Abstract
Background
Decoy receptor 3 (DcR3) is a soluble protein that binds to and inactivates the death ligand CD95L. Here, we studied a possible association between DcR3 expression and prognosis in patients with renal cell carcinomas (RCCs).
Methods
A tissue microarray containing RCC tumor tissue samples and corresponding normal tissue samples was generated. Decoy receptor 3 expression in tumors of 560 patients was examined by immunohistochemistry. The effect of DcR3 expression on disease-specific survival and progression-free survival was assessed using univariate analysis and multivariate Cox regression analysis. Decoy receptor 3 serum levels were determined by ELISA.
Findings
High DcR3 expression was associated with high-grade (P = .005) and high-stage (P = .048) RCCs. The incidence of distant metastasis (P = .03) and lymph node metastasis (P = .002) was significantly higher in the group with high DcR3 expression. Decoy receptor 3 expression correlated negatively with disease-specific survival (P < .001) and progression-free survival (P < .001) in univariate analyses. A multivariate Cox regression analysis retained DcR3 expression as an independent prognostic factor that outperformed the Karnofsky performance status. In patients with high-stage RCCs expressing DcR3, the 2-year survival probability was 25%, whereas in patients with DcR3-negative tumors, the survival probability was 65% (P < .001). Moreover, DcR3 serum levels were significantly higher in patients with high-stage localized disease (P = .007) and metastatic disease (P = .001).
Interpretation
DcR3 expression is an independent prognostic factor of RCC progression and mortality. Therefore, the assessment of DcR3 expression levels offers valuable prognostic information that could be used to select patients for adjuvant therapy studies.
Introduction
Renal cell carcinoma (RCC), the most lethal of all urological cancers, account for approximately 3% of all adult malignancies [1]. The factors used to assess the prognosis of patients are mainly based on the TNM (tumor, node, metastasis) staging system from the American Joint Committee on Cancer (AJCC)/Union Internationale Contre le Cancer (UICC) [2]. However, there is still an urgent need to identify more refined markers, which will allow to anticipate the clinical outcome in RCCs and which could further serve as targets for novel therapies. Given the paramount role of antiapoptotic proteins in the development and progression of malignant tumors, apoptosis-related proteins are promising candidates in the search for new tumor markers. The death receptor CD95 and its ligand, CD95L, play important roles in the evolution and therapy resistance in RCCs. The CD95/CD95L system is variably expressed in RCCs and is described to mediate the host cytotoxic T cell-based antitumoral immune response as well as the cytotoxic actions of an interleukin (IL) 12/IL-2 therapy [3,4]. Therefore, defects in the CD95 receptor/ligand system are believed to contribute to the resistance against cytotoxic T cells and against biologic therapies of RCC cells.
The decoy receptor 3 (DcR3, also known as TR6 and M68), a soluble protein that interacts with CD95L, LIGHT, and TNF-like molecule 1A (TL1A), is overexpressed in many types of malignancies, e.g., brain tumors, colon carcinoma, and lung cancer [5–8]. Decoy receptor 3 expression has been suggested as a potential tumor marker for the early detection of gastric carcinoma and ovarian cancer [9,10]. However, a systematic study on DcR3 expression in RCCs has not been published to date.
Patients and Methods
Patients
Tissue samples from 838 patients with primary RCC treated at the Department of Urology at the University of Heidelberg between 1987 and 2005 were collected. The human tissue samples were provided by the Tumor Tissue Bank of the National Center for Tumor Diseases Heidelberg after approval by the ethics committee of the University of Heidelberg. Clinical follow-up was available for all cases. Patients were prospectively evaluated every 3 months for the first 2 years after treatment, every 6 months for the next 3 years, and yearly thereafter [chest x-ray or thoracic computed tomographic (CT) scan; abdominal sonography or CT scan or magnetic resonance imaging; serum chemistry]. No adjuvant treatment of localized disease was administered. Patients with metastasized disease and with a Karnofsky performance index of ≥80 and no medical contraindications received palliative interferon-alpha- and IL-2-based immunotherapy. No targeted therapeutic approaches were performed. Survival was calculated from the date of nephrectomy until last visit or death. All tissue samples were reviewed by experienced pathologists. The tumors were graded according to the four-tiered nuclear grading system [11] and pathologically staged based on the TNM classification (2002).
Tissue Micro Arrays
A tissue microarray containing 838 primary tumor and corresponding normal tissue samples of 838 patients was created. First, representative tissue blocks were selected as donor blocks for the tissue microarray. Sections were cut from each donor block and stained with hematoxylin and eosin. Then, a morphologically representative region was chosen from each of the RCC and normal renal tissue samples. Two cylindrical core tissue specimens per tumor block (diameter, 0.6 mm) were punched from these regions and arrayed into the recipient paraffin block using a semiautomatic system (Beecher Instruments, Silver Spring, MD). In total, 19 tissue arrays were generated, each containing 200 core tissue specimens, matching 50 patients per array.
Immunohistochemistry
The tissue microarray slides were dewaxed and rehydrated using xylene and a series of graded alcohols, followed by heat-induced antigen retrieval using a target retrieval solution (S2031; DakoCytomation, Glostrup, Denmark) in a pressure cooker for 10 minutes. Staining was performed using an automated staining system (Techmate 500; DakoCytomation) with anti-DcR3 antibody (0.01 mg/ml, RD-1257, clone MD3B1; BioVendor, Heidelberg, Germany) or anti-active caspase 3 antibody, clone C92-605 (5 µg/ml; BD Biosciences, San Jose, CA) for 30 minutes, and avidin-biotin complex peroxidase technique using aminoethylcarbazole for visualization and hematoxylin for counterstaining. In accordance with the manufacturers' instructions, the following solutions were used: ChemMate Detection Kit [K5003 (DakoCytomation) containing Dako REAL Link, ready-to-use biotinylated goat antimouse and antirabbit immunoglobulins, and Dako REAL AEC/H2O2 Substrate Solution], ChemMate Buffer Kit (K5006; DakoCytomation), and for the reduction of nonspecific avidin/biotin-related staining Avidin/Biotin Blocking Kit (SP-2001; Vector Laboratories, Burlingame, CA). Specificity controls for the anti-DcR3 antibody included colon carcinoma cells stably transfected with a DcR3 expression plasmid. As a negative control for the immunohistochemical staining procedure, the primary antibody was omitted or an isotype control antibody (IgG1) was used, with all other experimental conditions kept constant.
For the immunohistochemical semiquantitative assessment of DcR3 expression, the product of the scores of staining intensity and quantity of immunoreactive tumor cells was calculated based on the following scoring system: the intensity ranged from 0 = negative, 1 = low, 2 = medium to 3 = high; the quantity comprised 0 = no expression, 1 = positivity in less than 1%, 2 = positivity in 1% to 9%, 3 = positivity in 10% to 50%, and 4 = positivity in more than 50%. The final immunohistochemical score (IHS; ranging from 0 to 12) is obtained by multiplication of the intensity score and the quantity score. The cutoff IHS for DcR3 expression was determined after graphically depicting the survival curves of each of the scores separately. This analysis allowed a visual demonstration of how continuous the association was between DcR3 expression and survival time of patients. In the case of DcR3, a distinct gap in the survival curves was observed between the IHSs <6 and ≥6 (Figure W1). This cutoff value was used in all univariate and multivariate analyses. The arrays were independently scored by two pathologists (S.M.-G. and W.R.) blinded to tissue annotations and patient outcomes. In the few instances of discrepant scoring, a consensus score was determined. Only cases with two properly stained tumor tissue specimens (duplicates) were included in the subsequent analyses. The discordance rates between the two cores regarding the IHS were as follows: in 64% of cases, an identical IHS was obtained; in 31% of cases, the discordance was ≤4 IHS units; and in 5% of cases, the discordance was >4 IHS units. In case of discordance, the average of the two IHS was used for further analysis.
Enzyme-Linked Immunosorbent Assay
The level of DcR3 in human serum was measured by quantitative ELISA specific for human soluble DcR3 [12]. Briefly, 100 µl of samples (50 µl of serum plus 50 µl of PBS) were added in duplicate to wells of 96-well ELISA plates that were coated with MD3E2 capture monoclonal antibody and blocked with 3% BSA. The standard DcR3 protein, twofold serially diluted in 3% BSA, was also added in duplicate to wells. Plates were incubated overnight at 4°C. After washing, biotinylated MD3B1 detection monoclonal antibody was incubated to wells for 2 hours at room temperature. After washing, peroxidase-conjugated streptavidin (Vector Laboratories) was added to the wells for 1 hour at room temperature. Color was developed by adding TMB peroxidase substrate solution (KPL, Gaithersburg, MD). After stopping the color reaction with 1 N H2SO4, plates were read at an absorbance of 450 nm in an Emax ELISA plate reader. Decoy receptor 3 levels were calculated in reference to the standard curve (a linear graph with range from 12.5 ng/ml to 12 pg/ml and R2 = 0.999) generated by the SOFTmax PRO 4.3.1 LS computer program accompanying the plate reader. Both the plate reader and the program were from Molecular Device (Sunnyvale, CA).
Fluorescence In Situ Hybridization
A DNA probe containing the human DcR3 gene was constructed from BAC clones RP4-583P15 and CTD-3184A7 (German Resource Center for Genome Research, Berlin, Germany) and labeled using the ALEXA 488 ULS kit (Invitrogen, Freiburg, Germany). Deparaffinized slides containing RCC tissue were subjected to heat pretreatment in 10 mM Na-citrate, pH 8 in a microwave oven (800 W for 10 minutes), allowed to cool down for 30 minutes, and digested using 0.005% pepsin (Sigma, Taufkirchen, Germany) for 20 minutes at 37°C. After rinsing in 2x SSC, slides were postfixed in 4% paraformaldehyde, briefly rinsed in 2x SSC and H2O, and air-dried. Tissue and DNA probes were codenatured in an in situ thermocycler (Perkin Elmer, Darmstadt, Germany) at 85°C for 5 minutes, cooled down to 37°C, and hybridized in a humid chamber overnight. After posthybridization washing in 2x SSC, 0.3% NP-40 (at 75°C for 2 minutes), slides were briefly rinsed in H2O and coverslipped using fluorescence mounting medium containing di-amidino-phenyl-indole (MP, Strasbourg, France).
Statistical Methods
Data were analyzed using the R software package (version 2.5.1, http://www.rproject.org). For count data, Fisher's exact test (two-sided) was used. The Kaplan-Meier method was applied to calculate survival probabilities for both progression-free and cancer-specific overall survival. The cuminc function in the R package cmprsk was used to perform a competing risk analysis. For univariate and multivariate analyses, the Cox proportional hazards regression model was used. Internal validation was performed using 1000 bootstrap resamples. Univariate survival data were tested for significance using the Mantel-Haenszel log rank test. The Wilcoxon rank sum test was used to compare DcR3 serum concentrations or the percentages of apoptotic cells with DcR3 IHSs. P < .05 were considered significant.
Results
To identify prognostic markers for RCCs in a large cohort of patients, we constructed a tissue microarray containing approximately 3500 tissue samples. Tumor tissue and corresponding normal renal tissue samples were used from 838 patients with RCCs. Decoy receptor 3 expression was analyzed using a commercially available mouse monoclonal antihuman DcR3 antibody. A total of 560 cases was successfully scored for DcR3 expression in two different areas of the tumor. The remaining 278 cases with insufficient tumor tissue or fixation artifacts were excluded from further analyses. The median follow-up time was 40 months (mean, 55 months). At the last follow- up, of 560 patients, 182 (33%) had died of RCC, 52 (9%) had died of unrelated causes, and 326 (58%) are still alive. Table 1 provides a summary of the clinical and pathologic features.
Table 1.
Summary of Clinical and Pathologic Features.
| Feature | Number of Patients | % of Patients |
| Sex | ||
| Male | 348 | 62 |
| Female | 212 | 38 |
| Age at surgery (median, 63 years; range, 25–89 years) | ||
| <65 years | 310 | 55 |
| ≥65 years | 250 | 45 |
| Karnofsky performance status scale | ||
| ≥80% | 512 | 91 |
| <80% | 48 | 9 |
| Tumor extent (TNM 2002) | ||
| pT1 | 304 | 54 |
| pT2 | 59 | 11 |
| pT3 | 186 | 33 |
| pT4 | 11 | 2 |
| Regional lymph node metastasis (TNM 2002) | ||
| N0/pN0 | 519 | 93 |
| pN1, pN2 | 41 | 7 |
| Distant metastasis (TNM 2002) | ||
| Yes | 87 | 16 |
| No | 473 | 84 |
| Grade of malignancy* | ||
| G1 | 146 | 26 |
| G2 | 321 | 58 |
| G3 | 87 | 16 |
| G4 | 3 | <1 |
| Histopathologic subtype | ||
| Clear-cell (conventional) RCC | 464 | 83 |
| Papillary (chromophil) RCC | 48 | 9 |
| Chromophobe RCC | 25 | 4 |
| Spindle cell carcinoma | 5 | <1 |
| Collecting duct carcinoma | 2 | <1 |
| Unclassified RCC | 16 | 3 |
| Type of surgery | ||
| Partial nephrectomy | 81 | 14 |
| Radical nephrectomy | 479 | 86 |
| Treatment for metastasized disease† | ||
| Yes | 25 | 29 |
| No | 62 | 71 |
Three patients with tumors of unknown grade.
Interferon-alpha. and IL-2.based immunotherapy.
DcR3 Expression Assessed by Immunohistochemistry
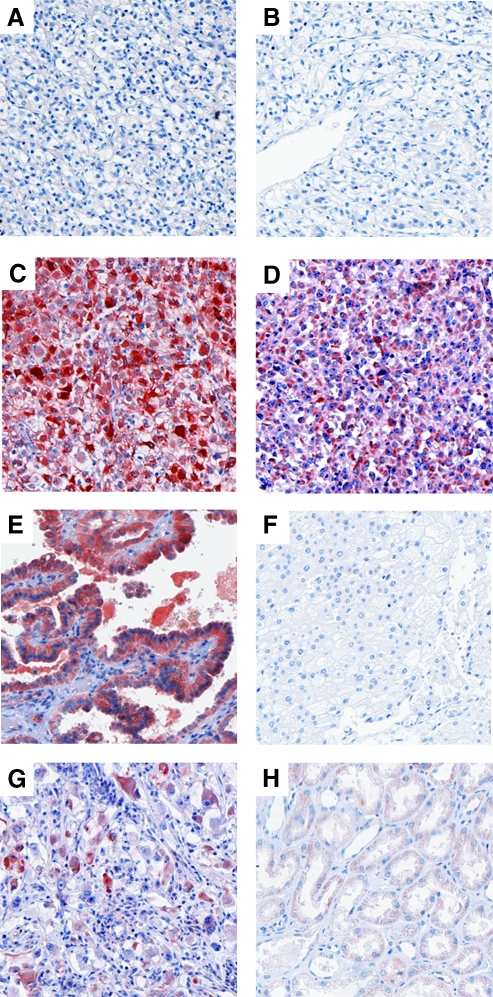
High DcR3 expression (defined as an IHS ≥6) was determined as the cutoff level after graphically depicting the survival curves of each of the scores separately (see Patients and Methods). High DcR3 expression was observed in 52 patients (9.3%). The subcellular expression pattern of DcR3 was mainly diffusely cytoplasmic (Figure 1, A–G). The normal renal tissue was negative or weakly positive for DcR3 (Figure 1H). No significant association was observed between DcR3 expression and the presence of apoptotic tumor cells as assessed by immunohistochemical detection of active caspase 3 (Figure W2). Using Fisher's exact tests, high DcR3 expression levels were significantly associated with tumor size, regional lymph node metastasis, distant metastasis, and grade of malignancy (Table 2). Further, DcR3-positive RCCs occurred significantly more frequently in male patients. In contrast, there was no consistent relationship between DcR3 expression and Karnofsky performance status or histopathologic subtype of the RCCs. Similarly, an association between age at surgery and DcR3 expression was not observed using the Wilcoxon rank sum test (P = .9; high DcR3: median age 62 years, range 36–79 years; low DcR3: median age 63 years, range 25–89 years). In the subgroup of patients without distant metastasis, DcR3 expression was associated with regional lymph node metastasis and sex. No significant associations were obtained in the subgroup of patients with distant metastasis, possibly due to the limited number of patients (n = 87).
Figure 1.
Immunohistochemical detection of DcR3 protein in RCCs of diverse histopathologic subtypes (original magnification, x100). (A–D) Clear-cell (conventional) RCCs with different grades of malignancy (A: G1, B: G2, C: G3, D: G4); (E) papillary (chromophil) RCC; (F) chromophobe RCC; (G) collecting duct carcinoma; (H) normal tubular renal tissue. The IHSs were as follows: A, 0; B, 0; C, 12; D, 12; E, 12; F, 0; G, 9.
Table 2.
Comparison of DcR3 Expression Levels and Clinical and Pathologic Features.
| Feature | All Patients | Without Distant Metastasis (M0) | With Distant Metastasis (M1) | ||||||||||||
| Low (n = 508, 91%) | High (n = 52, 9%) | P | Low (n = 435, 92%) | High (n = 38, 8%) | P | Low (n = 73, 84%) | High (n = 14, 16%) | P | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Sex | .004 | .015 | .5 | ||||||||||||
| Male | 306 | 55 | 42 | 8 | 253 | 53 | 30 | 6 | 53 | 61 | 12 | 14 | |||
| Female | 202 | 36 | 10 | 2 | 182 | 39 | 8 | 2 | 20 | 23 | 2 | 2 | |||
| Age at surgery | 1 | .9 | |||||||||||||
| <65 years | 281 | 50 | 29 | 5 | 231 | 49 | 21 | 4 | 50 | 57 | 8 | 9 | .5 | ||
| ≥65 years | 227 | 41 | 23 | 4 | 204 | 43 | 17 | 4 | 23 | 26 | 6 | 7 | |||
| Karnofsky performance status scale | .6 | 1 | .4 | ||||||||||||
| ≥80% | 463 | 83 | 49 | 9 | 400 | 85 | 35 | 7 | 63 | 73 | 14 | 16 | |||
| <80% | 45 | 8 | 3 | 1 | 35 | 7 | 3 | 1 | 10 | 11 | 0 | 0 | |||
| Tumor extent* | .048 | .1 | .8 | ||||||||||||
| pT1/2 | 336 | 60 | 27 | 5 | 306 | 65 | 22 | 5 | 30 | 35 | 5 | 6 | |||
| pT3/4 | 172 | 31 | 25 | 4 | 129 | 27 | 16 | 3 | 43 | 49 | 9 | 10 | |||
| Regional lymph node metastasis* | .002 | .02 | .2 | ||||||||||||
| N0/pN0 | 477 | 84 | 42 | 8 | 418 | 88 | 33 | 7 | 59 | 68 | 9 | 10 | |||
| pN1, pN2 | 31 | 6 | 10 | 2 | 17 | 4 | 5 | 1 | 14 | 16 | 5 | 6 | |||
| Distant metastasis* | .03 | - | - | ||||||||||||
| No | 435 | 78 | 38 | 7 | - | - | - | - | - | - | - | - | |||
| Yes | 73 | 13 | 14 | 3 | - | - | - | - | - | - | - | - | |||
| Grade of malignancy† | .005 | .2 | .1 | ||||||||||||
| G1/2 | 430 | 77 | 35 | 6 | 386 | 82 | 30 | 7 | 44 | 51 | 5 | 6 | |||
| G3/4 | 76 | 14 | 16 | 3 | 47 | 10 | 7 | 1 | 29 | 33 | 9 | 10 | |||
| Histopathologic subtype‡ | .1 | .8 | .06 | ||||||||||||
| Clear-cell RCC | 425 | 76 | 39 | 7 | 362 | 76 | 30 | 6 | 63 | 73 | 9 | 11 | |||
| Papillary RCC | 42 | 8 | 6 | 1 | 36 | 8 | 5 | 1 | 6 | 7 | 1 | 1 | |||
| Chromophobe RCC | 23 | 4 | 2 | <1 | 23 | 5 | 1 | <1 | 0 | 0 | 1 | 1 | |||
| Spindle cell carcinoma | 3 | 1 | 2 | <1 | 1 | <1 | 0 | 0 | 2 | 2 | 2 | 2 | |||
| Collecting duct carcinoma | 0 | 0 | 2 | <1 | 0 | 0 | 1 | <1 | 0 | 0 | 1 | 1 | |||
| Unclassified RCC | 15 | 3 | 1 | <1 | 13 | 3 | 1 | <1 | 2 | 2 | 0 | 0 | |||
| Type of surgery | .7 | .7 | .2 | ||||||||||||
| Radical nephrectomy | 433 | 77 | 46 | 8 | 360 | 76 | 33 | 7 | 73 | 84 | 13 | 15 | |||
| Partial nephrectomy | 75 | 14 | 6 | 1 | 75 | 16 | 5 | 1 | 0 | 0 | 1 | 1 | |||
P values < .05 are shown in bold.
TNM 2002.
Three patients with tumors of unknown grade.
Clear-cell (conventional) RCC versus other types.
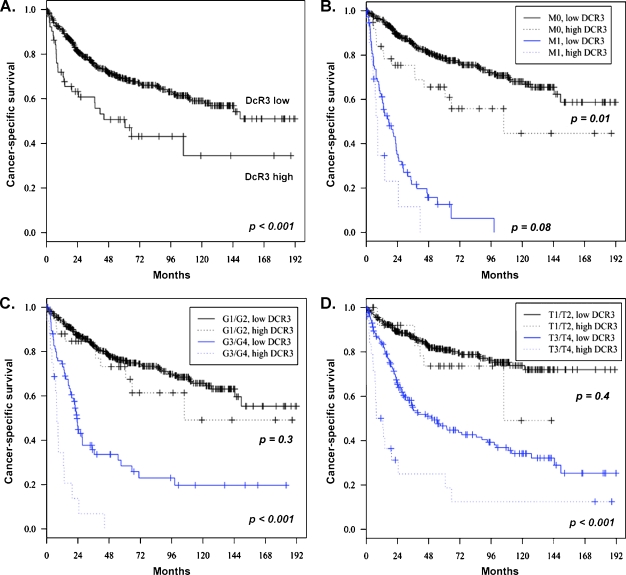
Univariate survival analyses showed that high DcR3 expression is associated with the risk of death from RCC both in the whole patient cohort and in the group of patients with nonmetastasized disease (M0; Figure 2, A and B). In metastatic disease (M1), a trend toward shorter survival times (P = .08) was observed (Figure 2B). Similarly, DcR3 was a significant prognostic factor for tumor-specific survival in the subgroups of patients with high-grade (G3/G4) RCCs (Figure 2C) and high-stage (T3/T4) RCCs (Figure 2D). In contrast, DcR3 expression was not significantly associated with the cancerspecific outcome in G1/G2 or in T1/T2 RCCs (P = .3 and P = .4, respectively). Moreover, high DcR3 expression increased the risk of RCC progression (n = 206) as defined by the first occurrence of either lymph node metastasis, distant metastasis, local tumor recurrence, or death due to RCC (Figure W3). The hazard ratio of the univariate Cox regression analyses was 2.1. To internally validate the data, we performed bootstrapping analysis by resampling the study cohort 1000 times with replacement (hazard ratio: mean, 2.1; SD, 0.49). Further, to confirm that the Kaplan-Meier method is applicable to our setting, we performed a competing risk analysis. Cumulative incidence curves for high and low DcR3 expression were significantly different for tumor-specific mortality (P < .001), whereas a significant difference was not observed regarding death due to tumor-unrelated causes (P = .4).
Figure 2.
Analysis of cancer-specific survival depending on DcR3 expression levels. (A) All patients (n = 560). (B) Patients with nonmetastatic disease (M0, n = 473) versus metastatic disease (M1, n = 87). (C) Patients with high-grade RCCs (G3/G4, n = 92) versus low-grade RCCs (G1/G2, n = 465). (D) Patients with extended primary tumor (T3/T4, n = 197) versus limited primary tumor (T1/T2, n = 363).
Next, we investigated the impact of DcR3 expression on the RCC-related cancer-specific survival and the progression-free survival by multivariate analysis. Multivariate Cox regression analysis on RCC outcome included DcR3 expression, Karnofsky performance status, tumor size, regional lymph node metastasis, distant metastasis, grade of malignancy, histopathologic subtype, type of surgery, and sex. This analysis revealed that DcR3 status is an independent prognostic factor for both cancer-specific survival (Table 3) and progression-free survival (Table W1). Apart from DcR3, tumor extent, metastatic disease, malignancy grade, and gender emerged as significant prognostic factors, whereas the Karnofsky status, histopathologic subtype, and type of surgery were not correlated with the clinical outcome. Moreover, we performed multivariate Cox regression analyses in the subgroups of patients with or without distant metastasis. Decoy receptor 3 expression was an independent prognostic factor for progression-free survival in nonmetastasized disease (P = .04) and showed a trend toward a shorter cancer-specific survival in metastatic disease (P = .07; Table W2).
Table 3.
Multivariate Cox Regression Analysis of DcR3 Expression and Clinical/Pathologic Features for the Prediction of Cancer Specific Survival.
| Feature | Multivariate Cox Regression Analysis* | ||
| Hazard Ratio | 95% CI | P | |
| DcR3 expression† | 1.8 | 1.2–2.8 | .006 |
| Karnofsky performance status‡ | 1.5 | 1.0–2.3 | .09 |
| Tumor extent§ | 2.0 | 1.5–3.0 | <.001 |
| Regional lymph node metastasis¶ | 1.4 | 0.9–2.2 | .1 |
| Distant metastasis# | 5.0 | 3.5–7.1 | <.001 |
| Grade of malignancy** | 1.8 | 1.3–2.6 | <.001 |
| Histopathologic subtype†† | 1.1 | 0.7–1.7 | .7 |
| Type of surgery‡‡ | 2.1 | 1.0–4.6 | .06 |
| Sex§§ | 0.7 | 0.5–0.9 | .02 |
CI indicates confidence interval.
Three patients with tumors of unknown grade of malignancy were excluded.
Immunohistochemical score ≥6 versus <6.
<80% versus ≥80%.
pT3/pT4 versus pT1/pT2.
pN1/pN2 versus N0/pN0.
M1/pM1 versus M0/pM0.
G3/G4 versus G1/G2.
Clear-cell (conventional) RCC versus other types.
Radical versus partial nephrectomy.
Female versus male.
DcR3 Expression Assessed by ELISA and FISH
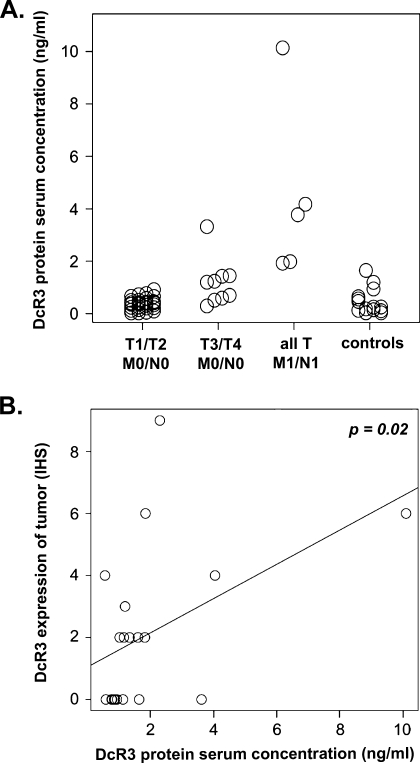
Further, we investigated serum DcR3 protein levels in an independent cohort of RCC patients by ELISA. Decoy receptor 3 serum levels ranged between 0.03 and 1.65 in normal controls (n = 15) and between 0.03 and 10.14 in patients with RCCs (n = 42). Serum DcR3 levels were significantly elevated in patients with high-stage, nonmetastatic RCCs and in patients with metastasized RCCs compared to controls (Figure 3A). In contrast, no significant difference was observed between normal controls and low-stage, nonmetastatic RCCs. Moreover, high DcR3 serum levels were significantly associated with high DcR3 expression in the tumor tissue as assessed by immunohistochemistry (P = .02; Figure 3B).
Figure 3.
(A) Comparison of DcR3 serum levels in patients (n = 42; for clinical and pathologic features, see Table W3) with low stage localized disease, high-stage localized disease, metastatic disease, and in normal individuals. Compared to controls, DcR3 levels were significantly higher in patients with high-stage localized disease (P = .007) as well as metastasized disease (P = .001). In contrast, DcR3 serum concentrations were not significantly higher in patients with low-stage localized disease (P = .7) compared to healthy control individuals. (B) Comparison between DcR3 serum concentrations and DcR3 expression in RCCs (n = 20; for clinical and pathologic features, see Table W4). The DcR3 expression levels are presented as IHSs.
To investigate the mechanisms of DcR3 overexpression in RCCs, we assessed the DcR3 gene copy number in 20 RCC samples (high DcR3 expression, n = 10; low DcR3 expression, n = 10) by fluorescence in situ hybridization. In these 20 tumors, DcR3 gene amplifications were not observed.
Discussion
Renal cell carcinoma cells are known to be largely multidrug-resistant. Apart from effective drug detoxification mechanisms and up-regulated expression of P-glycoprotein, RCC cells exhibit a pronounced resistance to CD95-mediated apoptosis, although they constitutively express CD95 and CD95L [13–15]. At the same time, CD95/CD95L interactions might constitute a key mechanism in the process of cytotoxic T lymphocytes killing RCC cells [4]. An elevated expression of the soluble decoy receptor for CD95L, DcR3, may contribute to the resistance of RCC cells to death ligand-induced apoptosis. Decoy receptor 3 is a 35-kDa protein that lacks a transmembrane domain and that is secreted into the extracellular space. Decoy receptor 3 binds to the ligands CD95L and LIGHT, thereby neutralizing their proapoptotic actions [5,7]. Importantly, DcR3 is frequently overexpressed in cancer, e.g., in malignant tumors of the brain, lung, and gastrointestinal tract [5,6,16–22]. Thus, it has been postulated that the activation of the CD95/CD95L system limits cancer growth and, consequently, that cells expressing higher levels of DcR3 are more likely to escape elimination. Decoy receptor 3 was prescribed an immunosuppressive role in diverse experimental models, e.g., in DcR3 transgenic mice exhibiting an attenuation of the TH1 response and a suppression of cell-mediated immunity [23]. Moreover, DcR3 inhibits T cell chemotaxis, down-regulates cytotoxic T lymphocyte activity in vitro and graft-versus-host responses in mice, and modulates dendritic cell differentiation and survival [24–28].
The suppression of immune responses by the above-mentioned mechanisms as well as by counteracting CD95L-mediated activation of apoptosis might play an important role in the evolution of RCCs. Therefore, we examined DcR3 expression in a large collection of human RCC samples by immunohistochemistry. In this study, we show that DcR3 expression in RCCs correlates significantly with the clinical outcome. In multivariate analyses including other prognostic factors (such as Karnofsky performance status, tumor size, distant metastasis, and grade of malignancy), DcR3 expression was prognostic for cancer-specific survival and progression-free survival. Further, DcR3 expression outperformed the Karnofsky performance status as a prognostic marker.
In addition to the tumor size and the presence of distant metastasis (T and M within the TNM staging system), the Karnofsky performance status score, grade of malignancy, sex, and microscopic tumor necrosis are considered to be prognostic markers in RCCs [29,30]. Moreover, many additional molecular and biochemical tumor markers have been tested in RCCs, including lactate dehydrogenase, serum calcium, ferritin, p53, IL-2, and others [31–33]. Nevertheless, more accurate and valid markers are urgently needed to allow an optimal patient selection for experimental adjuvant treatment strategies and better follow-up planning. Our results suggest DcR3 as a novel independent marker of RCC outcome. Interestingly, the apoptotic index (i.e., the percentage of apoptotic tumor cells) was reported to be an independent prognostic marker for overall survival in patients with RCCs [34]. Given the great importance of the CD95 system in the development of malignant tumors, a functional link between low apoptosis rates as well as high DcR3 expression in RCCs and poor clinical prognosis should be considered. However, we could not confirm an association between the presence of apoptotic tumor cells and the expression levels of DcR3 in RCCs. Because apoptosis is a rapidly executed process and only few cells might display features of apoptosis (such as activation of caspase 3) at a given time point, our results do not necessarily exclude a functional connection between DcR3 and inhibition of apoptosis in RCCs. Alternatively, CD95-independent immunosuppressive mechanisms triggered by DcR3 might play a role, such as suppression of CD8 T cell function by neutralization of LIGHT [35] or increased angiogenesis by neutralization of TL1A [36].
Further, the present study demonstrates a higher expression of DcR3 in male than in female patients. Although a gender-specific expression of DcR3 has not yet been reported, it is well known that the expression of several apoptosis-related proteins is regulated by hormone receptors. For example, the antiapoptotic proteins FLIP and protein kinase C delta are induced by androgen receptors [37,38]. Interestingly, androgen receptors are reported to be expressed in a subset of RCCs [39]. Thus, a possible androgen-dependent regulation of DcR3 expression should further be investigated.
The prognostic value of DcR3 expression might be specific for RCCs, because studies on urothelial carcinomas of the bladder/ureter as well as on colon carcinomas did not show a significant correlation between DcR3 expression and patient outcome [40,41]. However, the latter study found that amplification of the DcR3 gene was a predictive marker for the efficacy of a 5-fluorouracil-based adjuvant chemotherapy for colon carcinomas [41]. With DcR3 serving as a prognostic factor for RCC outcome, further investigations on the impact of DcR3 expression on the success of novel treatment strategies (e.g., targeted therapies) for RCCs are warranted.
Amplification of the DcR3 gene was described in several types of tumors, such as lung cancer, glioblastomas, gastric carcinomas, and colorectal cancer [5,9,17,41]. In other tumor types, a genomic amplification was not detectable [9,16]. We performed fluorescence in situ hybridizations of 20 RCC tissue samples, but we did not observe DcR3 gene amplification. Therefore, other mechanisms than genomic amplification may be responsible for the up-regulation of DcR3 in RCCs, such as stabilization of the DcR3 protein, methylation of the DcR3 promoter, or direct transcriptional activation.
Our data suggest that the immunohistochemical detection of DcR3 expression in RCC tissue corresponds well with DcR3 protein levels in the serum of patients as assessed by ELISA. Therefore, the ELISA-based measurement of DcR3 concentration in serum samples might even be a more feasible way to obtain prognostic information. However, the reliability of the DcR3 serum level as an independent prognostic marker needs confirmation in a larger study.
Finally, our data open up the possibility to develop novel treatment strategies based on the antagonization of DcR3. Given the strong expression of DcR3 in high-grade and high-stage RCCs, a recently described tumor vaccine approach based on cell surface expression of DcR3 might become an experimental treatment option for patients with RCCs [42].
Supplementary Material
Acknowledgments
The authors thank Maria Pritsch (Department of Medical Biometry, University of Heidelberg) for providing biostatistics support. The authors thank Elias Rüdiger, Barbara Schreiber, and Bettina Walter for expert technical assistance.
Footnotes
This work was supported by a grant from the Deutsche Krebshilfe to W.R. (German Cancer Aid, Max Eder Program), a Gerok scholarship (German Cancer Research Center) to N.W., the Tissue Bank of the National Center for Tumor Diseases Heidelberg, and National Institutes of Health grants GM079070 and DK076430 to S.K.
This article refers to supplementary materials, which are designated by Tables W1 to W4 and Figures W1 to W3 and are available online at www.neoplasia.com.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Greene FL, Page DL, Fleming ID, Fritz A. AJCC Cancer Staging Manual. 6th Edition. New York: Springer; 2002. [Google Scholar]
- 3.Wigginton JM, Gruys E, Geiselhart L, Subleski J, Komschlies KL, Park JW, Wiltrout TA, Nagashima K, Back TC, Wiltrout RH. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest. 2001;108:51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki N, Brooks AD, Carter CR, Back TC, Parsoneault EM, Smyth MJ, Wiltrout RH, Sayers TJ. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J Immunol. 2002;168:3484–3492. doi: 10.4049/jimmunol.168.7.3484. [DOI] [PubMed] [Google Scholar]
- 5.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 6.Roth W, Isenmann S, Nakamura M, Platten M, Wick W, Kleihues P, Bahr M, Ohgaki H, Ashkenazi A, Weller M. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–2765. [PubMed] [Google Scholar]
- 7.Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274:13733–13736. doi: 10.1074/jbc.274.20.13733. [DOI] [PubMed] [Google Scholar]
- 8.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Han B, Sheng H, Lin M, Moore PA, Zhang J, Wu J. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105:724–732. doi: 10.1002/ijc.11138. [DOI] [PubMed] [Google Scholar]
- 10.Simon I, Liu Y, Krall KL, Urban N, Wolfert RL, Kim NW, McIntosh MW. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol Oncol. 2007;106:112–118. doi: 10.1016/j.ygyno.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zhang L, Kim S. Quantification and detection of DcR3, a decoy receptor in TNFR family. J Immunol Methods. 2004;285:63–70. doi: 10.1016/j.jim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Wu XX, Mizutani Y, Kakehi Y, Yoshida O, Ogawa O. Enhancement of Fas-mediated apoptosis in renal cell carcinoma cells by adriamycin. Cancer Res. 2000;60:2912–2918. [PubMed] [Google Scholar]
- 14.Gerharz CD, Ramp U, Dejosez M, Mahotka C, Czarnotta B, Bretschneider U, Lorenz I, Muller M, Krammer PH, Gabbert HE. Resistance to CD95 (APO-1/Fas)-mediated apoptosis in human renal cell carcinomas: an important factor for evasion from negative growth control. Lab Invest. 1999;79:1521–1534. [PubMed] [Google Scholar]
- 15.Peduto Eberl L, Guillou L, Saraga E, Schroter M, French LE, Tschopp J, Juillerat-Jeanneret L. Fas and Fas ligand expression in tumor cells and in vascular smooth-muscle cells of colonic and renal carcinomas. Int J Cancer. 1999;81:772–778. doi: 10.1002/(sici)1097-0215(19990531)81:5<772::aid-ijc18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Bai C, Connolly B, Metzker ML, Hilliard CA, Liu X, Sandig V, Soderman A, Galloway SM, Liu Q, Austin CP, et al. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc Natl Acad Sci USA. 2000;97:1230–1235. doi: 10.1073/pnas.97.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arakawa Y, Tachibana O, Hasegawa M, Miyamori T, Yamashita J, Hayashi Y. Frequent gene amplification and overexpression of decoy receptor 3 in glioblastoma. Acta Neuropathol. 2005;109:294–298. doi: 10.1007/s00401-004-0956-6. [DOI] [PubMed] [Google Scholar]
- 18.Shen HW, Gao SL, Wu YL, Peng SY. Overexpression of decoy receptor 3 in hepatocellular carcinoma and its association with resistance to Fas ligand-mediated apoptosis. World J Gastroenterol. 2005;11:5926–5930. doi: 10.3748/wjg.v11.i38.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Zhang L, Lou H, Ding I, Kim S, Wang L, Huang J, Di Sant'Agnese PA, Lei JY. Overexpression of decoy receptor 3 in precancerous lesions and adenocarcinoma of the esophagus. Am J Clin Pathol. 2005;124:282–287. doi: 10.1309/XK59-4E4B-5WU8-2QR6. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji S, Hosotani R, Yonehara S, Masui T, Tulachan SS, Nakajima S, Kobayashi H, Koizumi M, Toyoda E, Ito D, et al. Endogenous decoy receptor 3 blocks the growth inhibition signals mediated by Fas ligand in human pancreatic adenocarcinoma. Int J Cancer. 2003;106:17–25. doi: 10.1002/ijc.11170. [DOI] [PubMed] [Google Scholar]
- 21.Ohshima K, Haraoka S, Sugihara M, Suzumiya J, Kawasaki C, Kanda M, Kikuchi M. Amplification and expression of a decoy receptor for fas ligand (DcR3) in virus (EBVor HTLV-I) associated lymphomas. Cancer Lett. 2000;160:89–97. doi: 10.1016/s0304-3835(00)00567-x. [DOI] [PubMed] [Google Scholar]
- 22.Takahama Y, Yamada Y, Emoto K, Fujimoto H, Takayama T, Ueno M, Uchida H, Hirao S, Mizuno T, Nakajima Y. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer. 2002;5:61–68. doi: 10.1007/s101200200011. [DOI] [PubMed] [Google Scholar]
- 23.Hsu TL, Wu YY, Chang YC, Yang CY, Lai MZ, Su WB, Hsieh SL. Attenuation of TH1 response in decoy receptor 3 transgenic mice. J Immunol. 2005;175:5135–5145. doi: 10.4049/jimmunol.175.8.5135. [DOI] [PubMed] [Google Scholar]
- 24.Shi G, Wu Y, Zhang J, Wu J. Death decoy receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo. J Immunol. 2003;171:3407–3414. doi: 10.4049/jimmunol.171.7.3407. [DOI] [PubMed] [Google Scholar]
- 25.Wan X, Shi G, Semenuk M, Zhang J, Wu J. DcR3/TR6 modulates immune cell interactions. J Cell Biochem. 2003;89:603–612. doi: 10.1002/jcb.10523. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Salcedo TW, Wan X, Ullrich S, Hu B, Gregorio T, Feng P, Qi S, Chen H, Cho YH, et al. Modulation of T-cell responses to alloantigens by TR6/DcR3. J Clin Invest. 2001;107:1459–1468. doi: 10.1172/JCI12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu TL, Chang YC, Chen SJ, Liu YJ, Chiu AW, Chio CC, Chen L, Hsieh SL. Modulation of dendritic cell differentiation and maturation by decoy receptor 3. J Immunol. 2002;168:4846–4853. doi: 10.4049/jimmunol.168.10.4846. [DOI] [PubMed] [Google Scholar]
- 28.You RI, Chang YC, Chen PM, Wang WS, Hsu TL, Yang CY, Lee CT, Hsieh SL. Apoptosis of dendritic cells induced by decoy receptor 3 (DcR3) Blood. 2008;111:1480–1488. doi: 10.1182/blood-2007-09-114850. [DOI] [PubMed] [Google Scholar]
- 29.Ficarra V, Galfano A, Mancini M, Martignoni G, Artibani W. TNM staging system for renal-cell carcinoma: current status and future perspectives. Lancet Oncol. 2007;8:554–558. doi: 10.1016/S1470-2045(07)70173-0. [DOI] [PubMed] [Google Scholar]
- 30.Cohen H, McGovern F. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 31.Kashyap MK, Kumar A, Emelianenko N, Kashyap A, Kaushik R, Huang R, Khullar M, Sharma SK, Singh SK, Bhargave AK, et al. Biochemical and molecular markers in renal cell carcinoma: an update and future prospects. Biomarkers. 2005;10:258–294. doi: 10.1080/13547500500218534. [DOI] [PubMed] [Google Scholar]
- 32.Zellweger T, Miyake H, July LV, Akbari M, Kiyama S, Gleave ME. Chemosensitization of human renal cell cancer using antisense oligonucleotides targeting the antiapoptotic gene clusterin. Neoplasia. 2001;3:360–367. doi: 10.1038/sj.neo.7900174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diegmann J, Junker K, Loncarevic IF, Michel S, Schimmel B, von Eggeling F. Immune escape for renal cell carcinoma: CD70 mediates apoptosis in lymphocytes. Neoplasia. 2006;8:933–938. doi: 10.1593/neo.06451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter EN, Oevermann K, Buentig N, Storkel S, Dallmann I, Atzpodien J. Primary apoptosis as a prognostic index for the classification of metastatic renal cell carcinoma. J Urol. 2002;168:460–464. [PubMed] [Google Scholar]
- 35.Tamada K, Ni J, Zhu G, Fiscella M, Teng B, van Deursen JM, Chen L. Cutting edge: selective impairment of CD8+ T cell function in mice lacking the TNF superfamily member LIGHT. J Immunol. 2002;168:4832–4835. doi: 10.4049/jimmunol.168.10.4832. [DOI] [PubMed] [Google Scholar]
- 36.Yang CR, Hsieh SL, Teng CM, Ho FM, Su WL, Lin WW. Soluble decoy receptor 3 induces angiogenesis by neutralization of TL1A, a cytokine belonging to tumor necrosis factor superfamily and exhibiting angiostatic action. Cancer Res. 2004;64:1122–1129. doi: 10.1158/0008-5472.can-03-0609. [DOI] [PubMed] [Google Scholar]
- 37.Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005;19:1792–1802. doi: 10.1210/me.2004-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gavrielides MV, Gonzalez-Guerrico AM, Riobo NA, Kazanietz MG. Androgens regulate protein kinase Cdelta transcription and modulate its apoptotic function in prostate cancer cells. Cancer Res. 2006;66:11792–11801. doi: 10.1158/0008-5472.CAN-06-1139. [DOI] [PubMed] [Google Scholar]
- 39.Langner C, Ratschek M, Rehak P, Schips L, Zigeuner R. Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. J Urol. 2004;171:611–614. doi: 10.1097/01.ju.0000108040.14303.c2. [DOI] [PubMed] [Google Scholar]
- 40.Yamana K, Bilim V, Hara N, Kasahara T, Itoi T, Maruyama R, Nishiyama T, Takahashi K, Tomita Y. Prognostic impact of FAS/CD95/APO-1 in urothelial cancers: decreased expression of Fas is associated with disease progression. Br J Cancer. 2005;93:544–551. doi: 10.1038/sj.bjc.6602732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mild G, Bachmann F, Boulay JL, Glatz K, Laffer U, Lowy A, Metzger U, Reuter J, Terracciano L, Herrmann R, et al. DCR3 locus is a predictive marker for 5-fluorouracil-based adjuvant chemotherapy in colorectal cancer. Int J Cancer. 2002;102:254–257. doi: 10.1002/ijc.10711. [DOI] [PubMed] [Google Scholar]
- 42.Shi G, Mao J, Yu G, Zhang J, Wu J. Tumor vaccine based on cell surface expression of DcR3/TR6. J Immunol. 2005;174:4727–4735. doi: 10.4049/jimmunol.174.8.4727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.