+ electrochemical gradient,
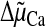 (Equation 1).
(Equation 1).
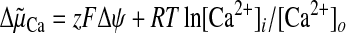 |
(Eq. 1) |
In respiring coupled mitochondria, the inside-negative Δψ favors
accumulation of Ca2+, which is transported with a net charge of 2
(23,
24). Ca2+-dependent
depolarization is compensated by H+ extrusion, causing matrix
alkalinization that prevents the recovery of Δψ, limiting the
further ability to accumulate Ca2+
(25–27).
Uptake of substantial amounts of Ca2+ therefore requires (i)
buffering of matrix pH to allow regeneration of the Δψ and (ii)
buffering of matrix Ca2+ to prevent the buildup of a
Ca2+ concentration gradient
(25–27).
Buffering of matrix pH is achieved by the simultaneous uptake of protons and
anions via diffusion of the undissociated acid (as in the case of acetate) or
of CO2 (which regenerates bicarbonate and H+ in the
matrix) or through transport proteins (like the H+-Pi
symporter) (28); buffering of
matrix Ca2+ depends on the cotransported anion and is maximal for
Pi, so in its presence, matrix free [Ca2+] becomes
invariant with matrix Ca2+ load
(18). In mitochondria
energized in the presence of Pi (which is by far the most used
anion in vitro and is likely to play a major role in situ),
the 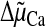 thus favors the accumulation of large loads of both Ca2+ and
Pi (29). Because
Ca2+ is essential for PTP opening
(30) and because Pi
is a classic inducer of the permeability transition
(31), it has been difficult to
sort the effects of Pi from those of Ca2+ and to
understand why, despite its effect of lowering matrix free
[Ca2+], Pi behaves as a PTP inducer
(18). The results of the
present work contribute to clarifying these longstanding problems and identify
a novel feature of Pi as an inhibitor of the PTP that mediates the
effects of CsA and of CyP-D ablation.
thus favors the accumulation of large loads of both Ca2+ and
Pi (29). Because
Ca2+ is essential for PTP opening
(30) and because Pi
is a classic inducer of the permeability transition
(31), it has been difficult to
sort the effects of Pi from those of Ca2+ and to
understand why, despite its effect of lowering matrix free
[Ca2+], Pi behaves as a PTP inducer
(18). The results of the
present work contribute to clarifying these longstanding problems and identify
a novel feature of Pi as an inhibitor of the PTP that mediates the
effects of CsA and of CyP-D ablation.
Pi as a PTP Inducer—Increasing Pi concentrations decrease matrix free [Ca2+], which should in turn decrease the probability of PTP opening (30). We suspect that the inducing effect of Pi, which is shared by its analogs Asi and Vi, is due in part to the decrease in matrix free [Mg2+], which, like all divalent cations but Ca2+, decreases the probability of PTP opening (32), and in part to buffering of matrix pH at ∼7.3, i.e. the optimum for opening of the PTP, the probability of opening of which declines at both lower and higher matrix pH values (33). An additional possibility is that matrix Pi gives rise to the formation of polyphosphate, which promotes the permeability transition (34). Because the inducing properties of Pi are shared by Asi and Vi with the same concentration dependence, it will be interesting to test if the latter can substitute for Pi in the formation of complexes with Ca2+ and polyhydroxybutyrate, which mimic the ion-conductive properties of the PTP (35).
Pi as a PTP Inhibitor—The most important result of this study is the demonstration that Pi has a desensitizing effect on the PTP. This can be detected as a shift of the threshold matrix Ca2+ required for pore opening at higher Ca2+ loads, provided that CsA is added or CyP-D is absent. Asi, Vi, and bicarbonate could not replace Pi, suggesting that the effect is not mediated by changes of matrix free Mg2+ and/or pH. Indeed, desensitization was observed at increasing Pi even when the total [Pi + Asi]or[Pi + Vi] was kept constant, indicating that only Pi is able to bind a PTP regulatory site that is not accessible when CyP-D is present. This finding suggests that CyP-D may not be a PTP inducer per se, but rather a factor that prevents the inhibitory action of Pi from being exerted. We have documented an inhibitory effect of Pi also after treatment of wild-type mitochondria with CsA, which is known to detach CyP-D from its membrane binding site(s) (36, 37). Thus, Pi rather than CsA appears to be the actual PTP desensitizer also in the presence of the drug. Inhibition by CsA can also be observed in de-energized mitochondria incubated in thiocyanate-based medium (6). Under these conditions, diffusion of SCN– provides the driving force for Ca2+ uptake (38), which is then readily followed by PTP opening (6). We suspect that the lipophilic SCN– anion may have access to the Pi-binding site and substitute for Pi, particularly at the very high concentrations (typically 150 mm) used in this type of swelling assays.
A Role for the Pi Carrier?—Quite recently, it has been proposed that the Pi carrier itself may constitute the PTP, possibly in association with the adenine nucleotide translocator (39). Although this is certainly a possibility, we think that the present results do not directly bear on the issue. Indeed, (i) although Pi, Asi, and Vi are all inducers of the PTP (Fig. 1), only Pi behaves as a PTP inhibitor (Fig. 3), yet all these anions are transported by the Pi carrier. (ii) SCN– shares the inhibitory properties of Pi in the presence of CsA, yet it does not need a carrier to cross the lipid bilayer. (iii) Ubiquinone 0 and Ro 68-3400 are reported to inhibit the Pi carrier in de-energized mitochondria incubated with 40 mm Pi (39), but this is probably not relevant to PTP inhibition in energized mitochondria that accumulate Ca2+ at the physiological concentration of 1 mm Pi (40, 41); indeed and as invariably observed with the Pi carrier inhibitors N-ethylmaleimide (26) and mersalyl (29), inhibition of Pi uptake should have resulted in a dramatic decrease in the rate and extent of Ca2+ uptake (see also the first paragraph under “Discussion”), which instead is never observed in mitochondria treated with any inhibitory ubiquinone analog (17, 42, 43) or with Ro 68-3400 (44). (iv) Ubiquinone 0 (17) and Ro 68-3400 (44), which inhibit the PTP independent of CyP-D (8), desensitize the PTP to Ca2+ even when Pi is replaced by Asi or Vi (data not shown). Thus, our results do not necessarily implicate the Pi carrier as the mediator of the complex effects of Pi on the PTP.
Implications for in Vivo Studies—CyP-D inhibition is an important pharmacological target for PTP desensitization, as demonstrated by the striking therapeutic efficacy of CsA in many disease models (see Ref. 2 for review), in patients with collagen VI muscular dystrophies (13, 14), and in heart infarction (45) and by the effect of CyP-D ablation in murine models of multiple sclerosis (11) and muscular dystrophy (15). By cautious extrapolation of the present results to the conditions prevailing in vivo, we can conclude that the concentration of Pi in the animal models, in collagen VI muscular dystrophy patients, and in heart infarction is high enough for CsA to be effective, in keeping with current estimates of cellular free Pi in the 1 mm range (40, 41). This may not be the case in all conditions, however. For example, in perfused rabbit hearts during KCl arrest, tissue free Pi drops below 0.1 mm (40). It is also remarkable that the pharmacological effect of CsA is not as pronounced as that of CyP-D ablation (Fig. 3), suggesting that extreme caution should be exerted before concluding that the PTP is not involved in a given paradigm of mitochondrial dysfunction only because CsA does not exert a protective effect.
It is fortunate that Pi was included in mitochondrial swelling assays of PTP in vitro so that the desensitizing effect of CsA was not missed. On the other hand, the Pi dependence of PTP inhibition by CsA may represent just one example of the factors that control the PTP sensitivity to inhibitors in situ. It is very striking that, in the absence of Pi, the sensitivity of the PTP to Ca2+ and inducers is identical in wild-type and CyP-D-null mitochondria, confirming our earlier conclusion that the basic features of the PTP are not affected by the presence of CyP-D (8). Thus, extreme prudence should be exerted in extrapolating results from in vitro studies to the status of the PTP in vivo, and we suspect that the PTP may be involved in even more paradigms of cell death than is currently believed.
Acknowledgments
We thank Robert S. Balaban and Michel Rigoulet for helpful discussions on the determination of cellular Pi levels.
This work was supported, in whole or in part, by National Institutes of Health Grant GM69883 from the United States Public Health Service (to M. A. F. and P. B.). This work was also supported by the Ministero per l'Universitàe la Ricerca, Italy (to P. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PTP, permeability transition pore; CyP-D, cyclophilin D; CsA, cyclosporin A; Vi, vanadate; Asi, arsenate; MOPS, 4-morpholinepropanesulfonic acid; CRC, calcium retention capacity.
References
- 1.Kroemer, G., Galluzzi, L., and Brenner, C. (2007) Physiol. Rev. 87 99–163 [DOI] [PubMed] [Google Scholar]
- 2.Bernardi, P., Krauskopf, A., Basso, E., Petronilli, V., Blachly-Dyson, E., Di Lisa, F., and Forte, M. A. (2006) FEBS J. 273 2077–2099 [DOI] [PubMed] [Google Scholar]
- 3.Fournier, N., Ducet, G., and Crevat, A. (1987) J. Bioenerg. Biomembr. 19 297–303 [DOI] [PubMed] [Google Scholar]
- 4.Crompton, M., Ellinger, H., and Costi, A. (1988) Biochem. J. 255 357–360 [PMC free article] [PubMed] [Google Scholar]
- 5.Broekemeier, K. M., Dempsey, M. E., and Pfeiffer, D. R. (1989) J. Biol. Chem. 264 7826–7830 [PubMed] [Google Scholar]
- 6.Halestrap, A. P., and Davidson, A. M. (1990) Biochem. J. 268 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines, C. P., Kaiser, R. A., Purcell, N. H., Blair, N. S., Osinska, H., Hambleton, M. A., Brunskill, E. W., Sayen, M. R., Gottlieb, R. A., Dorn, G. W., Robbins, J., and Molkentin, J. D. (2005) Nature 434 658–662 [DOI] [PubMed] [Google Scholar]
- 8.Basso, E., Fante, L., Fowlkes, J., Petronilli, V., Forte, M. A., and Bernardi, P. (2005) J. Biol. Chem. 280 18558–18561 [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa, T., Shimizu, S., Watanabe, T., Yamaguchi, O., Otsu, K., Yamagata, H., Inohara, H., Kubo, T., and Tsujimoto, Y. (2005) Nature 434 652–658 [DOI] [PubMed] [Google Scholar]
- 10.Schinzel, A. C., Takeuchi, O., Huang, Z., Fisher, J. K., Zhou, Z., Rubens, J., Hetz, C., Danial, N. N., Moskowitz, M. A., and Korsmeyer, S. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 12005–12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forte, M., Gold, B. G., Marracci, G., Chaudhary, P., Basso, E., Johnsen, D., Yu, X., Fowlkes, J., Rahder, M., Stem, K., Bernardi, P., and Bourdette, D. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 7558–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin, W. A., Bergamin, N., Sabatelli, P., Reggiani, C., Megighian, A., Merlini, L., Braghetta, P., Columbaro, M., Volpin, D., Bressan, G. M., Bernardi, P., and Bonaldo, P. (2003) Nat. Genet. 35 267–271 [DOI] [PubMed] [Google Scholar]
- 13.Angelin, A., Tiepolo, T., Sabatelli, P., Grumati, P., Bergamin, N., Golfieri, C., Mattioli, E., Gualandi, F., Ferlini, A., Merlini, L., Maraldi, N. M., Bonaldo, P., and Bernardi, P. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 991–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merlini, L., Angelin, A., Tiepolo, T., Braghetta, P., Sabatelli, P., Zamparelli, A., Ferlini, A., Maraldi, N. M., Bonaldo, P., and Bernardi, P. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 5225–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millay, D. P., Sargent, M. A., Osinska, H., Baines, C. P., Barton, E. R., Vuagniaux, G., Sweeney, H. L., Robbins, J., and Molkentin, J. D. (2008) Nat. Med. 14 442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emaus, R. K., Grunwald, R., and Lemasters, J. J. (1986) Biochim. Biophys. Acta 850 436–448 [DOI] [PubMed] [Google Scholar]
- 17.Fontaine, E., Ichas, F., and Bernardi, P. (1998) J. Biol. Chem. 273 25734–25740 [DOI] [PubMed] [Google Scholar]
- 18.Chalmers, S., and Nicholls, D. G. (2003) J. Biol. Chem. 278 19062–19070 [DOI] [PubMed] [Google Scholar]
- 19.DeLuca, H. F., and Engstrom, G. W. (1961) Proc. Natl. Acad. Sci. U. S. A. 47 1744–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasington, F. D., and Murphy, J. V. (1962) J. Biol. Chem. 237 2670–2677 [PubMed] [Google Scholar]
- 21.Lehninger, A. L., Rossi, C. S., and Greenawalt, J. W. (1963) Biochem. Biophys. Res. Commun. 10 444–448 [DOI] [PubMed] [Google Scholar]
- 22.Carafoli, E., Rossi, C. S., and Lehninger, A. L. (1964) J. Biol. Chem. 239 3055–3061 [PubMed] [Google Scholar]
- 23.Scarpa, A., and Azzone, G. F. (1970) Eur. J. Biochem. 12 328–335 [DOI] [PubMed] [Google Scholar]
- 24.Wingrove, D. E., Amatruda, J. M., and Gunter, T. E. (1984) J. Biol. Chem. 259 9390–9394 [PubMed] [Google Scholar]
- 25.Azzone, G. F., Pozzan, T., Massari, S., Bragadin, M., and Dell'Antone, P. (1977) FEBS Lett. 78 21–24 [DOI] [PubMed] [Google Scholar]
- 26.Nicholls, D. G. (1978) Biochem. J. 176 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardi, P., and Pietrobon, D. (1982) FEBS Lett. 139 9–12 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell, P. (1979) Science 206 1148–1159 [DOI] [PubMed] [Google Scholar]
- 29.Harris, E. J., and Zaba, B. (1977) FEBS Lett. 79 284–290 [DOI] [PubMed] [Google Scholar]
- 30.Haworth, R. A., and Hunter, D. R. (1979) Arch. Biochem. Biophys. 195 460–467 [DOI] [PubMed] [Google Scholar]
- 31.Hunter, D. R., Haworth, R. A., and Southard, J. H. (1976) J. Biol. Chem. 251 5069–5077 [PubMed] [Google Scholar]
- 32.Bernardi, P., Vassanelli, S., Veronese, P., Colonna, R., Szabo, I., and Zoratti, M. (1992) J. Biol. Chem. 267 2934–2939 [PubMed] [Google Scholar]
- 33.Nicolli, A., Petronilli, V., and Bernardi, P. (1993) Biochemistry 32 4461–4465 [DOI] [PubMed] [Google Scholar]
- 34.Abramov, A. Y., Fraley, C., Diao, C. T., Winkfein, R., Colicos, M. A., Duchen, M. R., French, R. J., and Pavlov, E. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 18091–18096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlov, E., Zakharian, E., Bladen, C., Diao, C. T., Grimbly, C., Reusch, R. N., and French, R. J. (2005) Biophys. J. 88 2614–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connern, C. P., and Halestrap, A. P. (1994) Biochem. J. 302 321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicolli, A., Basso, E., Petronilli, V., Wenger, R. M., and Bernardi, P. (1996) J. Biol. Chem. 271 2185–2192 [DOI] [PubMed] [Google Scholar]
- 38.Selwyn, M. J., Dawson, A. P., and Dunnett, S. J. (1970) FEBS Lett. 10 1–5 [DOI] [PubMed] [Google Scholar]
- 39.Leung, A. W. C., Varanyuwatana, P., and Halestrap, A. P. (July 30, 2008) J. Biol. Chem. 283 26312–26323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz, L. A., Swain, J. A., Portman, M. A., and Balaban, R. S. (1988) Am. J. Physiol. 255 H189–H196 [DOI] [PubMed] [Google Scholar]
- 41.Bose, S., French, S., Evans, F. J., Joubert, F., and Balaban, R. S. (2003) J. Biol. Chem. 278 39155–39165 [DOI] [PubMed] [Google Scholar]
- 42.Walter, L., Nogueira, V., Leverve, X., Bernardi, P., and Fontaine, E. (2000) J. Biol. Chem. 275 29521–29527 [DOI] [PubMed] [Google Scholar]
- 43.Walter, L., Miyoshi, H., Leverve, X., Bernardi, P., and Fontaine, E. (2002) Free Radic. Res. 36 405–412 [DOI] [PubMed] [Google Scholar]
- 44.Cesura, A. M., Pinard, E., Schubenel, R., Goetschy, V., Friedlein, A., Langen, H., Polcic, P., Forte, M. A., Bernardi, P., and Kemp, J. A. (2003) J. Biol. Chem. 278 49812–49818 [DOI] [PubMed] [Google Scholar]
- 45.Piot, C., Croisille, P., Staat, P., Thibault, H., Rioufol, G., Mewton, N., Elbelghiti, R., Cung, T. T., Bonnefoy, E., Angoulvant, D., Macia, C., Raczka, F., Sportouch, C., Gahide, G., Finet, G., André-Fouët, X., Revel, D., Kirkorian, G., Monassier, J.-P., Derumeaux, G., and Ovize, M. (2008) N. Engl. J. Med. 359 473–481 [DOI] [PubMed] [Google Scholar]