Abstract
Bone tissues respond to mechanical loading/unloading regimens to accommodate (re)modeling requirements; however, the underlying molecular mechanism responsible for these responses is largely unknown. Previously, we reported that connexin (Cx) 43 hemichannels in mechanosensing osteocytes mediate the release of prostaglandin, PGE2, a crucial factor for bone formation in response to anabolic loading. We show here that the opening of hemichannels and release of PGE2 by shear stress were significantly inhibited by a potent antibody we developed that specifically blocks Cx43-hemichannels, but not gap junctions or other channels. The opening of hemichannels and release of PGE2 are magnitude-dependent on the level of shear stress. Insertion of a rest period between stress enhances this response. Hemichannels gradually close after 24 h of continuous shear stress corresponding with reduced Cx43 expression on the cell surface, thereby reducing any potential negative effects of channels staying open for extended periods. These data suggest that Cx43-hemichannel activity associated with PGE2 release is adaptively regulated by mechanical loading to provide an effective means of regulating levels of extracellular signaling molecules responsible for initiation of bone (re)modeling.
The skeleton regulates its architecture and mass to meet structural and metabolic needs. To fulfill its structural functions, this complex tissue must adapt to loading and unloading while simultaneously regulating the metabolic demands of the skeleton. Numerous in vivo animal studies show the essential role of mechanical loading for bone formation and remodeling; however, the underlying molecular mechanisms, in particular how bone cells adapt to mechanical stimulation, remain largely uncharacterized.
When mechanical forces are applied to bone, several potential stimuli occur including changes in hydrostatic pressure, direct cell strain, fluid flow, and electric potentials. These changes lead to fluid movement through the bone (1–3). Shear stress induced by mechanical loading facilitates the exchange of nutrients and bone modulators, and elicits biochemical responses. Osteocytes are well positioned in the bone to sense the magnitude of mechanical strain and are essential for the skeleton adaptive response to load. Experimental studies have shown that osteocytes are sensitive to stress applied to both intact bone tissue and in cell culture (4–6). Encased within mineralized tissue, their dendritic morphology allows them to connect through small tunnels called canaliculi to form a three-dimensional network not only with adjacent osteocytes but also to connect to cells on the bone surface and bone marrow.
Connexins (Cx),2 gap junction-forming proteins, belong to a multigene family expressing four transmembrane domains. The regions corresponding to transmembrane and extracellular domains are highly conserved. Cx43 has been identified in most types of bone cells (7–13) and is the major connexin expressed in osteocyte-like MLO-Y4 cells and primary osteocytes (14–16). A role for connexins, independent of the formation of gap junction channels, has been suggested by the existence of hemichannels recently shown to exist in the nonjunctional plasma membrane (17). Functional Cx43-hemichannels have been observed in neural progenitor cells and neurons (18, 19), astrocytes (20, 21), heart (22), and especially, osteoblasts and osteocytes (23–25). Cx43-hemichannels appear to function as transducers of bisphosphonates, drugs used in the treatment of bone diseases by opening Cx43-hemichannels (24). We recently demonstrated that fluid flow shear stress (FFSS) induced the rapid opening of hemichannels, which in turn mediate the release of PGE2 in osteocytic MLO-Y4 cells (25). A recent study showed the release of ATP and PGE2 in MLO-Y4 cells induced by oscillating fluid flow (26). Prostaglandins have been shown to be involved in the response of bone tissue and cells to mechanical loading (27). More importantly, PGE2 can increase bone mass in mammals when used at the appropriate doses, and bone formation and remodeling caused by mechanical loading are attenuated by inhibition of prostaglandin biosynthesis (28–30). However, how the release of prostaglandins is regulated by changes of mechanical loading is unclear. In the present work, the dynamics of hemichannel opening associated with PGE2 release in response to FFSS was examined, and an antibody we developed specific for hemichannels, and not gap junctions, was used to validate this biological response to FFSS according to this mechanism.
EXPERIMENTAL PROCEDURES
Cell Culture and PGE2 Measurement—MLO-Y4 cells were cultured on collagen-coated (rat tail collagen type I, 0.15 mg/ml) surfaces and were grown in α-modified essential medium (α-MEM) supplemented with 2.5% fetal bovine serum and 2.5% BCS and incubated in a 5% CO2 incubator at 37 °C as described previously (15). After fluid flow treatment at specific time and shear stress levels, the conditioned media were collected, and the extracellular PGE2 released into the media was measured using a PGE2 EIA kit according to the manufacturer's instructions (Cayman Biochemicals, Ann Arbor, MI). Intracellular PGE2 was measured after the cells were thoroughly washed three times with PBS and lysed. PGE2 release after post-stress incubation was determined by subjecting the MLO-Y4 cells to FFSS and then incubating the cells in media at 37 °C, 5% CO2. The medium was collected at the respective time points as indicated and measured using the kit described above. The PGE2 measurement at FFSS (0 h) was then subtracted from the accumulated amount at each time point yielding a net PGE2 measurement.
Fluid Flow Shear Stress—FFSS experiments were conducted as previously described (31), and stress levels of 4, 8, and 16 dynes/cm2 were generated. Controls consist of MLO-Y4 cells not subjected to FFSS. A cell surface area of 5 cm2 was used for dye uptake, and 90 cm2 was used for PGE2 measurement and for the biotinylation assay. During post-stress chase experiments, the cells were maintained in α-MEM at 37 °C.
Dye Uptake Assay—MLO-Y4 cells were grown at low initial density to ensure that the majority of the cells were not physically in contact. After treatment, dye uptake experiments were conducted in the presence of 0.2% Lucifer yellow (LY) or 0.2% Alexa 594 along with 0.2% rhodamine dextran (RD) for 5 min, and cells were rinsed and fixed with 1% paraformaldehyde. LY and Alexa 594 were used as a tracer for hemichannel activity, and RD was used as a negative control. Similar fields were observed under the fluorescence microscope. Approximately 7 images per slide per treatment group were collected. From the phase images, the total number of cells was counted up to 300 cells. The number of fluorescent cells was then counted from the corresponding fluorescent images. Dye uptake is presented as the number of fluorescent cells divided by the total number of cells and normalized to 1 for control.
Immunofluorescence Labeling and Fluorescence Microscopy—The cells cultured on the microscopic slides were fixed in 2% paraformaldehyde in PBS for 30 min at room temperature. The cells were incubated in blocking solution (2% normal goat serum, 2% fish skin gelatin, and 1% bovine serum albumin in PBS) in the absence or presence of 1% Triton X-100 for 30 min and then overnight at 4 °C with affinity-purified antibodies against Cx43(E2), Cx43(CT), preimmune (1:300 dilutions), or anti-Cx43 antibody pre-absorbed with antigen Cx43 (E2) fusion protein (0.6 mg/ml) in blocking solution. Cells were then incubated for 1 h with fluorescein-conjugated goat anti-rabbit IgG (1:300 dilution) in blocking solution. Fluorescence microscopy was performed using an Olympus B-MAX microscope (Olympus, Tokyo, Japan) and recorded on a “Spot II” digital camera (Diagnostic Instruments, Tokyo, Japan). For dye uptake, LY was detected using the filter set for fluorescein at excitation wavelengths of 505–525 nm, and Alexa 594 and RD by using the filter set for rhodamine with a corresponding excitation wavelength of 560 nm. Mean fluorescence intensity was acquired by taking representative fluorescence images from various regions of each microscope slide. Using Image J program (Image J Software, NIH) the threshold was adjusted to clearly distinguish fluorescent cells from non-fluorescent cells. Threshold conditions were kept constant for each experimental condition. The program measured the intensity of the signal coming from all fluorescent cells in the field; the output is given as mean fluorescent intensity.
Cell Surface Biotinylation—Biotinylation of MLO-Y4 cells was performed based on a modification of previously published procedures (25, 32). Briefly, MLO-Y4 cells were treated with and without FFSS for 30 min and then post-FFSS incubated for 30 min, 2, 4, 8, and 24 h or continuous FFSS for 0, 2, 4, and 24 h. These cells were labeled with or without 1 mg/ml EZ-link Sulfo-NHS-LC-Biotin (Pierce Biotechnology) in PBS at 4 °C for 20 min. The cells were washed with PBS containing Ca2+, Mg2+, and glycine and lysed in lysis buffer (133 mm NaCl, 5 mm KCl, 1% dextrose, and 20 mm HEPES) plus 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, and protease inhibitors. Cell lysates were mixed with monomeric avidin beads and incubated for 1 h at room temperature. The beads were then washed with PBS, and biotinylated proteins were eluted by boiling for 5 min in sample loading buffer containing 1% SDS and 2% 2-mercaptoethanol, and proportional amounts of sample prior to and after avidin bead elution were loaded on SDS-PAGE and analyzed by Western blotting by using affinity-purified anti-Cx43 antibody. The intensity of Cx43 bands was quantified by densitometry (NIH Image J).
Generation of Cx43(E2) Antibody and Use in Blocking Experiments—An antibody (Cx43(E2)) against the second extracellular loop region, E2, of Cx43 between amino acid residues 185 and 206 was generated. Initially, the corresponding cDNA fragment was amplified by PCR, subcloned into pGEX-2T vector (Amersham Biosciences), sequenced, and the protein was expressed in Escherichia coli. The fusion protein was isolated using glutathione-Sepharose 4B beads (Amersham Biosciences) and was used to generate antiserum in rabbits (Pocono Rabbit Farm & Laboratory, Canadensis, PA). Both preimmune sera and anti-Cx43 were collected from the same rabbit. Cx43(E2) was affinity-purified using a GST column, followed by a Cx43(E2) fusion protein-conjugated column with sequential washing with PBS followed by elution buffer containing 0.1 m glycine at pH 2.5. MLO-Y4 cells were incubated with affinity-purified anti-Cx43(E2) (28 μg/ml), anti-Cx43 C-terminal domain(CT) (30 μg/ml), anti-Cx43(E2) antibody pre-absorbed with antigen Cx43 (E2) fusion protein (0.6 mg/ml), or preimmune antibodies for 30 min prior to FFSS at 16 dynes/cm2 for 30 min. Dye uptake experiments were conducted as described above.
Scrape-loading Dye Transfer Assay for Gap Junction-mediated Intercellular Communication—The scrape-loading dye transfer was based on a published protocol (33). Briefly, cells were scratched in the presence of two types of fluorescence dyes: RD (Mr ∼ 10,000) and LY (Mr ∼ 457). RD is too big to pass through gap junction channels and therefore serves as a tracer molecule for the cells originally receiving dyes. MLO-Y4 cells were treated in the absence or presence of 28, 56, and 100 μg/ml of anti-Cx43(E2) antibody for 4 h, and then 1% RD and 1% LY were applied to the cells after scratching lightly with a surgical blade. After incubation for 5 min, cells were washed with Hank's balanced salt solution three times and finally fixed in freshly made 2% paraformaldehyde (from 16% stock) for 30 min. The dye transfer results were examined with an Olympus fluorescence microscope (Tokyo, Japan), and the degree of dye transfer was calculated based on the percentage of cells transferring LY dye.
Isolation of Chick Primary Osteocytes—Preparation of primary osteocytes from chick calvaria was based on previously published procedures (34) with some modifications. Briefly, calvarial bone was dissected from 16-day embryonic chicks and minced into small pieces. The soft tissues and osteoid were removed by collagenase treatment followed by decalcification using EDTA. The final particles were treated with collagenase and vigorously agitated to release osteocytes. The larger sized osteoblast/fibroblast cells were separated from osteocytes by incubating the cells with collagen-coated culture plates for 10 min, of which osteoblast/fibroblast were attached to the plates.
Statistical Analysis—Data were analyzed with one-way analysis of variance and Newman-Keuls multiple comparison test along with a biostatistics program (Prism). Data are presented as the mean ± S.E. of multiple measurements. Asterisks represent the degree of significance compared with control (*, p < 0.05; **, p < 0.005; ***, p < 0.001). Some of the data presented were normalized to controls for easy comparison, and the variables were analyzed as described above.
RESULTS
Hemichannels Are Dynamic and Finely Regulated by Mechanical Loading—We quantitated hemichannel activity with increasing magnitudes of FFSS. The opening of hemichannels, as detected by LY (Mr ∼ 457) dye uptake, was directly proportional to the magnitude of FFSS applied (Fig. 1, A and B). Similar dye uptake results were obtained when these experiments were repeated with a larger probe, Alexa 594 (Mr ∼ 760) (Fig. 1C), showing that hemichannel function was not limited by either the properties or the smaller size of LY. The lack of RD uptake (Mr ∼10,000), a dye too big to pass through hemichannels, excludes the possibility of membrane leakage or the cells being unhealthy. In addition, a close correlation between hemichannel opening with increasing FFSS and increased release of PGE2 was also observed (Fig. 1D). To determine the effect of different types of FFSS on hemichannel function, steady (16 dynes/cm2) and pulsatile (8 ± 8 dynes/cm2) flow were applied (Fig. 1E). As compared with non-treated controls, both steady and pulsatile FFSS treatment showed a similar increase in hemichannel opening. There was no significant difference in hemichannel opening between these two different flow types. Together, these results suggest that hemichannel opening, associated with the release of PGE2, is directly correlated to the magnitude of FFSS applied.
FIGURE 1.
Opening of hemichannels and release of PGE2 are correlated with the magnitude of FFSS applied. A, MLO-Y4 cells were subjected to FFSS at 0, 4, 8, and 16 dynes/cm2 for 30 min. Immediately after flow, LY dye uptake was performed, and images were captured for quantitation. B, the number of MLO-Y4 cells with LY dye uptake in the presence or absence of FFSS were counted and quantified using a 10× objective. 4, 8, and 16 dynes/cm2 versus 0 dynes/cm2: **, p < 0.01; ***, p < 0.001. C, Alexa 594 dye uptake was performed after 30 min of FFSS at 16 dynes/cm2, and the number of MLO-Y4 cells with Alexa 594 dye uptake were counted and quantified. 16 dynes/cm2 versus 0 dynes/cm2: ***, p < 0.001. D, MLO-Y4 cells were subjected to FFSS at 0, 4, 8, and 16 dynes/cm2 for 30 min, and the media were collected. The amount of PGE2 in media was measured using a PGE2 EIA kit. 4, 8, and 16 dynes/cm2 versus 0 dynes/cm2: **, p <0.01; ***, p < 0.001. E, MLO-Y4 cells were subjected to control with no flow, C, steady flow, FF(St), at 16 dynes/cm2 or pulsatile flow, FF(Pu), at 8 ± 8 dynes/cm2 for 30 min and LY dye uptake was measured. FF(St) and FF(Pu) versus C: ***, p < 0.001. All data are presented as mean ± S.E. and n = 3.
To determine the function of hemichannels after FFSS, we assessed hemichannel opening at various incubation periods after exposure to FFSS (Fig. 2). For all post-shear stress incubation periods (0–8 h), except 24 h, a similar number of cells took up the LY dye (Fig. 2A). Although the relative number of cells taking up the dye was significantly increased over control, there was no significant difference in dye uptake between 0 and 8 h after FFSS. At 24 h, however, the number of cells taking up dye returned to control levels. To assess the relative level of functional hemichannels in individual cells after FFSS, the intensity of fluorescence signals was measured (Fig. 2B). The levels of dye uptake reached a maximum immediately following FFSS (0 h) and gradually decreased over time (0.5–8 h), but was still elevated at significant levels compared with control and eventually returned to control levels after 24 h. These results suggest that up to 8 h after FFSS, even though the total number of cells that have functional hemichannels remains the same, the number of open hemichannels in individual cells gradually declines. The release of PGE2 appeared to be closely associated with hemichannel function (Fig. 2C). This decrease in hemichannel function could be caused by either the gradual closure of hemichannels or/and decreased expression of Cx43 protein and therefore decreased numbers of hemichannels on the cell surface. To distinguish between these possibilities, Cx43 protein exposed on the cell surface was determined using a biotinylation assay (Fig. 2D). A significant increase in Cx43 protein on the cells surface was not observed until 2-h post-shear stress, which returned to control levels by 24-h post-shear stress. The results shown in Fig. 2 suggest that there is little direct correlation between Cx43 expression with either number of LY+ cells uptaking dye (Fig. 2A) or with dye intensity (Fig. 2B), not with PGE2 release (Fig. 2C). These results suggest that decreased function of hemichannels up to 8 h after post-stress is primarily caused by a decrease in numbers of open hemichannels. However at 24 h, hemichannel closure does appear to correspond to the decreased cell surface expression of Cx43. Reduced expression of Cx43 may be partially responsible for the 24-h response.
FIGURE 2.
Hemichannel activity, PGE2 release and surface expression of Cx43 were assessed at various time points after exposure of MLO-Y4 cells to 16 dynes/cm2 FFSS for 30 min. A, analysis of LY dye uptake was performed at 0, 0.5, 2, 4, 8, and 24 h after FFSS for 30 min or non-FFSS control (C). The number of cells with dye uptake were counted and quantified. Post-shear stress periods 0, 0.5, 2, 4, 8, and 24 h versus untreated control, C: ***, p < 0.001. All data are presented as mean ± S.E. and n = 3. B, the intensity of the fluorescence signal from cells at the same time points as A was analyzed using ImageJ software (NIH). Post-shear stress periods 0, 0.5, 2, 4, 8, and 24 h versus untreated control, C: ***, p < 0.001 and *, p < 0.05. All data are presented as mean ± S.E. and n = 3. C, conditioned media were collected after various post-FFSS periods, and the amount of PGE2 was measured using a PGE2 EIA kit. Post-shear stress periods 0, 0.5, 2, 4, 8, and 24 h versus untreated control, C: ***, p < 0.001 and **, p < 0.01. All data are presented as mean ± S.E. and n = 3. D, biotinylation was conducted for MLO-Y4 cells at various time points after FFSS at 16 dynes/cm2 for 30 min. The cells were lysed, and equal amounts of total protein of control, C and FFSS samples were applied to avidin-conjugated beads. The biotin-labeled samples were purified after binding to avidin-conjugated beads. Cell lysates prior to loading on avidin-conjugated beads (preloaded, upper panel) and bound to avidin beads (biotinylated, lower panel) were detected by Western blot by using anti-Cx43 antibody. The relative ratio of biotinylated to total Cx43 was quantified using densitometric measurements of the band intensity (lower panel). The data are presented as mean ± S.E. and n = 3.
To determine if response to reloading would affect hemichannel function, FFSS (16 dynes/cm2 for 30 min) was applied to MLO-Y4 cells after 0.5, 4, and 24 h incubation after FFSS. The reapplication of 30 min of FFSS significantly re-stimulated the function of hemichannels as detected by dye uptake in osteocytes that showed modest (0.5 and 4 h) or significant (24 h) reduction in dye uptake with post-FFSS incubation (Fig. 3A). Although the effects of re-applying FFSS are similar and significant in cells from each of the post-stress periods, they are more dramatic after the longer post-FFSS incubation periods, i.e. after the hemichannels have had longer time to recover (Fig. 3, 24 h (–) versus 24h(+)). However, reapplication of FFSS results in a similar level of response regardless of length of the post-FFSS period.
FIGURE 3.
Hemichannel response to repeated or continuous FFSS. A, MLO-Y4 cells were subjected to FFSS at 16 dynes/cm2 for 30 min, followed by incubation in the absence of flow (Post FFSS) for 0.5, 4, and 24 h (1st FFSS), and then treated by the second round of fluid flow (2nd FFSS). Dye uptake with LY was performed either at the respective post-FFSS incubation period or right after the 2nd FFSS treatment. The intensity of the fluorescent signal from cells at the same time points was analyzed using ImageJ software (NIH). 2nd FFSS versus non-2nd FFSS at identical post-FFSS time periods: ***, p < 0.001. B, MLO-Y4 cells were subjected to FFSS at 4 dynes/cm2 for 0, 0.5, 2, 4, 8, and 24 h, and immediately after flow, LY dye uptake was conducted. The number of cells with dye uptake was counted and quantified. 0.5, 2, 4, 8, and 24 h continuous FFSS versus non-flow control (0 h): ***, p < 0.001; **, p < 0.01; and *, p < 0.05. All data are presented as mean ± S.E. and n = 3. C, biotinylation was conducted for MLO-Y4 cells under continuous FFSS at 16 dynes/cm2 for 0, 2, 4, and 16 h. The cells were lysed, and equal amounts of total protein from various continuous FFSS samples were applied to avidin-conjugated beads. Cell lysates prior to loading on avidin-conjugated beads (preloaded) and bound to avidin beads (biotinylated) were detected by Western blot by using anti-Cx43 or anti-β-actin antibody (left panel). The relative ratio of biotinylated to total Cx43 was quantified using densitometric measurements of the band intensity (right panel). 2 and 4 h continuous FFSS versus non-flow control (0 h): ***, p < 0.001 and **, p < 0.01. All data are presented as mean ± S.E. and n = 3.
To investigate how hemichannels respond to sustained mechanical stimulation, MLO-Y4 cells were subjected to continuous FFSS for various time periods. As shown in Fig. 1, low shear stress resulted in lower dye uptake than that from high stress (Fig. 1B), but still above control levels. We subjected MLO-Y4 cells to FFSS at 4 dynes/cm2 to ensure cell attachment during the 24 h of treatment. Compared with control, FFSS increased dye uptake significantly at 0.5 and 2 h of FFSS (Fig. 3B). However, the induction of hemichannel opening by FFSS decreased with longer periods of shear stress (4, 8, and 24 h). The surface expression of Cx43 in response to continuous FFSS was examined by cell surface biotinylation (Fig. 3C). Biotinylated Cx43 protein increased at a significantly in response to continuous FFSS at 2 and 4 h of FFSS, but decreased to the control level at 24 h. (No biotinylation was detected for intracellular β-actin, therefore. the possibility of potential biotinylation of intracellular Cx43 is unlikely.) Similar to the biotinylation results shown in Fig. 2D, the pattern of cell surface-expressed Cx43 in response to continuous FFSS does not exactly correlate with hemichannel activity, further suggesting that the opening of hemichannels is not solely regulated by the level of Cx43 protein expressed on the cell surface. Together, the data suggest re-application of FFSS to previously stressed osteocytes leads to the re-activation of hemichannels as early as 30 min after the first round of FFSS stimulation. However, hemichannels appear to become less responsive to continuous FFSS and close over time in response to extended application of continuous shear stress. Similar to the post-stress experiments shown in Fig. 2, an increase in both cell surface protein expression and dye uptake occurs up to 4 h of continuous FFSS, but after 24 h, the decrease of dye uptake appears to correlate with a return to control levels of cell surface expression of Cx43, These results support the concept that a hemichannel gating mechanism is likely to be involved during the shorter periods of continuous FFSS, but reduced levels of cell surface-expressed Cx43 may be partially responsible for the decreased activity of hemichannels after 24 h of continuous FFSS.
Cx43-forming Hemichannels Play a Major Role in Regulating Osteocyte Response to FFSS—Clarification of the functional role(s) played by Cx43 hemichannels compared with gap junctions and other channels would be greatly advanced with specific blocking reagents for Cx43 hemichannels. Therefore, we generated a specific Cx43-hemichannel blocking antibody that targets the second extracellular loop (E2) of Cx43 (Fig. 4A). The generation of this antibody was not trivial as extracellular domains of connexins are highly conserved among different types of connexins from various species. This antibody (Cx43(E2)) recognizes Cx43, whereas no reactivity was observed using the preimmune antibody negative control (Fig. 4B). At the same dilution, the binding signal appeared to be even stronger than antibody against the C-terminal tail of Cx43 (Cx43(CT)), generated previously using a similar approach (35). Preincubating this antibody with its antigen, a GST fusion protein with the Cx43 E2 domain, but not GST protein alone, totally abolished this reactivity with Cx43 (Fig. 4C). This antibody failed to detect other connexins including Cx32, Cx26, Cx50, and Cx46 (data not shown). Immunostaining shows that in the presence of Triton X-100 (TX100) (Fig. 4D, panels D and F), comparable fluorescence signals are observed with Cx43(E2) antibody compared with that of Cx43(CT) antibody. While in the absence of detergent (panels C and E), only the labeling of Cx43(E2) antibody was observed, but no labeling for Cx43(CT) antibody, suggesting that the Cx43(E2) antibody recognizes the extracellular domain of Cx43 (Fig. 4D). The specificity of the Cx43(E2) antibody was further confirmed by preimmune and Cx43(E2) antibody pre-absorbed with antigen (Fig. 4D, panels A and B), where no specific signals were detected. Our previous preliminary study shows that gap junction activity appears not to be affected by this Cx43(E2) antibody (25). We showed here that gap junction function detected by scrape-loading dye transfer was not affected by increased concentrations of Cx43(E2) antibody (Fig. 4E). Cx43(E2) antibody significantly inhibited dye uptake induced by FFSS, but not by antibody against the cytosolic C-terminal domain of Cx43(CT) and preimmune antibody (Fig. 5A). In contrast, preincubating with antigen removed the capacity of Cx43(E2) antibody to block dye uptake induced by FFSS (Fig. 5B), further confirming the specificity of this antibody for hemichannels. Similar to MLO-Y4 cells, FFSS-induced dye uptake in primary osteocytes was significantly blocked by Cx43(E2) antibody, but not by preimmune antibody (Fig. 5C), showing that the MLO-Y4 cells function similarly to primary bone osteocytes. We have tested the specificity of this antibody in blocking hemichannnels, but not other membrane pores of similar sizes such as ATP-gated P2X7-associated pannexin channels (36). P2X7 channels were reported to be involved in bone cell response to mechanical stimulation (37). Treatment with 1 mm ATP induced dye uptake, possibly mediated through pannexin channels because of the activation of P2X7 receptor (36) (Fig. 5D). The opening of this channel was blocked by a P2X7 channel inhibitor, oxidized ATP (1 mm), but not by the Cx43(E2) blocking antibody, demonstrating that this antibody is specific for blocking Cx43-hemichannels but not pannexin or P2X7 channels. These results suggest that we have developed a potent antibody specific for Cx43-forming hemichannels and that FFSS-induced dye uptake is indeed mediated by the opening of Cx43-forming hemichannels.
FIGURE 4.
Generation of an antibody specific for the extracellular domain (E2) of Cx43 which showed no effect on gap junction channels. A, using a GST-fusion protein approach, a potent Cx43-hemichannel blocking antibody that targets the second extracellular loop domain (E2) of Cx43 was generated. B, MLO-Y4 cell lysates were immunoblotted with anti-Cx43 C-terminal domain (Cx43(CT)), anti-Cx43 E2 domain (Cx43(E2)), or preimmune (Pre) antibody. C, MLO-Y4 cell lysates were immunoblotted with anti-Cx43(E2) antibody (Cx43(E2)) (1.06 μl/ml), Cx43(E2) antibody (1.06 μg/ml) premixed with GST-Cx43 E2 domain fusion protein (Cx43(E2)+E2) (11.2 μg/ml) Cx43(E2) antibody premixed with GST protein (Cx43(E2)+GST) (11.2 μg/ml) or anti-β-actin antibody. D, MLO-Y4 cells were treated in the absence (panels C and E) or presence (panels A, B, D, and F) of Triton X-100 (TX100), then immunostained with anti-Cx43(E2) (panels C and D), anti-Cx43(CT) (panels E and F), preimmune (Pre)(panel A) antibody, or Cx43(E2) antibody pre-absorbed with antigen (Cx43(E2)Ab+E2) (panel B), and followed by labeling with FITC-conjugated anti-rabbit IgG secondary antibody (n = 4). E, scrape-loading dye transfer analysis was conducted in the absence (0) or presence of anti-Cx43(E2) antibody (Ab) at 28, 56, and 100 μg/ml for 4 h.
FIGURE 5.
Blockade of dye uptake in response to FFSS by the hemichannel blocking Cx43(E2) antibody. A, MLO-Y4 cells were treated with or without preimmune (Pre), Cx43(E2), or Cx43(CT) antibody in the absence (C), or presence of FFSS at 16 dynes/cm2 (FF) for 30 min. Immediately after FFSS, LY dye uptake was performed, and the number of cells with dye uptake were counted and quantified. FF+Cx43(E2) versus FF, FF+Cx43(CT), and FF+Pre: ***, p < 0.001. B, cells were treated with or without premix of Cx43(E2) antibody with GST-Cx43 E2 domain fusion protein (Cx43(E2)+E2) in the absence and presence of FFSS at 16 dynes/cm2 (FF) for 30 min. FF and FF+Cx43(E2)+E2 versus C: ***, p < 0.001. C, primary chick osteocytes were treated with or without Cx43(E2) antibody in the absence (C), or presence of FFSS at 16 dynes/cm2 (FF) for 30 min. FF+Cx43(E2) versus FF, *, p < 0.05. D, MLO-Y4 cells were treated with or without (control, C) 1 mm ATP, 1 mm ATP plus Cx43(E2) antibody (ATP+Cx43(E2)), or 1 mm ATP plus 10 mm oxidized ATP (ATP+oATP). LY dye uptake was conducted, and the numbers of cells uptaking dye were counted and quantified. ATP+Cx43(E2) and ATP versus C, ATP+oATP: **, p < 0.01. All data are presented as mean ± S.E. and n = 3.
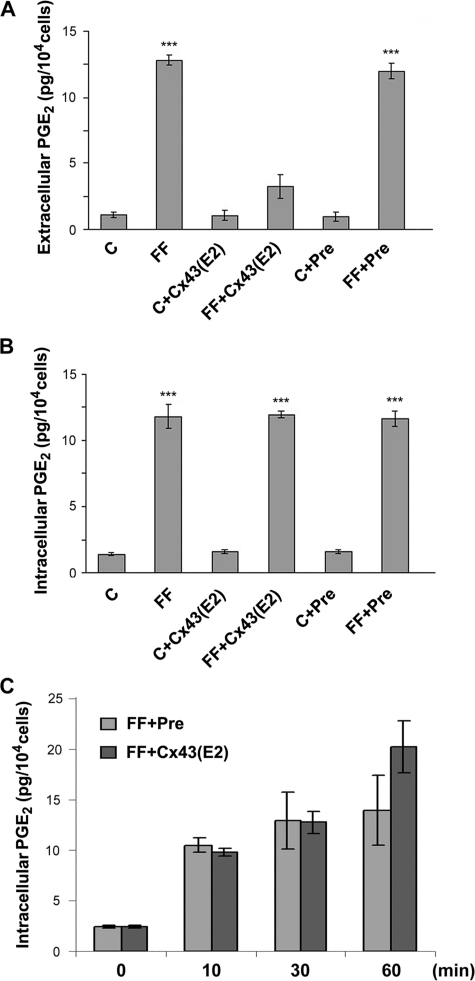
Similar to the effects shown for dye uptake, FFSS-induced release of PGE2 from hemichannels was significantly inhibited by Cx43(E2) but unaffected by preimmune antibody (Fig. 6A). Intracellular PGE2 was also increased in response to FFSS (FF) (Fig. 6B); however, there was no difference in intracellular PGE2 levels in Cx43(E2) or preimmune antibody-treated cells, which implies a potential feedback mechanism in maintaining stable levels of PGE2 inside the cell. To test this hypothesis, the intracellular PGE2 levels in cells exposed to FFSS for various time periods was measured (Fig. 6C). Interestingly, intracellular PGE2 levels were increased even after 10 min of stress and continued to increase after 30 min and 1 h in the absence and presence of Cx43(E2) antibody. After 1 h of FFSS, intracellular PGE2 in the Cx43(E2) antibody-treated cells was higher than preimmune antibody-treated cells, whereas no significant difference was observed during other time periods. These data suggest that intracellular PGE2 levels are tightly regulated, maintaining a constant, possibly saturated level through a feedback inhibition of biosynthesis by the substrate or by degradation mechanisms. Together, these results show we have developed a potent antibody specific for blocking hemichannels formed by Cx43, which will prove invaluable to identify other functional aspects of hemichannels. More importantly, Cx43-forming hemichannels play an essential role in regulating the release of prostaglandins in response to mechanical stimulation.
FIGURE 6.
Inhibition of PGE2 release, but not intracellular levels of PGE2, by Cx43(E2) antibody in response to FFSS. MLO-Y4 cells were treated with or without preimmune (Pre) or Cx43(E2) antibody in the absence (C) or presence of FFSS (FF) at 16 dynes/cm2 for 30 min (A and B) or for 10, 30, and 60 min. The media and cells were collected. The amount of extracellular (A), and intracellular (B and C) PGE2 was measured using a PGE2 EIA kit. In A, extracellular PGE2 in FF and FF+Pre versus FF+Cx43(E2), C, C+Cx43(E2) and C+Pre: ***, p < 0.001. In B, intracellular PGE2 in FF, FF+Cx43(E2) and FF+Pre versus C, C+Cx43(E2) and C+Pre: ***, p < 0.001. All data are presented as mean ± S.E. and n = 3.
DISCUSSION
In this study, we found that Cx43-hemichannels are dynamic channels capable of responding to different forms, magnitudes, and duration of mechanical loading. The opening of hemichannels is closely correlated with the release of PGE2, a factor directly involved in and essential for bone formation and remodeling in response to mechanical loading. Modulated release of cytoplasmic molecules, such as PGE2, by functional hemichannels in response to mechanical stimulation is likely to play a novel, important role in regulating anabolic/catabolic activities of bone cells. Moreover, an understanding of the dynamic aspects of hemichannels is important for finding associations between osteocyte response and bone adaptation, as they are shown here to be important for the temporal response to mechanical stimuli.
We show here that both uptake and release are magnitude-dependent with increasing response to increased applied shear stress most likely to accommodate an increased demand for exchange of signaling molecules in response to shear stress. Several theoretical models have merged over the years suggesting that bone cells can distinguish between different types of flow patterns, steady or pulsatile fluid flow (38–40). Similar to our previous observations for gap junction channels (15), we observed no significant differences in hemichannel response with either steady or pulsatile fluid flow. These results do not support any one flow pattern as an exclusive player in regulating hemichannel response to mechanical loading.
During post-FFSS periods, although the number of cells that have functional hemichannels remained relatively constant up to 8 h after flow, the number of open hemichannels in individual cells, however, appeared to decrease. Using a biotinylation assay, we found a delayed increase of Cx43 protein on the cell surface compared with the opening of hemichannels during the same incubation time after FFSS. This delayed response may be due to the time for Cx43 to migrate to the cell surface to accommodate the demand for hemichannel function. In addition, our previous studies showed increased Cx43 protein expression during post-stress periods (15), reducing the possibility of reduced hemichannel closure because of a degradation of Cx43 protein, although the possibility of the relocation of Cx43 cannot be ignored. These results indicate that closure of hemichannels may occur via two phases; acute and chronic, through the decreased cell surface expression of Cx43. The acute early phase of channel opening involves Cx43 already expressed at the plasma membrane, whereas the chronic, delayed phase is related to trafficking of additional Cx43 molecules to the membrane in response to FFSS. This is most likely the reason for a lack of close correlation between activities of hemichannels and levels of cell surface-expressed Cx43. Another possibility may involve an association of Cx43 with other protein(s) capable of regulating hemichannel functions. This area of research is currently under investigation in our laboratory.
The tightly regulated opening/closing of Cx43-hemichannels observed in this study may have important implications for bone function in vivo. A continuously opened hemichannel may lead to continuous release of prostaglandin, which could be detrimental to bone. Although prostaglandins are known to be skeletal anabolic agents, they also have catabolic effects on bone and have been shown to stimulate osteoclast formation, activation, and resorption at higher concentrations and for longer durations (41–44). Inappropriate opening of hemichannels may increase metabolic stress and subsequently lead to cell death, which would compromise the mechanosensory function of the osteocyte network.
Functionally adaptive remodeling is a process by which bone is continuously optimized to serve its mechanical needs throughout life (45). Previous in vivo models demonstrate a positive linear relationship exists between applied strain magnitude and a change in bone mass (46, 47). Additional studies showed that bone can recover its sensitivity to mechanical stimuli to the same degree as previous exposure to load with the inclusion of rest periods (48). We demonstrate that this homeostatic control mechanism occurs at the level of the individual osteocyte and involves hemichannel function, when repeated mechanical loading enhanced the hemichannel response after the cells were permitted to recover. The highly responsive nature of hemichannels to mechanotransduction is likely to modulate the ability of osteocytes to adapt to repeated mechanical stimulation.
Hemichannel opening was decreased with increased duration of continuous flow. Similar to post-FFSS periods, the pattern of cell surface-expressed Cx43 does not exactly correlate with the activity of hemichannels. However, the reduction of hemichannel opening 24 h of flow could be attributed to a return to control levels of Cx43 protein on cell surface. The hemichannel response to continuous stimulation may be a mechanism to prevent persistent opening of these channels that could be detrimental to osteocytes. As the strain sensor in bone, exposure of osteocytes to short changes in FFSS may be all that is necessary to trigger a substantial osteogenic response. In addition, several in vivo studies have shown static strains fail to cause an osteogenic response in bone tissue and cultured cells (48–50). Collectively, these studies support the hypothesis that hemichannels could function as mechanosensors of osteocytes, adjusting their activities in response to insertion of rest periods or to continuous shear, ultimately accommodating the needs of bone remodeling.
One of the major challenges for hemichannel research is related to the development of hemichannel, but not gap junction, specific reagents. A diverse range of chemical compounds and connexin mimetic peptides inhibit gap junction intercellular communication, but also act on hemichannels (25, 51–53). Connexin extracellular loop domain mimetic peptides, GAP26 and GAP27, were developed and shown to block hemichannels when treated for a short period of time (10–30 min), while longer treatment (more than 1 h) inhibits gap junctions as well (54, 55). In contrast to smaller molecules, such as chemical compounds and peptides, an immunoglobulin is a much larger protein (150K); therefore, access to gap junctional space is almost impossible. We found that 4 h of treatment with higher concentrations of Cx43(E2) antibody was still specific for blocking hemichannel, but not gap junction functions. Use of this antibody Cx43(E2) provides definitive evidence that the exit of PGE2 from osteocytes is mediated through hemichannels based on the following: 1) specificity of the antibody only for hemichannels, 2) no reactivity or interference with gap junctions nor with other channels of comparable pore size, such as pannexin hemichannels; 3) the antibody is effective in blocking dye uptake, and 4) highly effective in blocking PGE2 release.
Consistent with our previous studies using general gap junction/hemichannel inhibitors (25), dye uptake and PGE2 release were not completely blocked by this antibody under both basal and shear stress conditions. This could be interpreted as the incomplete effectiveness of blocking antibody or inhibitors used. Alternatively, this could be due to expression and function of other connexins even though Cx43 is the major connexin expressed in these cells. We previously found that the uptake and release of PGE2 were not attenuated by inhibitors of other channels such as oxidized ATP for the purinergic receptor P2X7 or the prostaglandin transporter PGT (25). The possible mechanism involved in mediating such low levels of dye uptake in MLO-Y4 osteocytes that is not blocked by this hemichannel-specific antibody will be pursued in future investigations.
Acknowledgments
We thank Nidhi Batra for assistance in RT-PCR analysis and members of Dr. Jiang's laboratory for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants F31 AR053468 (to A. J. S.-J.) and P01 AR46798 (to J. X. J., L. F. B., and E. S.). This work was also supported by the Welch Foundation Grant AQ-1507 (to J. X. J.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Cx, connexin; RD, rhodamine dextran; LY, Lucifer yellow; FFSS, fluid flow shear stress; PBS, phosphate-buffered saline; GST, glutathione S-transferase.
References
- 1.Knothe Tate, M. L. (2003) J. Biomech. 36 1409–1424 [DOI] [PubMed] [Google Scholar]
- 2.Zeng, Y., Cowin, S. C., and Weinbaum, S. (1994) Ann. Biomed. Eng. 22 280–292 [DOI] [PubMed] [Google Scholar]
- 3.Ajubi, N. E., Klein-nulend, J., Nijweide, P. J., Vrijheid-lammers, T., Alblas, M. J., and Burger, E. H. (1996) Biochem. Biophys. Res. Comm. 225 62–68 [DOI] [PubMed] [Google Scholar]
- 4.Cowin, S. C., Moss-Salentijin, L., and Moss, M. L. (1991) J. Biomed. Eng. 113 191–197 [DOI] [PubMed] [Google Scholar]
- 5.Aarden, E. M., Burger, E. H., and Nijweide, P. J. (1994) J. Cell. Biochem. 55 287–299 [DOI] [PubMed] [Google Scholar]
- 6.Burger, E. H., and Klein-nulend, J. (1999) FASEB J. 13 S101-S112 [PubMed] [Google Scholar]
- 7.Schirrmacher, K., Schmiz, I., Winterhager, E., Traub, O., Bremmer, F., Jones, D., and Binbmann, D. (1992) Calcif. Tissue Int. 51 285–290 [DOI] [PubMed] [Google Scholar]
- 8.Civitelli, R., Beyer, E. C., Warlow, P. M., Roberson, A. J., Geist, S. T., and Steinberg, T. H. (1993) J. Clin. Investig. 1888–1896 [DOI] [PMC free article] [PubMed]
- 9.Chung, D. J., Castro, C. H., Watkins, M., Stains, J. P., Chung, M. Y., Szejnfeld, V. L., Willecke, K., Theis, M., and Civitelli, R. (2006) J. Cell Sci. 119 4187–4198 [DOI] [PubMed] [Google Scholar]
- 10.Presley, C. A., Lee, A. W., Kastl, B., Igbinosa, I., Yamada, Y., Fishman, G. I., Gutstein, D. E., and Cancelas, J. A. (2005) Cell Commun. Adhes. 12 307–317 [DOI] [PubMed] [Google Scholar]
- 11.Mason, D. J., Hillam, R., and Skerry, T. M. (1996) J. Bone Miner. Res. 11 350–357 [DOI] [PubMed] [Google Scholar]
- 12.Donahue, H. J., Guilak, F., Mcleod, K. J., Rubin, C. T., Grande, D. A., and Brink, P. R. (1995) J. Bone Miner. Res. 10 1359–1364 [DOI] [PubMed] [Google Scholar]
- 13.Schwab, W., Hofer, A., and Kasper, M. (1998) Histochem. J. 30 413–419 [DOI] [PubMed] [Google Scholar]
- 14.Kato, Y., Windle, J. J., Koop, B. A., Mundy, G. R., and Bonewald, L. F. (1997) J. Bone Miner. Res. 12 2014–2023 [DOI] [PubMed] [Google Scholar]
- 15.Cheng, B., Zhao, S., Luo, J., Sprague, E., Bonewald, L. F., and Jiang, J. X. (2001) J. Bone Miner. Res. 16 249–259 [DOI] [PubMed] [Google Scholar]
- 16.Thi, M. M., Kojima, T., Cowin, S. C., Weinbaum, S., and Spray, D. C. (2003) Am. J. Physiol. (Cell Physiol.) 284 C389–C403 [DOI] [PubMed] [Google Scholar]
- 17.Goodenough, D. A., and Paul, D. L. (2003) Nature Rev. 4 1–10 [Google Scholar]
- 18.Hofer, A., and Dermietzel, R. (1998) Glia 24 141–154 [DOI] [PubMed] [Google Scholar]
- 19.Boucher, S., and Bennett, S. A. L. (2003) J. Neuro. Res. 72 393–404 [DOI] [PubMed] [Google Scholar]
- 20.Contreras, J. E., Saez, J. C., Bukauskas, F. F., and Bennett, M. V. L. (2003) Proc. Nat. Acad. Sci. U. S. A. 100 11388–11393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez, J. C., Contreras, J. E., Bukauskas, F. F., Retamal, M. A., and Bennett, M. V. L. (2003) Acta Physiol. Scand. 179 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John, S., Cesario, D., and Weiss, J. N. (2003) Acta Physiol. Scand. 179 23–31 [DOI] [PubMed] [Google Scholar]
- 23.Romanello, M., and D'Andrea, P. (2001) J. Bone Miner. Res. 16 1465–1476 [DOI] [PubMed] [Google Scholar]
- 24.Plotkin, L. I., Manolagas, S. C., and Bellido, T. (2002) J. Biol. Chem. 277 8648–8657 [DOI] [PubMed] [Google Scholar]
- 25.Cherian, P. P., Siller-Jackson, A. J., Gu, S., Wang, X., Bonewald, L. F., Sprague, E., and Jiang, J. X. (2005) Mol. Biol. Cell 16 3100–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genetos, D. C., Kephart, C. J., Zhang, Y., Yellowley, C. E., and Donahue, H. J. (2007) J. Cell. Physiol. 212 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein-nulend, J., Bacabac, R. G., and Mullender, M. G. (2005) Pathologie Biologie 53 576–580 [DOI] [PubMed] [Google Scholar]
- 28.Jee, W. S., and Ma, Y. F. (1997) Bone 21 297–304 [DOI] [PubMed] [Google Scholar]
- 29.Thompson, D. D., and Rodan, G. A. (1988) J. Bone Miner. Res. 3 409–414 [DOI] [PubMed] [Google Scholar]
- 30.Forwood, M. R. (1996) J. Bone Miner. Res. 11 1688–1693 [DOI] [PubMed] [Google Scholar]
- 31.Prasad, A. R., Logan, S. A., Nerem, R. M., Schwartz, C. J., and Sprague, E. A. (1993) Circ. Res. 72 827–836 [DOI] [PubMed] [Google Scholar]
- 32.Daniels, G. M., and Amara, S. G. (1998) Methods Enzymol. 296 307–318 [DOI] [PubMed] [Google Scholar]
- 33.El-Fouly, M. H., Trosko, J. E., and Chang, C. C. (1987) Exp. Cell Res. 168 422–430 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka, K., Yamaguchi, Y., and Hakeda, Y. (1995) J. Bone Miner. Res. 13 61–70 [Google Scholar]
- 35.He, D. S., Jiang, J. X., Taffet, S. M., and Burt, J. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 6495–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelegrin, P., and Surprenant, A. (2006) EMBO J. 25 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, J., Liu, D., Ke, H. Z., Duncan, R. L., and Turner, C. H. (2005) J. Biol. Chem. 280 42952–42959 [DOI] [PubMed] [Google Scholar]
- 38.Klein-nulend, J., Van Der Plas, A., Semeins, C. M., Ajubi, N. E., Frangos, J. A., Nijweide, P. J., and Burger, E. H. (1995) FASEB J. 9 441–445 [DOI] [PubMed] [Google Scholar]
- 39.Jacobs, C. R., Yellowley, C. E., Davis, B. R., Zhou, Z., Cimbala, J. M., and Donahue, H. J. (1998) J. Biomech. 31 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, L., Fritton, S. P., Weinbaum, S., and Cowin, S. C. (2003) J. Biomech. 36 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins, D. A., and Chambers, T. J. (1991) J. Bone Miner. Res. 6 157–164 [DOI] [PubMed] [Google Scholar]
- 42.Collins, D. A., and Chambers, T. J. (1992) J. Bone Miner. Res. 7 555–561 [DOI] [PubMed] [Google Scholar]
- 43.Raisz, L. G. (1999) Osteoarthritis Cartilage 7 419–421 [DOI] [PubMed] [Google Scholar]
- 44.Raisz, L. G. (2005) J. Clin. Investig. 115 3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skerry, M. (2006) J. Musculoskelet. Neuronal Interact. 6 122–127 [PubMed] [Google Scholar]
- 46.Rubin, C. T., and Lanyon, L. E. (1985) Calcif. Tissue Int. 37 411–417 [DOI] [PubMed] [Google Scholar]
- 47.Rubin, C. T., and Lanyon, L. E. (1987) J. Orthop. Res. 5 300–310 [DOI] [PubMed] [Google Scholar]
- 48.Robling, A. G., Burr, D. B., and Turner, C. H. (2000) J. Bone Miner. Res. 15 1596–1602 [DOI] [PubMed] [Google Scholar]
- 49.Lanyon, L. E., and Rubin, C. T. (1984) J. Biomech. 17 897–905 [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan, S., Weimer, D. A., Agans, S. C., Bain, S. D., and Gross, T. S. (2002) J. Bone Miner. Res. 17 1613–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kondo, R. P., Wang, S.-Y., John, S. A., Weiss, J. N., and Goldhaber, J. I. (2000) J. Mol. Cell Cardiol. 32 1859–1872 [DOI] [PubMed] [Google Scholar]
- 52.Dahl, G., Nonner, W., and Werner, R. (1994) Biophys. J. 67 1816–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braet, K., Vandamme, W., Martin, P. E. M., Evans, W. H., and Leybaert, L. (2003) Cell Calcium 33 37–48 [DOI] [PubMed] [Google Scholar]
- 54.Chaytor, A. T., Evans, W. H., and Griffith, T. M. (1997) J. Physiol. 503 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans, W. H., de Vuyst, E., and Leybaert, L. (2006) Biochem. J. 397 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]