Summary
Dietary restriction (DR) is well known as a nongenetic intervention that robustly extends lifespan in a variety of species; however, its underlying mechanisms remain unclear. We have found in Caenorhabditis elegans that dietary deprivation (DD) during adulthood, defined as removal of their food source Escherichia coli after the completion of larval development, increased lifespan and enhanced thermotolerance and resistance to oxidative stress. DD-induced longevity was independent of one C. elegans SIRTUIN, sir-2.1, which is required for the effects of DR, and was independent of the daf-2/insulin-like signaling pathway that independently regulates longevity and larval diapause in C. elegans. DD did not significantly alter lifespan of fem-1(hc17); eat-2(ad465) worms, a genetic model of DR. These findings suggest that DD and DR share some downstream effectors. In addition, DD was detrimental for longevity when imposed on reproductively active young adults, suggesting that DD may only be beneficial in the absence of competing metabolic demands, such as fertility. Adult-onset DD offers a new paradigm for investigating dietary regulation of longevity in C. elegans. This study presents the first evidence that long-term DD, instead of being detrimental, can extend lifespan of a multicellular adult organism.
Keywords: ad libitum, aging, Caenorhabditis elegans, calorie restriction, dietary restriction, reproduction
Introduction
Lifespan is regulated by diverse genetic and environmental factors (Kenyon, 2005; Kirkwood, 2005; Masoro, 2005). One of the most robust environmental manipulations of lifespan is dietary restriction (DR) (Masoro, 2005). DR has been shown to extend lifespan in many species, ranging from invertebrates to mammals. In addition, DR enhances resistance to a variety of stresses, such as heat and oxidative stress, and delays onset of age-related diseases in murine models for human cancer and diabetes (Masoro, 2005). Several genetic components of the DR longevity pathway have been identified. Foremost among these are the NAD-dependent protein deacetylase, SIR2 and other SIRTUIN family members (Guarente, 2005; Sinclair, 2005), and TOR (target of rapamycin), a protein kinase that coordinates cell growth in response to nutrient availability (Vellai et al., 2003; Kapahi et al., 2004; Kaeberlein et al., 2005). In Drosophila, insulin-like signaling converges with DR and may also be a downstream like target of DR (Clancy et al., 2002). However, DR and insulin-like signaling appear to be independent in Caenorhabditis elegans (Lakowski & Hekimi, 1998; Houthoofd et al., 2003).
The degree of calorie reduction can be a critical factor modu-lating the effect of DR on lifespan (Everitt et al., 1982; Houthoofd et al., 2002). In rodents, DR, often referred to as calorie restriction, is normally achieved by relatively precise control of food or calorie intake (Masoro, 2005). DR to the level of 20–50% reduction of the ad libitum (AL) calorie intake extends lifespan, while further caloric restriction is detrimental (Everitt et al., 1982). Furthermore, imposing DR during development can be detrimental (Houthoofd et al., 2002). In yeast, worms, and flies, uncertainty over the amount of food intake has made DR problematic (Houthoofd et al., 2005). Historically, DR in these model systems is generally imposed by dilution of the caloric food source with low-calorie media (Klass, 1977; Houthoofd et al., 2002; Magwere et al., 2004). As observed for rodents, dilution studies have also shown that extreme caloric deprivation during development is detrimental (Klass, 1977; Henderson et al., 2006). DR has also been imposed using genetic mutations that interfere with nutrient uptake (Klass, 1977;Lakowski & Hekimi, 1998;Houthoofd et al., 2003; Kapahi et al., 2004; Kaeberlein et al., 2005). However, in some cases, longevity induced by these genetic mutations can be variable (Vellai et al., 2003; Walker et al., 2005; Henderson et al., 2006).
Previous studies in C. elegans imposed DR by food dilution prior to the completion of larval development (Houthoofd et al., 2002). However, adverse effects on development could arise even when DR is imposed during larval development, as suggested by findings that extreme food dilutions is usually detrimental (Avery & Horvitz, 1990; Henderson et al., 2006). This possibility, in combination with the uncertainty inherent in food dilution protocols and variable effects from genetic mutations that impose DR, led us to examine whether a new dietary regimen could affect longevity. Here we report that a dietary deprivation (DD) regimen, in which the food source Escherichia coli is removed from adult worm cultures, could prolong adult lifespan by up to 45%. Because this regimen involves removal of the food source, the problem of controlling food intake, which has hampered interpretation of past studies, is alleviated. Using this unambiguous method for dietary manipulation of longevity, we have investigated the genetic pathways necessary for lifespan extension by DD.
Results
Dietary deprivation extends lifespan
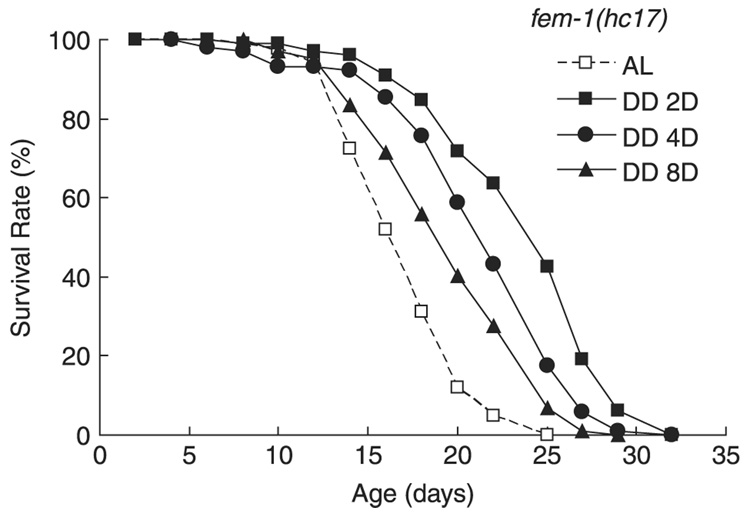
To examine the effects of DD on lifespan, we first used the C. elegans strain, fem-1(hc17), which is a temperature-sensitive mutant developing into semifertile adults at 20 °C and sterile adults at 25 °C. Lifespan analyses can be facilitated by utilizing sterile adult fem-1(hc17) populations, which have lifespans similar to those of wild-type animals (Nelson et al., 1978). We transferred sterile fem-1(hc17) adults on adult day 2 or older to agar plates with or without bacteria (E. coli OP50), which represent the AL or DD conditions, respectively. Both DD and AL media contained a mixture of 5-fluoro-2′-deoxyuridine (FUdR) and ampicillin to minimize egg formation and bacterial growth. Mean and maximum lifespan of control AL populations were similar to published results (Kenyon et al., 1993). In contrast contrast, the DD regimen significantly increased mean and maximum adult lifespan by up to 45% (Fig. 1).
Fig. 1. Extension of adult lifespan by dietary deprivation (DD).
DD was initiated in sterile fem-1(hc17) adults on adult day 2 (2D), 4 (4D) or 8 (8D). Lifespan under each DD regimen was extended compared to ad libitum (AL) controls, with the most effective extension observed on day 2 DD (42.5% and 41.4% increase mean and maximum; P < 0.0001 for both vs. AL controls (see Table 1). For AL conditions, worms were transferred onto agar plates with the same drug supplements as DD, except supplemented with E. coli OP50. AL controls that were initiated at adult day 2 were shown here; similar results were obtained when AL was initiated on adult day 4 or 8. n = approximately 30 worms tested in triplicate. Graph shows results from one representative of three independent experiments performed by two different individuals.
To assess the temporal requirements for initiation of DD, adult worms were maintained on AL conditions for different periods of time and then transferred to DD conditions for the remainder of their lives. The optimal effect of DD was observed when food was removed at adult day 2. Imposition of DD at later ages, in day 4 or 8 adults, elicited progressively weaker effects on longevity (Fig. 1; Table 1).
Table 1.
Effects of Dietary Deprivation Regimens on Lifespan
| Lifespan (days) | ||||||
|---|---|---|---|---|---|---|
| Dietary Regimens | Initiating age (day) | n | Mean | Change | Maximum | Change |
| Effects of DD Initiating Time on Lifespan | ||||||
| AL | 0 | 89 | 14.1 ± 0.5 | 18.0 ± 0.0 | ||
| 2 | 83 | 15.3 ± 0.4 | 19.1 ± 0.6 | |||
| 4 | 95 | 14.6 ± 0.5 | 20.0 ± 1.2 | |||
| 8 | 90 | 15.5 ± 0.4 | 19.5 ± 0.6 | |||
| DD | 0 | 69 | 18.4 ± 0.7*** | +30.5% | 24.4 ± 1.2** | +35.6% |
| 2 | 99 | 21.8 ± 0.5*** | +42.5% | 27.0 ± 0.0** | +41.4% | |
| 4 | 102 | 19.4 ± 0.5*** | +32.9% | 24.9 ± 0.1* | +24.5% | |
| 8 | 102 | 17.6 ± 0.4** | +13.5% | 23.0 ± 1.0* | +18.0% | |
| Effects of Refeeding on DD-Induced Longevity | ||||||
| AL | 1 | 120 | 14.7 ± 0.4 | 19.5 ± 0.5 | ||
| DD | 1 | 98 | 23.1 ± 0.4*** | 27.0 ± 0.0*** | ||
| DD5D,RF | 1 | 100 | 16.0 ± 0.3††† | −30.7 % | 21.7 ± 2.7 | |
| DD5D,RF 5D,DD | 1 | 98 | 16.9 ± 0.4††† | −26.8% | 22.0 ± 0.0† | −18.5% |
| DD5D,RF1D,DD5D,RF1D,DD | 1 | 100 | 18.7 ± 0.4††† | −19.0% | 23.6 ± 0.9† | −12.6% |
| DD5D,RF1D,DD | 1 | 105 | 20.2 ± 0.4††† | −12.6% | 25.4 ± 0.4† | −5.9% |
| DD10D, RF | 1 | 101 | 19.2 ± 0.4††† | −16.9% | 22.8 ± 0.8†† | −15.6% |
| DD15D, RF | 1 | 90 | 21.0 ± 0.4†† | −9.0% | 25.5 ± 0.5† | −5.6% |
Date are represented as mean ±SEM.
P<0.05
P<0.01
P<0.0001 vs. AL counterparts
P<0.05
P<0.01
P<0.0001 vs. DD
P-value of mean lifespan calculated by one-way ANOVA and post-hoc Fisher’s test
P-value of maximum lifespan calculated by Student’s t-test
AL, ad libitum; DD, dietary deprivation; RF, re-feed.
Dietary deprivation did not slow the rate of aging-related functional declines
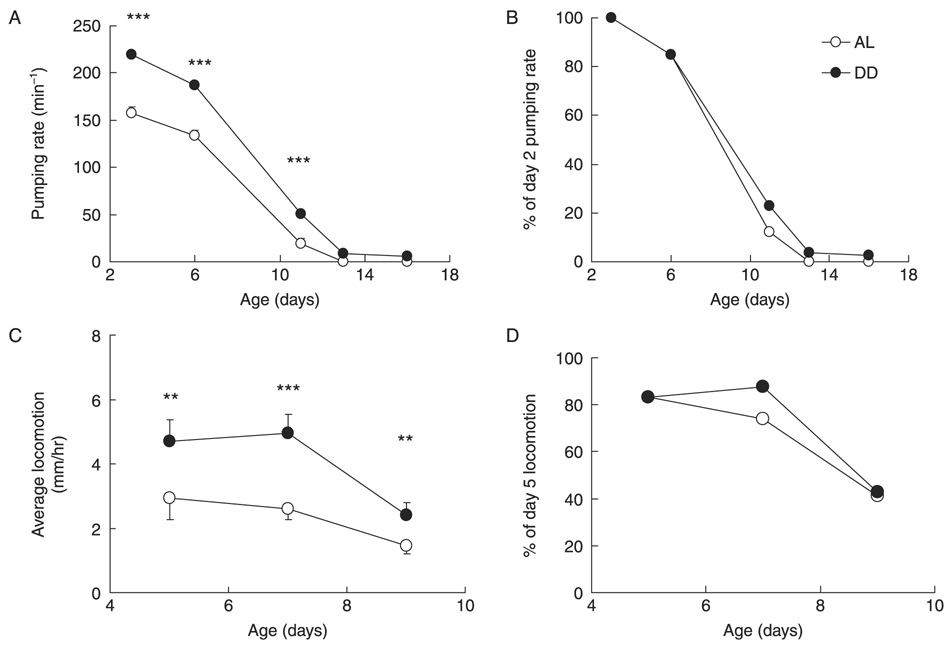
By assessing age-related functional declines in AL- and DD-treat treated worms, we next examined whether DD prolonged healthspan, rather than merely enhancing survival at old age. Two standard measures of functional aging in C. elegans are declines in pharynx pumping and locomotion (Johnson, 1987; Herndon et al., 2002; Glenn et al., 2004; Huang et al., 2004; Chow et al., 2006). In both AL- and DD-treated animals, pharynx-pumping rates progressively declined with age, although DD-treated animals maintained significantly higher pumping rates at all ages than AL-treated animals (Fig. 2A). The rate of decline of pharynx pumping with advancing age was similar in DD- and AL-treated animals (Fig. 2B). Similarly, spontaneous locomotion also declined progressively during aging of AL- and DD-treated animals, and DD-treated animals exhibited significantly greater spontaneous locomotion than AL-treated animals at all ages examined (Fig. 2C). As observed for pharynx pumping, locomotor activity declined at similar rates in DD- and AL-treated animals (Fig. 2D). This suggests that DD did not significantly slow the rate of aging in these tissues.
Fig. 2. Effects of dietary deprivation (DD) on aging-related functional decline.
DD or ad libitum (AL) treatments were initiated on adult day 1 in sterile adult fem-1(hc17) worms. (A) Pharynx-pumping rates were measured at indicated ages in DD- or AL-treated worms maintained at 25 °C. Pumping rates were higher in DD worms than age-matched AL worms (n = 6–12 worms/treatment group). (B) Decline in pharynx-pumping rate during aging relative to day 2 pumping rate. By this analysis, the rate of aging-related decline of pharynx pumping was similar in DD- and AL-treated worms. (C) Spontaneous locomotion in DD- or AL-treated worms. Spontaneous locomotion was measured as the average distance moved within 1 h (n = ~60 worms/treatment). (D) Change in spontaneous locomotion at indicated ages, relative to day 5 locomotion rate. Movement declined similarly in DD- and AL-treated worms. Curves are the results from one representative of three experiments; * P < 0.05; ** P < 0.01; *** P < 0.001.
Dietary deprivation enhances stress resistance
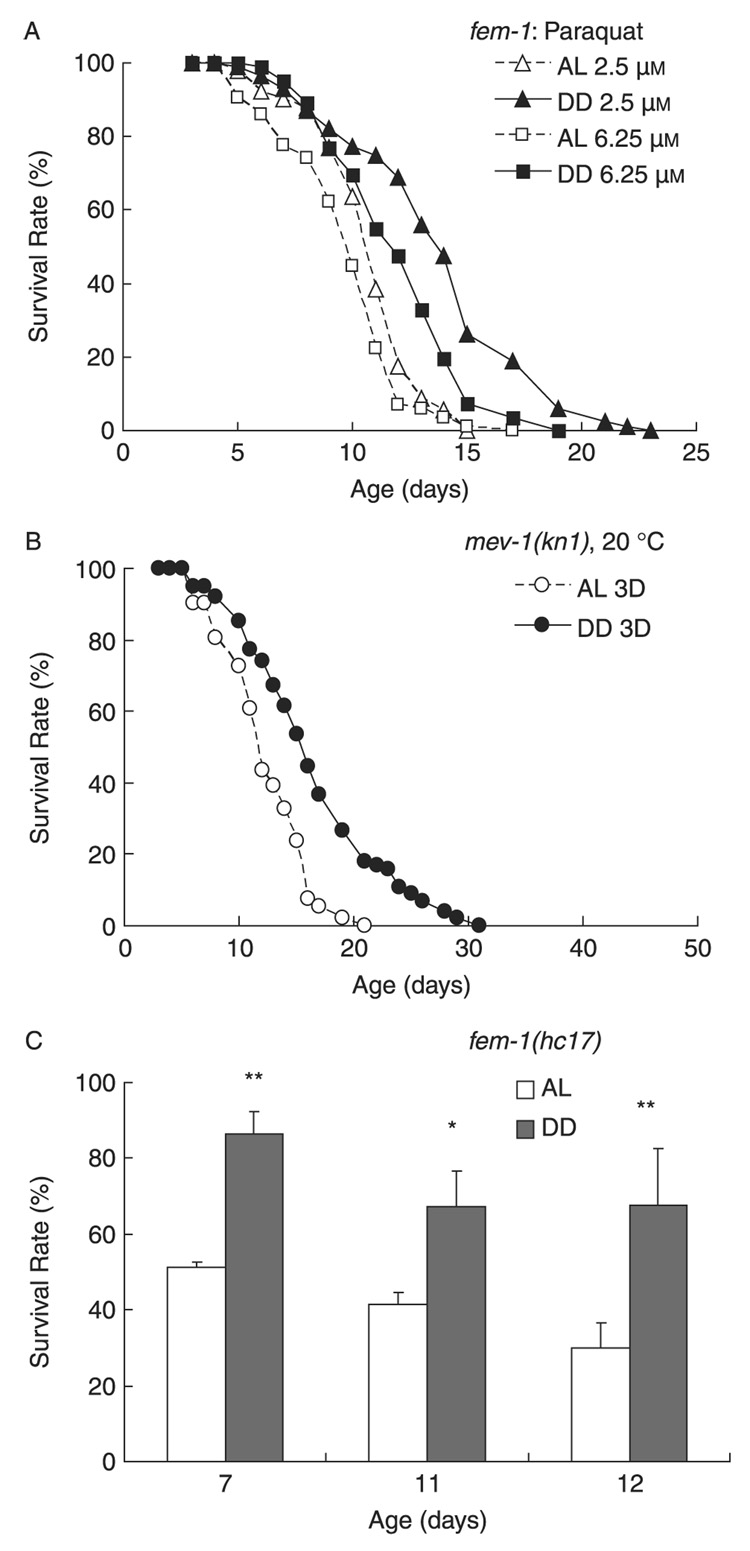
Extension of lifespan by genetic and nongenetic manipulations is often associated with increased resistance to stress (Finkel & Holbrook, 2000). Therefore, we measured resistance to oxidative and thermal stress for worms under DD. DD enhanced resistance to paraquat-induced oxidative stress compared to AL (Fig. 3A). In C. elegans, effects on oxidative stress can also be tested using a genetic mutant strain, mev-1(kn1) (Melov et al., 2000; Wilson et al., 2006). The mev-1(kn1) strain contains a mutant version of mitochondrial succinate dehydrogenase cytochrome b, which is associated with increased superoxide production, oxidative stress, and shortened lifespan (Ishii et al., 1998; Senoo-Matsuda et al., 2001). DD significantly increased mev-1(kn1) adult lifespan (Fig. 3B). This beneficial effect of DD in mev-1(kn1) worms could be due to either increased resistance to oxidative stress, reduced production of free radicals, or through independent effects that are beneficial to mev-1 animals. In addition, DD increased thermotolerance compared to AL in wildtype animals (Fig. 3C).
Fig. 3. Dietary deprivation (DD) enhanced stress resistance.
(A) DD enhanced resistance to oxidative stress from paraquat. Adult day 3 fem-1(hc17) worms were transferred to DD or ad libitum (AL) conditions with 2.5 or 6.25 µm paraquat. DD increased mean and maximum survival on both paraquat concentrations, 2.5 µm paraquat, 29.5% increased mean survival, P < 0.0001; 6.25 µm paraquat, 26.1% increase, P < 0.0001. n = approximately 30 worms tested in triplicate. Curves are created from one representative experiment of two independent experiments. (B) DD increased mean and maximum lifespan of mev-1(kn1) worms, which experienced increased oxidative stress. DD was initiated at adult day 3; increased mean lifespan by 35.7% (P < 0.0001). n = approximately 30 worms tested in triplicate of one experiment. (C) DD enhanced survival of fem-1(hc17) worms under thermal stress. Graph shows fractional survival at indicated ages after 17 h at 35 °C. DD enhanced thermotolerance at adult days 7, 11, and 12 by 68.8% (*P < 0.05), 62.9% (**P < 0.01), and 125% (***P < 0.001), respectively.n = approximately 20 worms/treatment tested in quadruplicate. Curves show results from one representative from three independent experiments.
Dietary deprivation and dietary restriction have overlapping effects
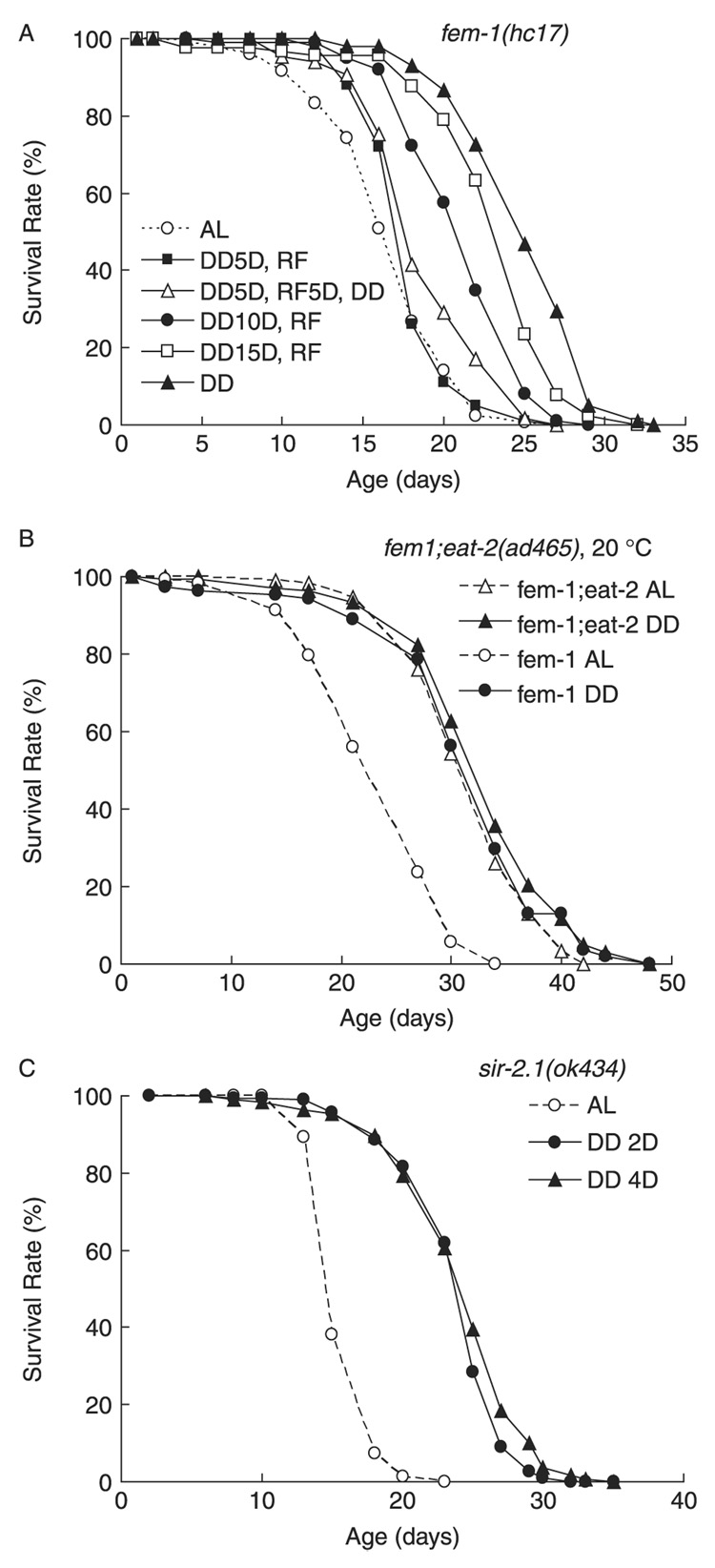
Next we compared DD to other DR regimens. It has been demonstrated that intermittent fasting extends lifespan in rodents onstrated (Goodrick et al., 1982, 1990; Anson et al., 2005). To compare DD to intermittent fasting, we subjected animals to alternating feeding and fasting regimens. We found that strict DD was superior in extending lifespan to any regimen with varied refeeding periods (Fig. 4A). Imposition of DD for only the first 10–15 days of adulthood could prolong lifespan, but to a lesser extent than the strict DD regimen over the adult lifespan. Interestingly, interrupting the DD regimen with AL feeding on adult days 5–10, and reimposing DD after day 10, negated the benefits of the DD regimen. This suggests that early to mid-adulthood is a critical period for DD to induce the full benefit on longevity.
Fig. 4. Effects of re-feeding (RF) and dietary restriction (DR) genetic pathways on dietary deprivation (DD) induced lifespan extension.
(A) DD was introduced at adult day 1 in sterile fem-1(hc17) worms and then either maintained (DD), or worms were transferred to AL conditions after 5 days (DD5D, RF), 10 days (DD10D, RF) or 15 days (DD15D, RF). RF reduced the DD-induced lifespan extension and the severity of reduction correlated with prolonged re-feeding time (1D vs. 5D) of RF (P < 0.0001). n = approximately 30 worms tested in triplicate in one experiment. (B) DD did not lengthen mean or maximum lifespan in slow-feeding fem-1(hc17); eat-2(ad465) worms, which are subject to DR, although DD significantly increased both mean and maximum lifespan in the fem-1(hc17) single mutant by 28.8% (P < 0.0001) and 25.2% (P < 0.01), respectively. Approximately 30 worms/treatment were tested in quadruplicate of one experiment. (C) DD initiated at adult day 2 extended both mean and maximum lifespan in sir-2.1(ok434) worms by 81.8% (P < 0.0001) and 121.8% (P < 0.0001), respectively, compared to AL; n = 30 worms/treatment tested in 5 replicates in one experiment.
To compare DD with genetically imposed DR, we examined the effect of DD on lifespan of fem-1(hc17);eat-2(ad465) animals, which have reduced food intake due to a mutational defect in pharynx pumping (Lakowski & Hekimi, 1998). DD fails to significantly extend lifespan of long-lived fem-1(hc17; eat-2(ad465) adults (Fig 1 and Fig 4C), suggesting that DD and eat-2 may affect longevity through a similar pathway.
We next examined the requirement for sir-2.1, a member of the conserved SIRTUIN family of proteins that regulate responses to DR in many organisms (Tissenbaum & Guarente, 2001; Guarente, 2005; Wang & Tissenbaum, 2006). We found that DD could extend lifespan of sir-2.1(ok434) animals, which lack one of the four C. elegans sirtuins, and the only one that has been implicated in regulating DR-induced longevity in C. elegans (Frye, 2000; Tissenbaum & Guarente, 2001). Together, these results suggest that, although DD prolongs lifespan through DR, as evidenced by lack of effect on long-lived fem-1(hc17);eat-2(ad465) adults, this effect does not apparently require sir-2.1. This may reveal a role for additional genes in mediating DD-induced longevity in C. elegans.
Lifespan extension by dietary deprivation is independent of insulin-like signaling
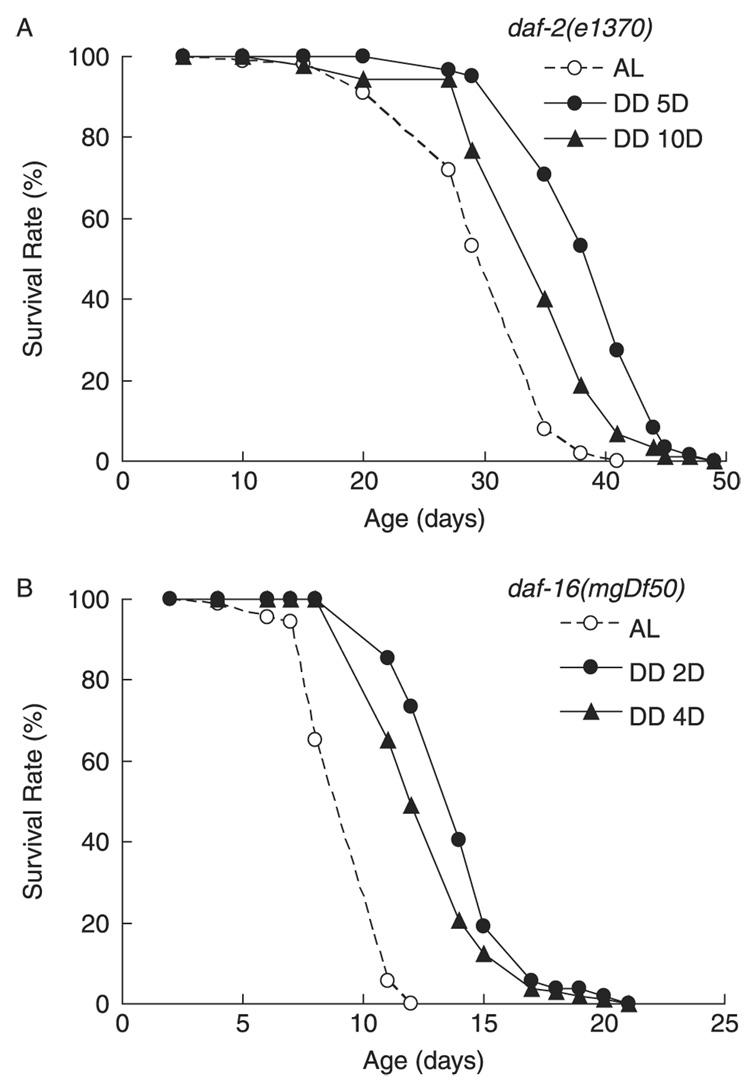
When the food source is scarce, C. elegans larvae can survive for long periods as diapaused dauer larvae (Albert et al., 1981; Riddle et al., 1981). An insulin-like signaling pathway regulates both dauer diapause and lifespan (Kenyon, 2005). To investigate the relationship between insulin-like signaling and DD-induced longevity, we evaluated the effects of DD in long-lived daf-2(e1370) worms, lacking DAF-2 insulin-like receptor activity. We also examined DD in short-lived daf-16(mgDf50) worms, lacking DAF-16/FOXO activity, which is the major downstream target of DAF-2/insulin-like signaling (Lin et al., 1997; Ogg et al., 1997). Mean and maximum lifespans of these two strains were significantly increased by DD (Fig. 5 A,B; Table 2), suggesting that lifespan extension by DD does not depend solely on DAF-2/ insulin-like signaling or DAF-16 and thus probably does not induce a dauer-like state in adult animals.
Fig. 5. Dietary deprivation (DD) increased lifespan of strains with defective insulin-like signaling.
(A) DD initiated at adult day 5 extended both mean and maximum lifespan of daf-2(e1370) worms by 35.2% (P < 0.0001) and 30.7% (P < 0.0001), respectively, compared to ad libitum (AL); n = approximately 30 worms tested in triplicate. Curves are created from one representative experiment of 2 independent experiments. (B) DD initiated at adult day 2 extended both mean and maximum lifespan in daf-16(mgDf50) worms by 62.3% (P < 0.0001) and 64.4% (P < 0.0001), respectively, compared to AL; n = approximately 30 worms tested in quadruplicate in one experiment. DD also extended both mean and maximum lifespan of both strains when initiated at older ages.
Table 2.
Effects of genetic mutations on DD compared to AL-induced longevity
| Lifespan (days) | ||||||
|---|---|---|---|---|---|---|
| Mutant | Initiating age (day) | n | Mean | Change | Maximum | Change |
| mev-1(knl) | ||||||
| AL | 3 | 92 | 11.2 ± 0.4 | 15.0 ± 0.0 | ||
| 5 | 92 | 14.3 ± 0.5 | 19.0 ± 0.0 | |||
| DD | 3 | 101 | 15.2 ± 0.6*** | +35.7% | 22.9 ± 2.0* | +52.7% |
| 5 | 100 | 18.20 ± 0.7*** | +27.3% | 25.9 ± 0.6** | +36.3% | |
| fem-1(hc17) | ||||||
| AL | 1 | 127 | 24.1 ± 0.5 | 30.6 ± 0.7 | ||
| DD | 1 | 88 | 30.8 ± 1.0*** | +28.8% | 38.3 ± 1.4** | +25.2% |
| fem-1(hc17); eat-2(ad465) | ||||||
| AL | 1 | 117 | 32.3 ± 0.5 | 39.3 ± 0.9 | ||
| DD | 1 | 137 | 33.4 ± 0.6 | +3.4% | 40.4 ± 1.2 | +2.8% |
| sir-2.1(ok434) | ||||||
| AL | 2 | 150 | 13.7 ± 0.2 | 15.6 ± 0.6 | ||
| 4 | 144 | 13.1 ± 0.2 | 15.6 ± 0.6 | |||
| DD | 2 | 191 | 24.9 ± 0.9*** | +81.8% | 34.6 ± 7.0* | +121.8% |
| 4 | 192 | 22.9 ± 0.4*** | +74.8% | 27.5 ± 0.7*** | +76.3% | |
| daf-2(e1370) | ||||||
| AL | 5 | 100 | 26.4 ± 0.6 | 30.6 ± 1.6 | ||
| 10 | 73 | 27.3 ± 0.2 | 29.0 ± 0.0 | |||
| DD | 5 | 62 | 35.7 ± 0.7*** | +35.2% | 40.0 ± 0.0* | +30.7% |
| 10 | 90 | 31.1 ± 0.7*** | +13.9% | 39.0 ± 1.0** | +34.5% | |
| daf-16(mgDf50) | ||||||
| AL | 2 | 89 | 7.7 ± 0.1 | 8.0 ± 0.0 | ||
| 6 | 90 | 8.3 ± 0.2 | 9.0 ± 0.8 | |||
| DD | 2 | 109 | 12.5 ± 0.3*** | +62.3% | 15.0 ± 0.0 | +87.5% |
| 6 | 111 | 12.0 ± 0.3*** | +11.6% | 14.8 ± 0.2** | +64.4% | |
Data are represented as mean ± SEM.
P < 0.05
P < 0.01
P < 0.0001 vs. AL counterparts.
P-value of mean lifespan calculated by one-way anova and post hoc Fisher’s test.
P-value of maximum lifespan calculated by Student’s t-test.
AL, ad libitum; DD, dietary deprivation.
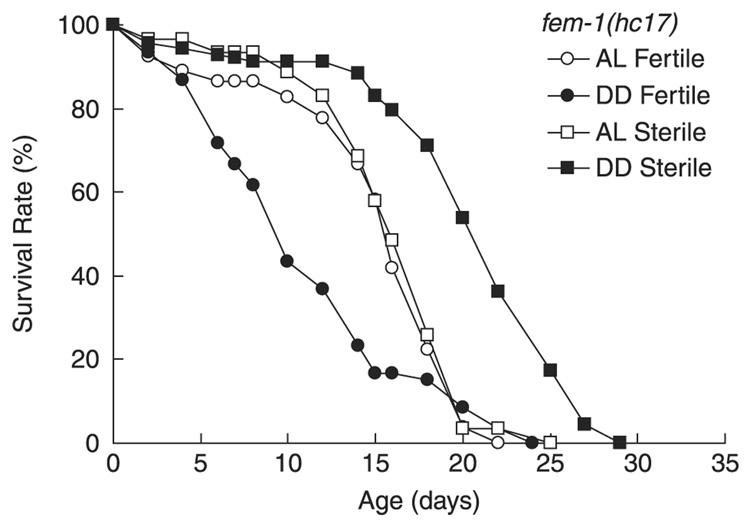
Reproductive status is critical for dietary deprivation to extend lifespan
In adult animals, a major sink for metabolic resources is reproduction. Adult C. elegans nematodes produce between 150 and 250 progeny during 3–5 days of reproduction. Therefore, reproduction constitutes a major metabolic demand that could modulate the effect of a DD regimen on parental lifespan. We evaluated the effect of reproductive status on DD-induced longevity by taking advantage of the temperature sensitive nature of fem-1(hc17) adult sterility (Spence et al., 1990). When cultured at a semipermissive temperature (20 °C), fem-1(hc17) worms develop into semifertile adults. These semifertile fem-1(hc17) adults were then transferred to 25 °C at adult day 0, and DD was imposed at either day 0, 2, or 4. Mean lifespan was dramatically decreased when DD was initiated on adult day 0, when the fertile adults were beginning to lay eggs. However, DD still increased lifespan of fertile fem-1(hc17) adults when imposed on adult day 2 or 4, when egg laying had markedly declined (Fig. 6; Table 1). This observation was confirmed in sir-2.1(ok434) fertile adults, which showed no benefits, or slightly reduced lifespan, when DD was imposed on fertile adult day 0 (Table 3). For the sterile adults raised from eggs grown at restricted temperature (25 °C), DD significantly extended mean and maximum lifespan of these animals when initiated on adult day 0 compared to AL (Fig. 6; Table 3). These findings suggest that DD is detrimental in animals that are capable of reproduction and suggest that DD can extend lifespan only when reproductive function is minimized.
Fig. 6. Role of reproductive status on DD.
(A) Lifespan of semifertile and sterile fem-1(hc17) adults when DD or AL treatments were initiated at adult day 0. When DD was initiated in fertile fem-1(hc17) adults, DD reduced mean lifespan by 29.5% (P < 0.0001) but did not statistically alter the maximum lifespan compared to AL (7.2%); n = approximately 30 worms/treatment were tested in triplicate. Curves are created from one representative experiment of two independent experiments.
Table 3.
Effect of reproduction on DD-induced longevity
| Lifespan (days) | ||||||
|---|---|---|---|---|---|---|
| Genotype | Initiating age (day) | n | mean | Change | Maximum | Change |
| fem-1(hc17) | ||||||
| AL | 0 | 85 | 13.2 ± 0.6 | 18.0 ± 0.0 | ||
| 2 | 80 | 15.2 ± 0.4 | 18.3 ± 0.3 | |||
| 4 | 89 | 14.5 ± 0.4 | 18.0 ± 0.0 | |||
| DD | 0 | 81 | 9.3 ± 0.8*** | −70.5% | 16.7 ± 2.2 | −7.2% |
| 2 | 96 | 20.3 ± 0.5*** | +33.6% | 26.0 ± 0.0*** | +42.1% | |
| 4 | 82 | 17.9 ± 0.7*** | +23.4% | 23.3 ± 1.3* | +29.4% | |
| sir-2.1(ok434) | ||||||
| AL | 0 | 150 | 13.7 ± 0.2 | 15.6 ± 0.6 | ||
| DD | 0 | 160 | 9.0 ± 0.3*** | −34.3% | 14.2 ± 0.5 | −9.0% |
Data are represented as mean ± SEM.
P < 0.05
P < 0.01
P < 0.0001 vs. AL counterparts.
P-value of mean lifespan calculated by one-way anova and post hoc Fisher’s test.
P-value of maximum lifespan calculated by Student’s t-test.
AL, ad libitum; DD, dietary deprivation.
Discussion
Lifespan is modulated by genetic and environmental factors, including diet and metabolic status (Kenyon, 2005; Kirkwood, 2005; Masoro, 2005). Here we have described an effective paradigm to study dietary regulation of longevity. We have demonstrated that removal of food, or dietary deprivation, in nonreproductive adults, is a robust dietary intervention to extend lifespan in C. elegans. The DD regimen offers several advantages to investigate molecular and cellular mechanisms of lifespan regulated by environmental factors. First, it is easy to implement by simply removing the food source, E. coli. This simple procedure minimizes complications due to the uncertainty of food intake in current DR regimens in C. elegans, which include bacterial dilution, axenic media, or utilizing genetic mutants with feeding defects (Houthoofd et al., 2005). It is important to acknowledge for the current DD protocol that we cannot provide conclusive evidence that worms under DD are completely deprived of nutrients since they are maintained on nematode growth medium (NGM) agar, which contains low amounts of soluble protein source (0.25% of Bacto-Pepton) and cholesterol (1 mm). However, it is unlikely that these extremely ). low concentrations of nutrients can have significant impact on food intake. Second, DD is not a strain-specific phenomenon. We showed that DD extended lifespan of a number of mutant strains. In particular, DD increased lifespan of long-lived daf 2-(e1370) animals, as well as two short-lived strains, mev-1(kn1) and daf-16(mgDf50) ). DD elicited a number of beneficial effects, which are commonly associated with longevity, such as enhanced stress resistance (Kenyon, 2005). These findings demonstrate that the DD regimen in C. elegans can be a powerful approach for identifying molecular and cellular mechanisms of lifespan regulation by dietary intake.
In this study, we investigated the genetic requirements for DD-induced longevity. We have found that DD was unable to extend lifespan of a DR mutant fem-1(hc17); eat-2(ad465), which has reduced food consumption, indicating that the effects of DD and DR may have some overlap (Lakowski & Hekimi, 1998). However, DD did extend lifespan of the C. elegans sirtuin mutant, sir-2.1(ok434), suggesting that lifespan extension induced by DD is sir2.1-independent. It has been demonstrated that SIRTUINs are required for DR to extend lifespan in several species ranging from yeast and worms to fruit flies (Tissenbaum & Guarente, 2001; Wood et al, 2004; Guarente, 2005). However, the role of SIRTUINs in DR remains controversial. Sir2, a ever, yeast SIRTUIN, has been shown to mediate DR response in some experimental conditions but not in others (Kaeberlein et al., 2004; Guarente, 2005). The discrepancy has been suggested to be due to differences in strain background, DR protocols, or sirtuin gene redundancy (Guarente, 2005). Considering that DD-induced longevity was independent of sir-2.1, we suggest that sir-2.1 function might be critical for lifespan extension by mild, but not by extreme calorie deprivation. However, further work needs to be done to determine whether the other three C. elegans sirtuins, sir-2.2, −2.3, and −2.4, are also dispensable for DD-induced longevity (Frye, 2000).
We also presented two pieces of evidence showing that DD probably does not increase longevity by inducing a dauer-like state in adults. First, adult worms under DD have higher pharynx-pumping rate and locomotor activity than AL-treated animals. Dauer larvae cease pharynx pumping and have reduced locomotor activity, inconsistent with our observations. Second, motor DD-induced longevity was independent of components of the insulin-like signaling pathway, which are involved in dauer formation (Riddle et al., 1981; Antebi, 2004). This evidence further demonstrates that the worms under DD are not in a dauer-like state, although we cannot exclude that similar downstream genes may be required for DD-induced longevity and dauer longevity.
Reproduction has been shown to be critical in regulating lifespan in C. elegans and Drosophila melanogaster (Partridge & Prowse, 1997; Hsin & Kenyon, 1999; Sgro & Partridge, 1999; Arantes-Oliveira et al., 2002). However, the relationship between reproduction and lifespan is far from clear. For example, in Drosophila melanogaster, dietary deprivation of yeast reduces, both fecundity and lifespan when applied to adults (Good & Tatar, 2001). However, deprivation of yeast only in the third instar larvae does not slow aging, although it reduces fecundity (Tu & Tatar, 2003). DD in C. elegans appeared to be detrimental when imposed on adult day 0, suggesting that the effects of DD could interact with reproduction. It is possible that DD delays aging by shifting energy use from reproduction to somatic maintenance, which is consistent with the disposable soma theory of aging (Drenos & Kirkwood, 2005). This type of energy shift has been suggested to be the mechanism by which DR extends lifespan (Drenos & Kirkwood, 2005; Kirkwood, 2005).
As far as we are aware, our study is the first to report that a multicellular adult organism can survive under long-term food deprivation conditions, which contrasts with the conventional view that DR is beneficial only up to a certain degree of deprivation. This effect may be limited depending on the particular metabolic demands imposed on a particular organism. For example, DD was detrimental in animals during reproductive periods, and starvation is detrimental in organisms with life histories that differ from that of C. elegans (Masoro, 2005; Henderson et al., 2006). Further studies of the DD paradigm in C. elegans should reveal mechanisms and genetic pathways governing longevity under different environmental conditions. Some of the mechanisms should be evolutionarily conserved and therefore applicable to other species.
Experimental procedures
Dietary deprivation assay
Caenorhabditis elegans stocks were obtained from the Caenorhabditis Genetics Center and maintained at 15 °C. For lifespan assays, worms were allowed to produce progeny for 6 h at 25 °C; the progeny developed from the L1 to L4 stage within 48 h (Duhon et al., 1996). We defined day 0 of adulthood as the first day following the L4 molting to adult. After hood being fed with E. coli OP50 during larval development, adult animals were transferred to 3.5 cm Petri dishes with or without a lawn of E. coli on NGM agar, which represented ad libitum (AL) or dietary deprivation (DD), respectively. NGM agar contains Bacto-Peptone (2.5 g L−1), NaCl (3 g L−1), cholesterol (5 mg L−1), CaCl2, (1 mm), MgSO4 (1 mm), KH2PO4 (25 mm, pH 6.0) and agar (21 g L−1). DD and AL plates were pretreated with 0.25 mL of 250 µg mL−1 5-fluoro-2′-deoxyuridine (FUDR) and ampicillin (Walker et al., 2005). The number of dead worms was recorded once every 2 or 3 days by touch and movement analysis. Surviving worms were transferred to fresh plates once every 6–8 days.
Pharynx-pumping rate assay
Pharynx-pumping rates (the number of contractions of the pharynx terminal bulb in 1 min) were assayed at room temperature as previously described with modifications (Huang et al., 2004). For pumping rate assays, worms were allowed to equilibrate to room temperature with light for at least 1 h and pumping rates were measured. This was recorded as the 0-h data point. Then 10–12 worms from each group were transferred to a 6-cm NGM plate with bacteria, and the pumping rate was recorded at 1, 2, and 5 h after transfer. Each assay was performed with at least 6 worms and was repeated at least twice.
Spontaneous locomotion assay
This assay was performed at room temperature as previously described with modifications (Glenn et al., 2004). At adult days 4, 6, and 8, worms subjected to either AL or DD from adult day 1 were washed with 0.5 mL of S-basal buffer containing 250 µg mL−1 of Amp to remove any residual bacteria and air-dried for 1 h on bacteria-free NGM agar plates. Then 20 worms were transferred to the center of a 6-cm NGM bacteria-free plate at room temperature. The distance each worm traveled from the center was measured at 0.5, 1, 2, and 5 h after the transfer by using concentric circles on a transparent film with 1 mm difference in diameter from the center. The average value was calculated based on the total number of worms inside the plate. All experiments were performed with three independent populations.
Oxidative stress assay
Paraquat CL tetrahydrate (Chem Service, West Chester, PA, USA) was dissolved in 5 µg mL−1 FUdR/amp solution to final concentrations of 10 and 20 mm. A 0.25 mL of each paraquat solution was added on the top of 5 mL agar medium in a 3.5-cm Petri dish with or without a lawn of E. coli. Therefore, the final concentrations of paraquat were 2.5 and 6.5 he µm plate plate−1, respectively (Yanase et al., 2002). Adult day 3 worms were then transferred onto the AL or DD plates treated with paraquat. The number of dead worms was counted every other day until all had died.
Thermotolerance assay
At adult day 7, 11, and 12, worms under AL or DD were transferred onto 6 cm Petri dishes containing NGM agar without bacterial lawn. The plates were sealed to maintain moisture and kept at 35 °C. After 17 h, the number of dead worms was recorded after the plates were cooled down to room temperature for 1 h.
Statistical methods
Lifespan data are expressed as mean or maximum ± SEM. Statistical significance was determined either using a one-way analysis of variance (ANOVA) with post hoc Fisher’s test for comparisons of mean lifespan and functional parameters, or using parisons Student’s t-test for comparison of maximum lifespan (defined as 10% survival) with assistance from STATVIEW software (SAS Institute, Inc.). The level of significance was accepted as P < 0.05.
Acknowledgments
We thank the Caenorhabditis Genetics Center for providing strains. This research was supported by the Intramural Research Program of National Institute on Aging at the National Institutes of Health. C.A.W. was also supported by the Ellison Medical Foundation.
References
- Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J. Comp. Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- Anson RM, Jones B, deCabo R. The diet restriction paradigm paradigm: a brief review of the effects of every-other-day feeding. AGE. 2005;27:17–25. doi: 10.1007/s11357-005-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi A. Inside insulin signaling, communication is key to long. Sci. Aging Knowledge Environ. 2004;2004:pe25. doi: 10.1126/sageke.2004.23.pe25. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of lifespan by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Chow DK, Glenn CF, Johnston JL, Goldberg IG, Wolkow CA. Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Exp. Gerontol. 2006;41:252–260. doi: 10.1016/j.exger.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Drenos F, Kirkwood TB. Modelling the disposable soma theory of ageing. Mech. Ageing Dev. 2005;126:103. doi: 10.1016/j.mad.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Duhon SA, Murakami S, Johnson TE. Direct isolation of longevity mutants in the nematode Caenorhabditis elegans. Dev. Genet. 1996;18:144–153. doi: 10.1002/(SICI)1520-6408(1996)18:2<144::AID-DVG7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Porter BD, Wyndham JR. Effects of caloric intake and dietary composition on the development of proteinuria, age-associated renal disease and longevity in the male rat. Gerontology. 1982;28:168–175. doi: 10.1159/000212530. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Frye RA. Biochem. Biophys. Res. Commun. Vol. 273. 2000. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins; pp. 793–798. [DOI] [PubMed] [Google Scholar]
- Glenn CF, Chow DK, David L, Cooke CA, Gami MS, Iser WB, Hanselman KB, Goldberg IG, Wolkow CA. J. Gerontol. A Biol. Sci. Med. Sci. Vol. 59. 2004. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty; pp. 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good TP, Tatar M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. J. Insect Physiol. 2001;47:1467–1473. doi: 10.1016/s0022-1910(01)00138-x. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and lifespan in rats. Gerontology. 1982;28:233–241. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech. Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Guarente L. Calorie restriction and SIR2 genes – towards a mechanism. Mech. Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Bonafe M, Johnson TE. daf-16 protects the nematode ematode Caenorhabditis elegans during food deprivation. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:444–460. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp. Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Lenaerts I, Brys K, De Vreese A, Van Eygen S, Vanfleteren JR. Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends lifespan in Caenorhabditis elegans. Exp. Gerontol. 2002;37:1371–1378. doi: 10.1016/s0531-5565(02)00173-0. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1125–1131. doi: 10.1093/gerona/60.9.1125. [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- Johnson TE. Aging can be genetically dissected into component processes using long-lived lines of Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1987;84:3777–3781. doi: 10.1073/pnas.84.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent lifespan extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild-type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing lifespan. Mech. Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: an HNF-3/forkhead family member that can function to double the lifespan of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on lifespan and mortality rates in female and male Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of lifespan with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Nelson GA, Lew KK, Ward S. Intersex, a temperature-sensitive mutant of the nematode Caenorhabditis elegans. Dev. Biol. 1978;66:386–409. doi: 10.1016/0012-1606(78)90247-6. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in duces C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Partridge L, Prowse N. The effects of reproduction on longevity and fertility in male Drosophila melanogaster. J. Insect Physiol. 1997;43:501–512. doi: 10.1016/s0022-1910(97)00014-0. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H, Hartman PS, Ishii N. A defect in the cytochrome b large zawa subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J. Biol. Chem. 2001;276:41552–41558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation regulation. Mech. Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Spence AM, Coulson A, Hodgkin J. The product of fem-1, a nematode sex-determining gene, contains a motif found in cell cycle control proteins and receptors for cell–cell interactions. Cell. 1990;60:981–990. doi: 10.1016/0092-8674(90)90346-g. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech. Ageing Dev. 2005;126:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect lifespan. Mech. Ageing Dev. 2002;123:1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]