Abstract
Oral cancer is a common neoplasm worldwide with tobacco and alcohol being the major etiological factors contributing to its pathogenesis. Epidermal growth factor receptor (EGFR) and ErbB2 are known to be involved in the development of oral cancer with the former up-regulated in up to 90% human cases. The goal of this study was to evaluate the chemopreventive effects of a dual inhibitor of EGFR and ErbB2 tyrosine kinases, GW2974, in the 7, 12-dimethylbenz[a]anthracene (DMBA)-induced hamster cheek pouch model. A short-term experiment (3-week topical DMBA followed by 1-week topical GW2974) was conducted to examine the effects of GW2974 on aberrant arachidonic acid (AA) metabolism and cell proliferation in the hamster oral epithelium. Topical application of 0.1ml GW2974 (160 µM, three times a week) significantly reduced the levels of prostaglandin E2 (PGE2), leukotriene B4 (LTB4), 5-, 12-, 15-hydroxyeicosatetraenoic acid (HETE), and cell proliferation (BrdU-labeling index). In a long-term post-initiation experiment (6-week topical DMBA followed by 18-week topical GW2974), GW2974 (4mM and 8mM) significantly inhibited the incidence, number and size of visible tumors. Under microscope, the numbers of oral lesions (hyperplasia, dysplasia, carcinoma) and the incidence of squamous cell carcinoma (SCC) were also significantly suppressed by GW2974. In summary, our study indicated that dual inhibition of EGFR and ErbB2 tyrosine kinases by GW2974 was effective in preventing oral carcinogenesis in DMBA-induced hamster cheek pouch model. GW2974 exerted its chemopreventive effects in part by suppressing aberrant AA metabolism.
Keywords: Oral cancer, EGFR, DMBA, GW2974
Introduction
Oral cancer mainly represents the cancers of the tongue, cheek and palate. It occurs predominantly in developing countries where it constitutes up to 25% of all kinds of cancers 1. In the United States about 34,360 new cases and 7,550 deaths are expected in 2007 2. Despite significant advances in various forms of treatment, the overall five-year survival rate for oral cancer patients has not improved over the past couple of decades and remains around 50% 3. Therefore, it is important to understand the pathogenesis of this disease and to design novel and efficacious chemopreventive strategies.
Epidermal growth factor receptor (EGFR) family, which includes EGFR/ErbB1, ErbB2, ErbB3 and ErbB4, are known to regulate cell proliferation, differentiation, migration, angiogenesis and apoptosis 4–6. When activated by various ligands, these receptors homo- or hetro-dimerize with each other to form active tyrosine kinases, and activate various downstream signaling molecules, such as MAPKs, PI3K and STATs 7. EGFR is expressed in normal oral squamous epithelial cells of humans and hamsters 8, 9. Amplification and overexpression of EGFR were shown to be early events during oral carcinogenesis, and paralleled progression of the disease 10. Expression levels of EGFR, ErbB2 and ErbB3 together have stronger prognostic value than either one of them alone 11–13. Among the four EGFR family members, EGFR and ErbB2 are most frequently co-expressed in the same cell layer of neoplastic epithelium 14. Patients who exhibit overexpression of both EGFR and ErbB2 tend to have more aggressive tumors and poorer clinical prognosis when compared to those expressing either one of them 13, suggesting an important role of EGFR/ErbB2 heterodimer in oral carcinogenesis. In addition, it was shown recently that hamsters vaccinated in the buccal pouch with a DNA coding for the ErbB2 receptor elicited an antibody response that suppressed the development and progression of SCC 15. Both in vitro and in vivo data have suggested that the dual inhibition of both EGFR and ErbB2 is more effective in cancer therapy than agents targeting individual receptors 16.
Aberrant arachidonic acid (AA) metabolism has been suggested to play an important role in the development of human oral cancer, with the involvement of both cyclooxygenase (Cox) and lipoxygenase (Lox) pathways 17, 18. The major metabolite of the Cox pathway is prostaglandin E2 (PGE2), while the Lox enzymes can give rise to a number of metabolites such as leukotriene B4 (LTB4) and 5-, 12-, 15-hydroxyeicosatetraenoic acid (HETE) depending upon the position of oxygen insertion in AA 19. These metabolites are known to be potent mediators of inflammation as they recruit and activate inflammatory cells, increase vascular permeability and induce smooth muscle contraction 20. We have shown previously that Cox-2 and 5-Lox were overexpressed in hamster and human oral cancer, and specific inhibitors of Cox-2 and 5-Lox were able to suppress the development of hamster cheek pouch carcinogenesis 18. We further demonstrated that LTB4 promoted oral carcinogenesis, while multiple inhibitors of 5-Lox pathway had chemopreventive effects on cancer development, in the same model system 21.
There is ample evidence in the literature to suggest a cross talk between the EGFR signaling cascade and AA metabolism. EGFR mediated activation of MAPK has been shown to upregulate Cox-2 gene transcription, while Cox-2 derived PGE2 can stimulate cell proliferation through EGFR transactivation 22. Activation of EGFR and ErbB2 has been shown to stimulate Cox-2 expression and translocation as well as PGE2 synthesis and mitogenesis 23, 24. It has been reported earlier that AA metabolites act as secondary messengers during EGF-induced cytoskeletal changes in rat fibroblasts and HeLa cells 25. Also in another study, an anti-EGF monoclonal antibody was able to reduce the levels of PGE2 in human amnion cells 26. Moreover, combined use of both EGFR and Cox-2 inhibitors has shown synergistic effects in cancer therapy.
The major goal of this study was to investigate the chemopreventive activity of GW2974, a dual inhibitor of EGFR and ErbB2 tyrosine kinases, in oral carcinogenesis. GW2974 was first tested in a short-term experiment for its effects on 7, 12-dimethylbenz[a]anthracene (DMBA)-induced aberrant AA metabolism and cell proliferation in hamster oral epithelium. A long-term experiment was then conducted to evaluate its chemopreventive efficacy in oral carcinogenesis.
Materials and Methods
Chemicals
DMBA and GW2974 were purchased from Sigma Chemical Company (St. Louis, MO). They were dissolved in mineral oil at appropriate concentrations for topical application.
Short-term effects of topical GW2974 on aberrant AA metabolism and cell proliferation in DMBA-treated hamster cheek pouch
This study was conducted at Rutgers University under Protocol number 91-024. Male Syrian golden hamsters (6 weeks old) weighing 60–80 g were purchased from Harlan (Indianapolis, IN) and housed 4 per cage in a room with controlled temperature and humidity with 12 h light: dark cycles. All animals were given AIN-93M diet (Research Diets, New Brunswick, NJ) and water ad libitum. After 1 week of acclimatization, the animals were divided into 2 groups, with Group 1A serving as the negative control (3 animals). The left cheek pouch of the remaining 12 hamsters was topically treated with 0.5% DMBA in 100 µl mineral oil using a paintbrush 3 times a week for 3 weeks. They were then randomly divided into 2 groups with Group 1B (6 animals) serving as the positive control and receiving no further treatment. Group 1C (6 animals) was treated topically with 160 µM GW2974 in 100 µl mineral oil 3 times per week for 1 week. The animals were sacrificed at the end of the experiment (Week 4), 6 hours after the last treatment. The animals were injected with bromodeoxyuridine (BrdU) i.p. at 50 mg/kg body weight 2 hours prior to sacrificing. The cheek pouch was harvested, one half being snap frozen in liquid nitrogen for analysis of AA metabolites, and the other half being fixed in 10% PBS-buffered formalin for histopathology.
Long-term effects of topical GW2974 on DMBA-induced carcinogenesis in hamster cheek pouch
The animals were housed under the same conditions as mentioned above. Group 2A (10 animals) served as the negative control. Other groups were topically treated with 100 µl of 0.5% DMBA in mineral oil using a paintbrush 3 times per week for 6 weeks. The animals were then randomly divided into 3 groups (30 animals each), with Group 2B receiving no further treatment. Groups 2C and 2D were treated with 4 mM and 8 mM of GW2974 respectively, 3 times per week for another 18 weeks. At the end of Week 24, all animals were sacrificed and the left hamster cheek pouch was harvested and fixed in 10% buffered formalin for histopathology.
Histopathological analyses
The whole cheek pouch was excised and flattened on a transparency plate for counting the number of visible tumors. The length, width and height of each tumor was measured with a caliper and the tumor volume calculated using the formula: volume=4/3πr3 (where r was the average radius of the three diameter measurements in mm). Formalin-fixed pouches were cut into 4–6 pieces of approximately equal width, Swiss-rolled, processed and embedded in paraffin. Thirty sections (5 µm) of each sample were cut and the 1st, 15th and 30th slides were stained with hematoxylin and eosin (H&E) for histopathological analysis. Basal cell hyperplasia, dysplasia, squamous cell carcinoma and papillomas were diagnosed using established criteria 27, 28.
Cell proliferation analysis
To assess cell proliferation in the squamous epithelium of hamster oral mucosa, bromodeoxyuridine (BrdU) immunostaining was performed on formalin-fixed, paraffin-embedded tissue sections. The avidin-biotin peroxidase method was used with a rat monoclonal antibody (Serotec, Raleigh, NC) at a concentration of 5 µg/ml. Three noncontiguous, randomly selected fields under 400x were photographed per sample, and the sum of all positive cells was divided by total number of cells to obtain the cell proliferation index. Image-Pro Plus 4.5 software (Media Cybernetics, Silver Spring, MD) was used for cell counting.
Profiling of AA metabolites with LC/MS/MS
After homogenization in a buffer containing 10 µM zileuton and indomethacin, tissues were extracted with hexane:ethyl acetate under reduced light conditions after the addition of an internal standard (PGE2-d4). The samples were dried under nitrogen, reconstituted in methanol:2mM ammonium acetate, and analyzed using an established method 29. The levels of AA metabolites (PGE2, LTB4, 5-HETE, 12-HETE, and 15-HETE) were determined and expressed as nanograms per milligram protein.
Statistical analysis
The tumor incidence was analyzed with χ2 test. One-way ANOVA test was used to compare body weight, the number of visible tumors, and the numbers of oral lesions. Tumor volume was analyzed using the Wilcoxon signed rank test. Statistical significance between the levels of AA metabolites among the various groups was analyzed using the Student’s t test.
Results
Overall the animals appeared healthy throughout the two experiments. However, DMBA-treated animals weighed less than untreated animals by 4–8% probably due to metabolic stress caused by inflammation or irritation in the cheek pouch. Body weight of the animals was monitored once a week and increased steadily with less than 10% variation among different groups (data not shown).
Inhibition of DMBA-induced aberrant AA metabolism and cell proliferation in hamster cheek pouch by short-term treatment of topical GW2974
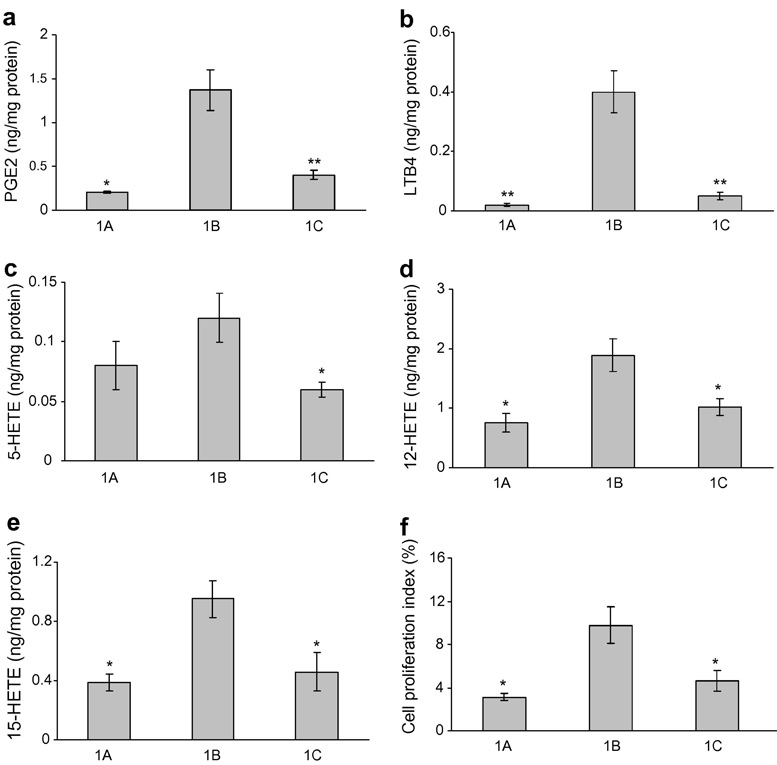
At the end of the short-term study (Week 4), DMBA-treated hamster cheek pouch appeared reddish, hyperkeratotic and inflamed. The levels of AA metabolites were found to be elevated in the positive control group (Group 1B), when compared to the negative control group (Group 1A). Topical application of 160 µM GW2974 (Group 1C) significantly reduced the levels of these metabolites (Figure 1a–e).
Figure 1.
Effects of topical GW2974 on AA metabolism and cell proliferation in DMBA-treated hamster cheek pouch. The values of AA metabolites are expressed as mean ± SD. Group 1A (negative control) and Group 1C (GW2974) are compared with Group 1B (positive control). *p<0.05 and **p<0.01.
BrdU immunohistochemical analysis showed that Group 1B had marked increase in the rate of cell proliferation when compared to Group 1A. This increase was significantly inhibited by treatment with GW2974 (p<0.05) (Figure 1f).
Chemopreventive effects of topical GW2974 on DMBA-induced oral carcinogenesis in hamster cheek pouch
Consistent with our previous studies 18, 21, 30, topical application of DMBA (100 µl, 0.5% in mineral oil, 3/week for 6 weeks) led to the development of oral lesions in the hamster cheek pouch at the end of 24 weeks. Topical application of GW2974 (4 mM and 8 mM, 100 µl, 3/week for 18 weeks) at the post-initiation stage significantly inhibited the incidence, number and size of DMBA-induced visible tumors. Under the microscope, GW2974 led to a significant decrease in the numbers of oral lesions (hyperplasia, dysplasia, and SCC) (Table 1).
Table 1.
Effects of topical GW2974 on DMBA-induced oral carcinogenesis in hamster cheek pouch a
| Group | Treatment | No. of animals | Visible Tumors |
Microscopic Observations |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence % (No.) | No. | Size (mm3) | No. of hyperplasia | No. of dysplasia | No. of SCC | Incidence of SCC % (No.) | |||
| 2A | Negative control | 10 | - | - | - | - | - | - | - |
| 2B | Positive control | 30 | 80% (24) | 1.00±0.88 | 256.84±442.53 | 8.07±4.39 | 7.57±3.37 | 1.83±1.91 | 70% (21) |
| 2C | GW2974 (4 mM) | 30 | 43.3% (13)** | 0.57±0.82* | 62.08±58.99 | 5.73±3.09** | 6.80±3.74 | 0.67±0.99* | 40% (12)* |
| 2D | GW2974 (8 mM) | 30 | 36.7% (11)** | 0.43±0.63** | 50.50±54.88 | 5.30±2.89** | 5.97±2.62* | 0.43±0.68** | 33% (10)** |
The number and size of visible tumors, and the numbers of oral lesions (hyperplasia, dysplasia, and SCC) were expressed as mean ± SD. χ2 test was used for comparison of incidences of visible tumor and SCC, signed rank test for tumor size, and one-way ANOVA for the numbers of visible tumors, hyperplasia, dysplasia and SCC. Values of Groups 2C and 2D were compared with that of Group 2B.
p<0.05
p<0.01.
Discussion
The role of ErbB family of receptors in oral carcinogenesis has been well established. Dual inhibition of EGFR/ErbB2 tyrosine kinases could be an effective approach for chemoprevention and therapy of oral cancer. In this study, we demonstrated the chemopreventive effects of GW2974, a dual inhibitor of both EGFR and ErbB2 tyrosine kinases, on DMBA-induced oral carcinogenesis in hamster cheek pouch.
As a quinazoline derivative, GW2974 has been shown previously to be highly potent in suppressing the growth of cancer cells in vitro and in vivo 31. It was effective in selectively inhibiting the growth of tumor cells in cancer cell lines overexpressing both EGFR and ErbB2. In addition, GW2974 also suppressed the growth of xenograft tumors in a dose-dependent manner due to selective inhibition of EGFR and ErbB2 receptor phosphorylation 31. A synergistic effect was observed when GW2974 was treated in combination with Bcl-2 inhibitors in suppressing the growth of various human breast cancer cell lines 32. In a separate in vivo study, GW2974 was more effective than gefitinib in significantly inhibiting the development of gallbladder carcinoma 33.
In our study, topical application of GW2974 thrice a week for 18 weeks to the hamster oral epithelium inhibited not only the number, size, and incidence of visible tumors, but also the numbers of oral lesions and the incidence of SCC. Higher dose of GW2974 (8 mM) was more effective in our study (Table 1). To our knowledge, this is the first study to evaluate the effect of dual inhibition of EGFR and ErbB2 tyrosine kinases for oral cancer chemoprevention in the DMBA-induced hamster cheek pouch model. However, this study has its limitations because of the hamster cheek pouch model although it mimics many aspects of human oral cancer development. The human mouth does not have a similar pouch in structure and histology, and DMBA is not a natural carcinogen related to human oral cancer development 34.
GW2974 may inhibit DMBA-induced oral carcinogenesis through modulation of aberrant AA metabolism and cell proliferation. It was reported by our group that aberrant AA metabolism is involved in the pathogenesis of oral cancer and can be targeted for its chemoprevention 18. In the present study, GW2974 led to a significant reduction in the levels of pro-inflammatory mediators such as PGE2, LTB4, and 5-HETE which were elevated due to aberrant AA metabolism induced by DMBA treatment (Figure 1a–e). These data are consistent with the observation that EGFR and AA metabolites interact with each other at multiple levels.
In summary, we show here that a dual inhibitor of EGFR/ErbB2 tyrosine kinases prevents oral carcinogenesis in the DMBA induced hamster cheek pouch model at the post-initiation stage. Its chemopreventive activity is in part due to modulation of AA metabolism and inhibition of cell proliferation, in addition to its inhibitory effects on EGFR/ErbB2.
Acknowledgments
Grant support: NIH grant CA101235, Beijing Natural Science Foundation No. 7032020, and National Natural Science Foundation of China No. 30271414
Abbreviations
- AA
arachidonic acid
- BrdU
bromodeoxyuridine
- Cox
cyclooxygenase
- DMBA
7,12-dimethylbenz[a]anthracene
- EGFR
epidermal growth factor receptor
- LC/MS/MS
high performance liquid chromatography/electrospray ionization tandem mass spectrometry
- LTB4
leukotriene B4
- 5-Lox
5-lipoxygenase
- PGE2
prostaglandin E2
- SCC
squamous cell carcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. J Natl Cancer Inst. 1993;85:862–874. doi: 10.1093/jnci/85.11.862. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Funk GF, Karnell LH, Robinson RA, et al. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24:165–180. doi: 10.1002/hed.10004. [DOI] [PubMed] [Google Scholar]
- 4.Sebastian S, Settleman J, Reshkin SJ, et al. The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta. 2006;1766:120–139. doi: 10.1016/j.bbcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Nicholas MK, Lukas RV, Jafri NF, Faoro L, Salgia R. Epidermal growth factor receptor - mediated signal transduction in the development and therapy of gliomas. Clin Cancer Res. 2006;12:7261–7270. doi: 10.1158/1078-0432.CCR-06-0874. [DOI] [PubMed] [Google Scholar]
- 7.Ono M, Kuwano M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin Cancer Res. 2006;12:7242–7251. doi: 10.1158/1078-0432.CCR-06-0646. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Han C, Chen J. Tea preparations protect against DMBA-induced oral carcinogenesis in hamsters. Nutr Cancer. 1999;35:73–79. doi: 10.1207/S1532791473-79. [DOI] [PubMed] [Google Scholar]
- 9.Wong DT, Gallagher GT, Gertz R, Chang AL, Shklar G. Transforming growth factor alpha in chemically transformed hamster oral keratinocytes. Cancer Res. 1988;48:3130–3134. [PubMed] [Google Scholar]
- 10.Shin DM, Gimenez IB, Lee JS, et al. Expression of epidermal growth factor receptor, polyamine levels, ornithine decarboxylase activity, micronuclei, and transglutaminase I in a 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch carcinogenesis model. Cancer Res. 1990;50:2505–2510. [PubMed] [Google Scholar]
- 11.Khan AJ, King BL, Smith BD, et al. Characterization of the HER-2/neu oncogene by immunohistochemical and fluorescence in situ hybridization analysis in oral and oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2002;8:540–548. [PubMed] [Google Scholar]
- 12.Werkmeister R, Brandt B, Joos U. Clinical relevance of erbB-1 and -2 oncogenes in oral carcinomas. Oral Oncol. 2000;36:100–105. doi: 10.1016/s1368-8375(99)00069-x. [DOI] [PubMed] [Google Scholar]
- 13.Xia W, Lau YK, Zhang HZ, et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. 1999;5:4164–4174. [PubMed] [Google Scholar]
- 14.Bei R, Pompa G, Vitolo D, et al. Co-localization of multiple ErbB receptors in stratified epithelium of oral squamous cell carcinoma. J Pathol. 2001;195:343–348. doi: 10.1002/path.965. [DOI] [PubMed] [Google Scholar]
- 15.Berta GN, Mognetti B, Spadaro M, et al. Anti-HER-2 DNA vaccine protects Syrian hamsters against squamous cell carcinomas. Br J Cancer. 2005;93:1250–1256. doi: 10.1038/sj.bjc.6602853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) Eur J Cancer. 2007;43:481–489. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 17.el-Hakim IE, Langdon JD. Arachidonic acid cascade and oral squamous cell carcinoma. Clin Otolaryngol Allied Sci. 1991;16:563–573. doi: 10.1111/j.1365-2273.1991.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Sood S, Wang S, et al. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 19.Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD. Arachidonic acid cascade in endothelial pathobiology. Microvasc Res. 2005;69:107–127. doi: 10.1016/j.mvr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Claria J, Romano M. Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways. Impact on inflammation and cancer. Curr Pharm Des. 2005;11:3431–3447. doi: 10.2174/138161205774370753. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z, Sood S, Li N, et al. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis. 2006;27:1902–1908. doi: 10.1093/carcin/bgl039. [DOI] [PubMed] [Google Scholar]
- 22.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 23.Coffey RJ, Hawkey CJ, Damstrup L, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadlamudi R, Mandal M, Adam L, et al. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999;18:305–314. doi: 10.1038/sj.onc.1202307. [DOI] [PubMed] [Google Scholar]
- 25.Peppelenbosch MP, Tertoolen LG, Hage WJ, de Laat SW. Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell. 1993;74:565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell MD. Epidermal growth factor actions on arachidonic acid metabolism in human amnion cells. Biochim Biophys Acta. 1987;928:240–242. doi: 10.1016/0167-4889(87)90127-3. [DOI] [PubMed] [Google Scholar]
- 27.Kramer I, Lucas R, Pindborg J, Sobin L. WHO, Collaborating center for oral precancerous lesions: definitions of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg. Oral Med. Oral Pathol. 1978;46:518–589. [PubMed] [Google Scholar]
- 28.Leininger I, KJokinen M. In: Pathology of tumors in laboratory animals. Turosov V, editor. Albany, NY: IARC; 1982. pp. 167–169. [Google Scholar]
- 29.Kempen EC, Yang P, Felix E, Madden T, Newman RA. Simultaneous quantification of arachidonic acid metabolites in cultured tumor cells using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Biochem. 2001;297:183–190. doi: 10.1006/abio.2001.5325. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Chen X, Liao J, et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis. 2002;23:1307–1313. doi: 10.1093/carcin/23.8.1307. [DOI] [PubMed] [Google Scholar]
- 31.Rusnak DW, Affleck K, Cockerill SG, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001;61:7196–7203. [PubMed] [Google Scholar]
- 32.Witters LM, Witkoski A, Planas-Silva MD, et al. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17:465–469. [PubMed] [Google Scholar]
- 33.Kiguchi K, Ruffino L, Kawamoto T, Ajiki T, Digiovanni J. Chemopreventive and therapeutic efficacy of orally active tyrosine kinase inhibitors in a transgenic mouse model of gallbladder carcinoma. Clin Cancer Res. 2005;11:5572–5580. doi: 10.1158/1078-0432.CCR-04-2603. [DOI] [PubMed] [Google Scholar]
- 34.Mognetti B, Di Carlo F, Berta GN. Animal models in oral cancer research. Oral Oncol. 2006;42:448–460. doi: 10.1016/j.oraloncology.2005.07.014. [DOI] [PubMed] [Google Scholar]