Abstract
Although the p53 tumor suppressor is most frequently inactivated by genetic mutations, exclusion from the nucleus is also seen in human tumors. We have begun to examine p53 nuclear importation by isolating a series of mutant cells in which the temperature-sensitive murine p53Val135 mutant is sequestered in the cytoplasm. We previously showed that that three of them (ALTR12, ALTR19, and ALTR25) constituted a single complementation group. Here, we found that ALTR12 cells are more sensitive to heat stress than either ALTR19 or ALTR25 and that there was a complete lack of induction of Hsp70 in response to heat shock. Western blot analysis showed no expression of the Hsf1 transcription factor, and neither heat shock nor azetidine could induce p53 nuclear localization in ALTR12 cells but did in parental A1–5 cells. Suppression of Hsf1 in A1–5 cells with quercetin or an Hsf1 siRNA reduced p53 nuclear importation and inhibited p53-mediated activation of a p21 reporter. Most convincingly, p53 nuclear importation could be restored in ALTR12 cells by introducing an exogenous Hsf1 gene. Collectively, our result suggests that Hsf1 is required for p53 nuclear importation and activation and implies that heat shock factors play a role in the regulation of p53.
Introduction
Nuclear localization of the p53 is a critical element in the activation of its transactivation function, and sequestration in the cytoplasm renders the protein nonfunctional. The p53 protein shuttles between nucleus and cytoplasm [1]. Nuclear export of p53 is mainly regulated by the MDM2 protein, which acts in conjunction with Crm1 to export p53 from nucleus through a nuclear export signal located in the C-terminus [2]. However, under conditions of genotoxic stress, nuclear p53 levels are increased, which results in the induction of downstream target genes that regulate cell cycle progression and induction of apoptosis [3].
The transportation of p53 into the nucleus is less well understood but requires a functional nuclear localization signal, the nuclear localization signal 1 (NLS1), located in the C-terminal domain of the wild type protein [4]. This motif binds importins alpha and beta, proteins that ferry their cargo across the nuclear membrane and into the nucleus. Mutations of NLS1 results in a p53 protein that remains sequestered in the cytoplasm [5]. However, mutations in the NLS1 have not been observed in sporadic human cancers, although p53 is found sequestered in the cytoplasm of some tumors. This suggests that the pathway that controls p53 nuclear importation may be a target for disruption during tumorigenesis.
A1–5 fibroblasts express a temperature-sensitive murine p53 (tsp53), which accumulates in the nucleus and acts as wild type p53 at 32°C but is sequestered in the cytoplasm at 37°C [6]. Previously, a series of A1–5 low temperature-resistant (ALTR) cell lines were generated from A1–5 cells by chemical mutagenesis, and in most of these cell lines, p53 was found to be sequestered in the cytoplasm. Among them, ALTR12, ALTR19, and ALTR25 were determined using an in vitro p53 nuclear importation assay system to constitute a complementation group [7]. Here, we showed for the first time that Hsf1, a major regulator of the heat shock response, is required for p53 nuclear importation and activation and found evidence that heat shock proteins play a role in p53 nuclear importation.
Materials and Methods
Cell Culture, Reagents, and Irradiation
A1–5 is a rat fibroblast cell line transfected with the temperature-sensitive murine p53Val135 gene [6]. SK-N-SH is a human neuroblastoma cell line expressing wild type p53. SK-N-SH, A1–5, and ALTR cell lines were maintained in complete DMEM consisting of 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco BRL, Gaithersburg, MD). SK-N-SH and A1–5 cells were incubated at 37°C and in an atmosphere containing 5% CO2 unless otherwise noted. ALTR cell lines were maintained under the same conditions as A1–5 cells except that they were normally incubated at 32°C. Cells were exposed to a 5-Gy ionizing radiation using 60Co source at an average dose rate of 47 cGy/min.
Quercetin and azetidine were from Sigma (St. Louis, MO). Antibodies specific for p53 (PAb421) were kindly provided by Dr. Arnold Levine. Anti-p53 (DO-1) and Anti-β-actin was from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-Hsf1 (SPA-950) and the anti-Hsp70 (SPA-810) antibodies were from Assay Designs/StressGen (Ann Arbor, MI). Anti-p21 (Ab-6) was from Calbiochem (La Jolla, CA).
Plasmids and Transfection
A 1.9-kb HindIII/XbaI DNA fragment encoding Neomycin resistance gene (Neor) from pCMV-Neo-Bam3 plasmid was inserted into the BamHI site of pWWP-Luc plasmid (Generously provided by Dr. Bert Vogelstein, The Johns Hopkins Oncology Center, Baltimore, MD), which encodes a firefly luciferase under the control of p21 promoter [8], to construct the pWWP-Luc-Neo plasmid. pSa244, which encodes a firefly luciferase under the control of a Rous sarcoma virus long terminal repeat promoter, was provided by Dr. S. Subramani, University of California, San Diego. pMMfluc, which encodes a firefly luciferase under the control of a MTV-GRE promoter and pRenilla-Luc, was provided by Dr. Roger L. Miesfeld, University of Arizona. pCDNA3.1-Hsf1 encoding wild type Hsf1 and pCDNA3.1-hHsf1 encoding a constitutively activated Hsf1 mutant were provided by Dr. R. Voellmy, University of Miami. Stable and transient transfections were performed using Superfect (QIAGEN, Valencia, CA) as described in the manufacturer's protocol. Stable lines were selected and maintained in DMEM supplemented with 10% FBS, penicillin/streptomycin, and 400 µg/ml G418 (Gibco BRL).
Heat Shock Survival Assay by Crystal Violet Staining
Cells grown on a 10-cm plate to 80% to 90% confluence were trypsinized and diluted to 104 per milliliter in complete DMEM that had been preheated to 42°C. After incubation at 42°C for different time points, 1-ml aliquots were diluted into 9 ml of PBS at room temperature. Five hundred-microliter (500 cells) aliquots of diluted cells were then put into a 60-mm plate with DMEM. After incubation at 37°C for ∼10 days, colonies were stained with crystal violet and read on the colony counter.
Luciferase Refolding Assay
Cells were transfected with pSa244 and incubated for 48 hours to allow for optimal luciferase expression. Cells were exposed to 42°C for 20 minutes in the presence of protein synthesis inhibitor cycloheximide (10 µg/ml; Sigma-Aldrich). Luciferase activity was determined either immediately after heating or after a 2- or 4-hour recovery period at 37°C.
Luciferase Assay
Cell lysates were collected, and luciferase assays or Dual-Luciferase reporter assays were performed using Promega (Madison, WI) kit as described in the manufacturer's protocol. A Monolight 3010 luminometer (Analytical Luminescence Laboratory, San Diego, CA) was used for measuring luciferase activity.
siRNA Transfection
A1–5 cells were grown in antibiotics-free DMEM supplemented with 5% FBS to be ∼70% confluent. Cells were then transfected with the rat hsf1 siRNA pool (D-081010-00; Dharmacon, Lafayette, CO), either of two hsf1 siRNA (D-081010-01 or D-081010-02; Dharmacon) or negative control siControl RISC-free siRNA and siControl Nontargeting siRNA#1 (Dharmacon). The DharmaFECT1 transfection reagent was used for transfection following the manufacturer's protocol (Dharmacon). The final siRNA concentration was 20 nM for each transfection. For SK-N-SH cells, human hsf1 siRNA pool (L-012109-00; Dharmacon) was transfected twice using the same protocol as above. At 48 hours after the last transfection, cells were collected and subjected for further analysis.
Indirect Immunofluorescence and Western Blot Analysis
For immunofluorescence, cells were plated on coverslips 20 hours before use. Next, the cells were processed and analyzed as described previously [9].
Western blot analysis was performed according to standard procedures using polyvinylidene fluoride membranes (Millipore, Bedford, MA). Signals were detected by the chemiluminescence method using Super Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Results
ALTR Cells Exhibit a Defect in Heat Shock Response and Reactivation of Luciferase
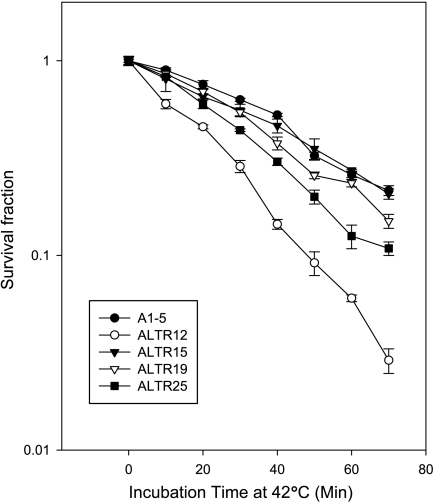
We previously described the derivation and characterization of a set of mutant cell lines, the ALTR cell lines, in which p53 nuclear localization was defective and showed using an in vitro nuclear importation assay that three lines, ALTR12, ALTR19, and ALTR25, belonged to the same complementation group [7]. Because nuclear localization of the tsp53 in parental A1–5 cells can be controlled by altering the incubation temperature, we reasoned that the cell's tolerance for heat stress might have been affected in the ALTR cells [6]. Hence, we tested the ability of ALTR12, ALTR19, and ALTR25 cells to survive heat shock. We also included the ALTR15 cell line, which we showed did not belong to the 12/19/25 complementation group [7]. When comparing the survival curves with A1–5 cells, the ALTR12 line showed the most sensitivity. The ALTR25 and ALTR19 cell lines exhibited intermediate sensitivity to heat shock, whereas the survival curve for ALTR15s was the same as that for A1–5 cells (Figure 1). This demonstrated that the heat stress response pathway was compromised in all three of the ALTR lines that belong to the 12/19/25 complementation group.
Figure 1.
Heat shock survival curve of A1–5 and ALTR cells. Cells (80% confluence) were heat-shocked and plated as described in the Materials and Methods section. After incubation at 37°C for ∼10 days, colonies were stained with crystal violet and read on a colony counter. Graphs show the mean surviving fraction ± SE and are from three independent experiments.
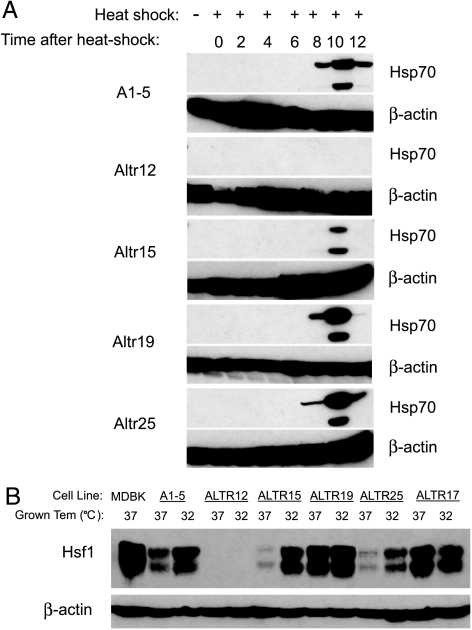
The Hsp70 protein is an essential component of the cellular response to heat stress and is induced in response to elevated temperatures [10]. Our observation that some ALTR cell lines exhibited reduced survival to heat stress prompted us to test for the induction of Hsp70 in the ALTR cell lines using Western blot analysis. Strikingly, there was no induction of Hsp70 in ALTR12 cells, whereas the other ALTR lines and A1–5 cells showed normal induction (Figure 2A). Because Hsf1 is the major transcription factor regulating induction of Hsp70 in response to heat shock response [11], we tested the ALTR lines for Hsf1 expression (Figure 2B). We found that Hsf1 expression was undetectable in ALTR12 cells suggesting that the gene for this factor had been inactivated. This is consistent with the impaired heat shock response in the ALTR12 cells.
Figure 2.
Expression of Hsp70 and HSF1 is impaired in ALTR12 cells. (A) Induction of Hsp70 in A1–5 and ALTR cells was examined by Western blot analysis. Cells at 80% confluence were heat-shocked for 20 minutes at 42°C. After incubation at 37°C for different time points, cells were harvested and analyzed by Western blot analysis using an anti-Hsp70 antibody. (B) The expression of Hsf1 in A1–5 and ALTR cells was examined by Western blot analysis. Confluent A1–5 and ALTR cells grown at 37°C or 32°C were harvested and analyzed by Western blot analysis using an anti-Hsf1 antibody.
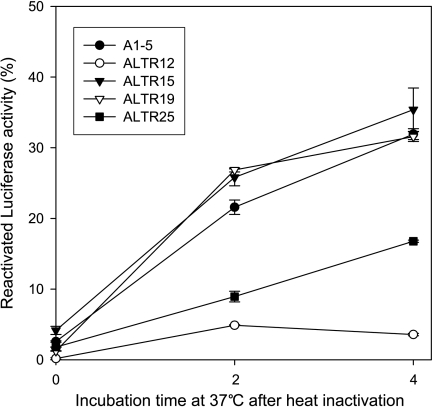
The heat shock system is required for refolding of heat-denatured proteins [12]. Consequently, we tested the ALTR cells for their ability to reactivate heat-inactivated luciferase. A plasmid encoding a firefly luciferase was transfected into each of the ALTR cell lines, and luciferase reactivation assays were performed as described in the Materials and Methods section. We found that the ability to reactivate luciferase was abolished in ALTR12 and was markedly decreased in ALTR25. However, the reactivation of heat-inactivated luciferase was unchanged in ALTR15 and ALTR19 when compared to parental A1–5 (Figure 3). This further supports the notion that the heat shock system is compromised in some of the ALTR lines.
Figure 3.
Luciferase refolding is defective in ALTR12 cells. A1–5 and ALTR cells were transfected with pSa244 and incubated for 48 hours to allow for optimal luciferase expression. Cells were then exposed to 42°C for 20 minutes in the presence of protein synthesis inhibitor cycloheximide (10 µg/ml). Luciferase activity was determined either immediately after heating or after a 2- or 4-hour recovery period at 37°C. Graphs show the mean ± SE from three independent experiments.
ALTR Cells That Are Defective for p53 Nuclear Importation Are Also Defective for Glucocorticoid Receptor Nuclear Translocation
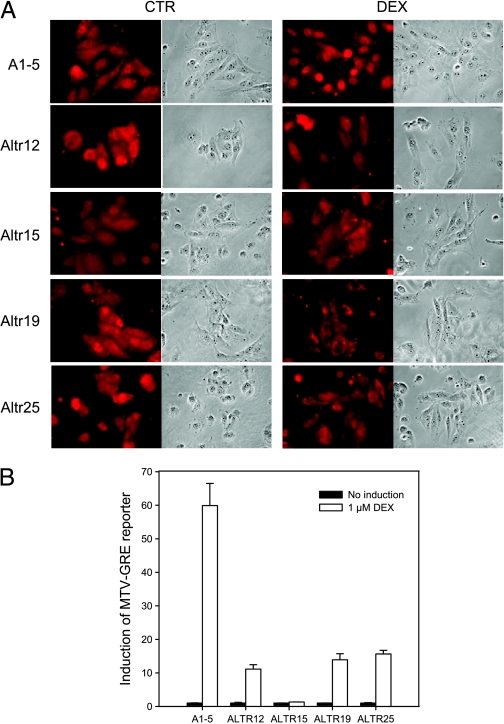
Hsp/Hsc70 is known to function in concert with other heat shock proteins in protein folding and in regulating the activity of enzymes including the glucocorticoid receptor (GR) [13]. Hence, we asked whether the deficiency in p53 nuclear localization affected the GR. Immunostaining the ALTR cells for GR after incubating with dexamethasone (DEX) showed that the GR remained in the cytoplasm in all four of the ALTR cell lines, whereas in parental A1–5 cells, the GR became concentrated in the nucleus (Figure 4A). This suggested that nuclear importation of both p53 and the GR are regulated similarly. To confirm the immunostaining results, the pMMTV plasmid, a reporter construct encoding a firefly luciferase under the control of a GR response element, was cotransfected with a plasmid encoding Renilla luciferase and assayed for dual luciferase activities of the cell lysate with or without DEX treatment. As shown in Figure 4B, whereas the GR MTV has a 70-fold induction by DEX in A1–5 cells, the induction of GR-MTV was decreased to 10-fold or less in ALTR cells. This suggests that the pathway that leads to p53 nuclear localization overlaps with the pathway that regulates GR nuclear localization.
Figure 4.
Nuclear localization of the GR was inhibited in ALTR cells. (A) A1–5 and ALTR cells were grown on coverslips. After treatment with or without DEX for 3 hours, GR localization were stained using an anti-GR antibody. Photomicrographs of a typical experiment are shown. (B) A1–5 and ALTR cells that were transfected with either the pMMfluc, which encodes a firefly luciferase reporter under the control of MTV-GRE promoter, or the pRenilla-Luc as internal control. At 24 hours after transfection, cells were treated with or without DEX, and luciferase activities were measured after 6 hours. Luciferase activities at any particular treatment were corrected for variations in transfection efficiency using the internal control. Graphs show the mean ± SE from three independent experiments.
Hsf1 Is Required for p53 Nuclear Localization
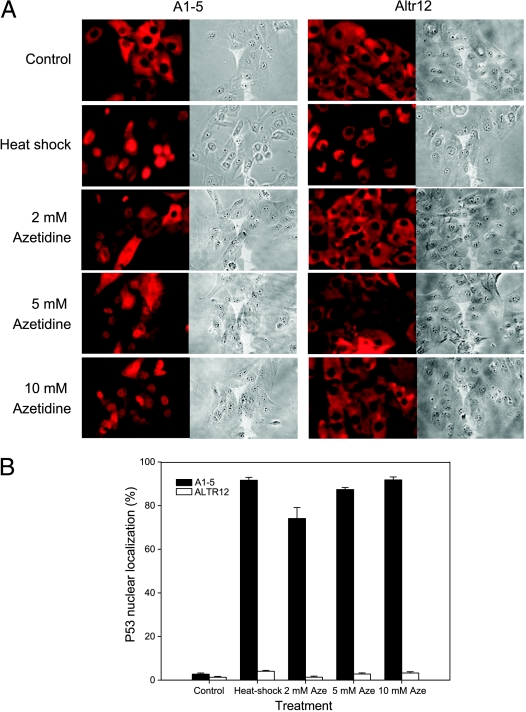
HSF-1, a transcription factor for the heat shock proteins, is a key regulator in response to heat shock stress. The defective heat shock response in ALTR12, ALTR19, and ALTR25 and the lack of Hsf1 expression in ALTR12 prompted us to ask whether Hsf1 regulates p53 nuclear importation. To address this question, we first asked if heat shock or the heat shock-trigging drug azetidine treatment might induce p53 nuclear translocation. Using immunostaining, the p53 localization was determined in A1–5 or ALTR12 cells treated with heat shock or azetidine (Figure 5A). As shown in Figure 5B, nuclear localized p53 was found in approximately 90% of A1–5 cells after heat shock treatment (42°C for 20 minutes), and treatment with 2, 5, or 10 mM azetidine induced nuclear localized p53 in a concentration-dependent manner (∼70%, 85%, and 90%, respectively). However, neither heat shock nor azetidine treatments induced p53 nuclear import in the ALTR12 cells, suggesting that the Hsf1 pathway is required for regulating p53 nuclear import.
Figure 5.
Heat shock-inducible p53 nuclear import is inhibited in ALTR12 cells. (A) A1–5 or ALTR12 cells were treated with heat shock (42°C for 20 minutes) or azetidine at the concentration indicated. After incubation at 37°C for 3 hours, cells were stained for p53 localization using PAb421 antibody. The photomicrographs shown represent a typical result. (B) The fraction of nuclear localized p53 in A was quantified. Graphs show the mean ± SE from three independent experiments.
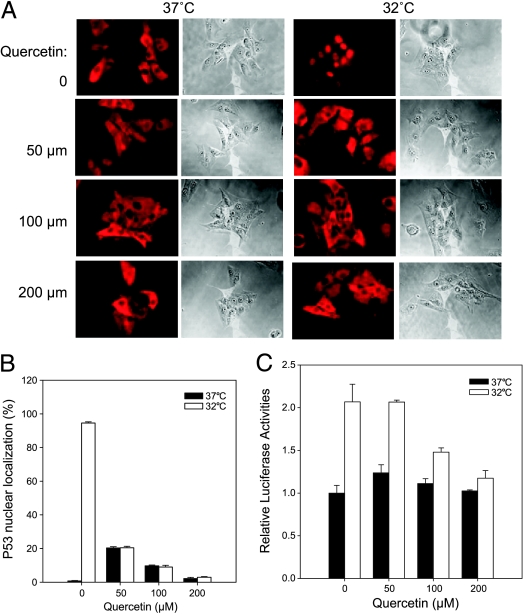
To confirm that Hsf1-mediated heat shock response regulates p53 nuclear importation, A15 cells was treated with an Hsf1 inhibitor, quercetin [14], and tested for p53 localization after a temperature shift to 32°C by immunostaining (Figure 6A). In the absence of quercetin, p53 was located to the nucleus of most A1–5 cells (∼95%) after 5 hours of incubation at 32°C. Interestingly, p53 nuclear localization induced by temperature shift treatment was inhibited by quercetin in a concentration-dependent manner. With 50, 100, or 200 µM quercetin treatment, the A1–5 cells with nuclear localized p53 was decreased to ∼20%, 10%, and 3%, respectively (Figure 6B). However, a low concentration (50 µM) of quercetin induced ∼20% of p53 nuclear localization at 37°C compared to untreated cells, indicating that quercetin itself may induce stress-mediated p53 nuclear importation. To confirm the immunostaining results, the pWWP-Luc-Neo plasmid, which encodes a firefly luciferase under the control of p21 promoter, was stably transfected into the A1–5 cell line and assayed for luciferase activities at 37 or 32°C with or without quercetin. Again, the induction of p21 reporter by temperature shift was decreased by quercetin in a concentration-dependent manner. With 100 or 200 µM quercetin treatment, the p21 induction was decreased from ∼2.1-fold to ∼1.5- and 1.2-fold, respectively, compared to untreated cells (Figure 6C). These results suggest that Hsf1-mediated heat shock response plays a role in regulating p53 nuclear importation.
Figure 6.
Quercetin suppressed p53 nuclear localization in A1–5 cells. (A) A1–5 cells were grown on coverslips at 37°C to 60% confluence. The cells were then treated with different concentrations of quercetin or left untreated for 2 hours at 37°C. The cells were then shifted to 32°C for 5 hours and then stained for p53 localization by PAb421 antibody. The photomicrographs shown represent a typical result. (B) The fraction of nuclear localized p53 in A was quantified. Graphs show the mean ± SE from three independent experiments. (C) A1–5 cells stably transfected with pWWP-Luc-Neo plasmid, which encodes a firefly luciferase under the control of p21 promoter, were grown at 37°C to 80% confluence. After treatment with different concentrations of quercetin for 2 hours at 37°C, the cells were shifted to a 32°C incubator for 6 hours and then assayed for luciferase activity. Graphs show the mean ± SE from three independent experiments.
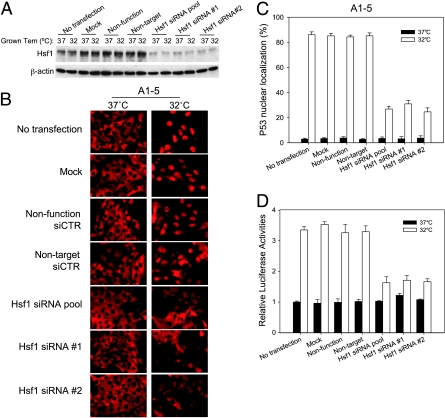
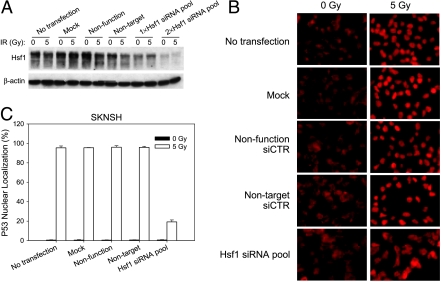
Because quercetin might have pleiotropic effects, we used Hsf1 siRNA to specifically knock down Hsf1 and test for p53 nuclear translocation. To address the question whether Hsf1 affects importation of endogenous wild type p53, a human neuroblastoma cell line expressing wild type p53 (SK-N-SH) was included in this test. The Hsf1 levels in A1–5 cells were effectively knocked down by siRNA pool, Hsf1 siRNA#1 and Hsf1 siRNA#2 (Figure 7A), and the Hsf1 level in SK-N-SH cells was also knocked down after twice transfection of Hsf1 siRNA pool (Figure 8A). After transfection with Hsf1 siRNA, p53 nuclear translocation was examined in A1–5 and SK-N-SH (Figures 7B and 8B). As expected, p53 nuclear localization was unchanged (∼85%) in A1–5 that were transfected with either of the control siRNA and were incubated at 32°C. In contrast, transfection of cells with Hsf1 siRNA resulted in a reduction of cells with nuclear localized p53 to ∼30% (Figure 7C). Similarly, transfection of SK-N-SH cells with Hsf1 siRNA resulted in a reduction of cells with nuclear accumulated p53 to ∼20% (Figure 8C). These results suggested that the Hsf1-mediated heat shock response is involved in regulating the nuclear importation of both temperature-sensitive p53 and endogenous wild type p53.
Figure 7.
Suppression of Hsf1 with siRNA inhibits p53 nuclear localization. (A) A1–5 cells were transfected with the indicated siRNA at 37°C. Forty-eight hours later, either the cells were harvested and extracts were prepared (37°C) or the incubation temperature was shifted to 32°C for 5 hours before preparing extracts (32°C). Levels of Hsf1 were determined by immunoblot analysis with the appropriate antibody. β-Actin levels were used as loading controls. (B) A1–5 cells were grown on coverslips at 37°C to 60% confluence and then transfected with the indicated siRNA. Forty-eight hours later, the coverslips were either immediately stained for p53 localization (37°C) or shifted to 32°C for 5 hours and then stained for p53 localization by PAb421. (C) The fraction of cells with nuclear localized p53 in panel (B) was quantified. Graphs show the mean ± SE from three independent experiments. (D) A1–5 cells stably transfected with pWWP-Luc-Neo plasmid were grown at 37°C to 60% confluence. Forty-eight hours after transfection with the indicated siRNA, either the cells were harvested immediately and assayed for luciferase activity (37°C) or the incubation temperature was shifted to 32°C for an additional 6 hours and then assayed for luciferase activity (32°C). Graphs show the mean ± SE from three independent experiments.
Figure 8.
Hsf1 knockdown in SK-N-SH cells suppresses p53 nuclear localization. (A) SK-N-SH cells were grown to 60% confluence and then transfected with the indicated siRNA either once (1x) or twice with a 24-hour interval (2x). Forty-eight hours after the last transfection of siRNA, the transfected cells were either treated with a 5-Gy ionizing radiation or left untreated. Cells were harvested, and extracts were prepared 3 hours after irradiation. Hsf1 expression was determined by Western blot analysis using an anti-Hsf1 antibody. β-Actin levels are depicted as the loading control. (B) SK-N-SH cells were grown on coverslips at 37°C to 60% confluence and were transfected with the indicated siRNA. The cells were transfected twice with the Hsf1 siRNA as in panel (A). Forty-eight hours later, the cells were either treated with a 5-Gy ionizing radiation or left untreated. p53 localization was stained using DO1 antibody 3 hours after irradiation. (C) The fraction of cells with nuclear localized p53 in panel (B) was quantified. Graphs show the mean ± SE from three independent experiments.
We also examined p53 activity in A1–5 cells where Hsf1 expression was reduced. Here, we used an A1–5 cell line that had been stably transfected with the pWWP-Luc-Neo, which contained the luciferase reporter under control of the p21 promoter. These cells were transfected with Hsf1 siRNA. Assaying for luciferase activity showed that compared to cells transfected with siRNA controls the Hsf1 siRNA resulted in a marked reduction in reporter activation of from ∼3.4- to 1.5-fold induction after temperature shift. As expected no difference in p21 induction was found in the mock- or control siRNA-transfected cells (Figure 7D). These results confirmed the involvement of Hsf1 in the regulation of p53 nuclear localization and activation.
Exogenous Hsf1 Restores p53 Nuclear Importation in ALTR12 Cells
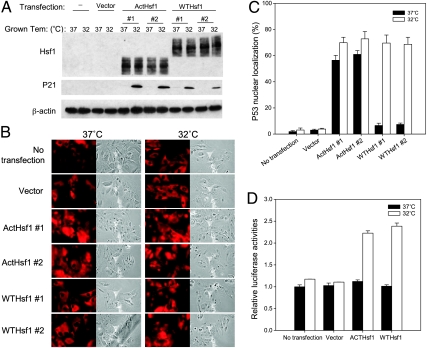
To confirm that the lack of Hsf1 expression in ALTR12 resulted in impaired p53 nuclear importation, a plasmid encoding either wildtype or a constitutively active Hsf1 mutant was stably transfected in ALTR12 cells and assayed for Hsf1 and p21 expression by Western blot analysis. Hsf1 was detected in the cell lysate of either wild type or constitutively active Hsf1-transfected ALTR12 cells but not in the lysate of nontransfected or vector-transfected cells (Figure 9A). Whereas no expression of endogenous p21 was detected in parental ALTR12 and vector transfected cells introduction of either wild type or constitutively active Hsf1 resulted in p21 induction at 32°C (Figure 9A). The localization of p53 was determined by immunostaining using PAb421 antibody (Figure 9B). After the introduction of constitutively active Hsf1, p53 was nuclear localized in ∼70% of cells at 32°C and 60% of the cells at 37°C. Interestingly, the ALTR12 cells transfected with wild type Hsf1 showed p53 nuclear localization (70%) at 32°C and not at 37°C, similar to what is seen in parental A1–5 cells (Figure 9C). Additionally, in assays using the p21 reporter, no activation of p21 reporter occurred in parental ALTR12 or in vector-transfected ALTR12 cells by temperature shift to 32°C. p21 expression was induced by ∼2.3-fold in the ALTR12 cells transfected with either wild type Hsf1 or constitutively active Hsf1 (Figure 9D), suggesting that introduction of Hsf1 could restore p53 nuclear importation and activation in ALTR12 cells.
Figure 9.
Exogenous Hsf1 restores p53 nuclear importation in ALTR12 cells. (A) Confluent ALTR12 or ALTR12 cells stably transfected with pCDNA3.1 vector (vector) or plasmids encoding either a constitutively active Hsf1 (ActHsf1) or the wild type hsf1 (WTHsf1) were grown at 37 or 32°C. Cells were harvested and analyzed for Hsf1 and p21 expression by Western blot analysis. (B) Parental ALTR12 or ALTR12 cells stably transfected with pCDNA3.1 vector or a vector encoding either a wild type hsf1 or a constitutively active Hsf1 were grown on coverslips at 37°C to 60% confluence. Cells were either immediately stained for p53 localization or shifted to 32°C for 5 hours and then stained for p53 using PAb421 antibody. (C) The fraction of nuclear localized p53 in B was quantified. Graphs show the mean ± SE from three independent experiments. (D) ALTR12 cells stably transfected with pWWP-Luc-Neo plasmid were grown at 37°C to 60% confluence. After transfection of pCDNA3.1 vector or plasmids encoding either a wild type hsf1 or a constitutively active Hsf1 for 48 hours at 37°C, cells were either retained at 37°C or shifted to 32°C for an additional 6 hours and then assayed for luciferase activity. Graphs show the mean ± SE from three separate experiments.
Discussion
In this study, we showed for the first time that the Hsf1-mediated pathway is involved in p53 nuclear importation and activation. In support of this, we showed that Hsf1 expression was abolished in ALTR12 cells (Figure 2B) and that treatment with heat shock and azetidine, which is an activator of Hsf1 [15], could not induce p53 nuclear localization in ALTR12 cells but did in parental A1–5 cells (Figure 5). Similarly, inhibition of Hsf1 by quercetin treatment or with an Hsf1 siRNA reduced p53 nuclear import and suppressed p53-mediated induction of a p21 reporter (Figures 6 and 7). Collectively, these observations indicate that ALTR12 cells lack a functioning Hsf1 and imply that Hsf1 plays an important role in regulating p53 subcellular localization. Importantly, we show that inhibiting Hsf1 expression in SK-N-SH cells, a human neuroblastoma-derived cell line, resulted in a similar reduction in accumulation of p53 in the nucleus. Finally, we showed that the p53 nuclear importation and activation in ALTR12 cells could be restored by introducing an exogenous Hsf1, confirming that Hsf1 is required for p53 nuclear importation and activation.
Additionally, analysis of our panel of ALTR cells provides insight into the mechanistic relationship between p53 and the stress response pathway. We showed previously that ALTR19 and ALTR25 belong to the same complementation group as ALTR12 cells [7]. Consistent with this, we show that these two cell lines also show a modest sensitivity to killing by heat shock suggesting that the heat shock response is compromised in these cells. However, Western blot analysis showed Hsp70 could be induced in both the ALTR19s and ALTR25s (Figure 2). Examination of the heat shock-induced stress response mechanism shows that multiple components function together to restore protein functionality [12]. Hence, it seems likely that a component of the heat stress response pathway other than Hsp70 that is induced by Hsf1 is defective in ALTR19s and ALTR25s cells.
Our results also suggest that activation of p53's capability for transactivation is separable from its translocation into the nucleus. Reintroduction of Hsf1 into ALTR12 cells restored the ability of p53 to be translocated into the nucleus. The wild type Hsf1 only caused nuclear localization of p53 when the transfected ALTR12 cells were incubated at 32°C, which recapitulates phenotype of parental A1–5 cells. The constitutively activated form of Hsf1 resulted in a constitutive nuclear localization of p53 at both 37 and 32°C (Figure 9C). However, p53 was only capable of activating expression of p21 at 32°C (Figure 9, A and D) indicating that localization to the nucleus is necessary but not sufficient for activation of p53's transactivation properties. This notion is supported by our previous studies, which show that the p53 in another ALTR line, ALTR17s, has a constitutively nuclear p53 that is not capable of activating gene expression [8].
The defect in ALTR15s is less clear. ALTR15 showed unchanged heat shock survival and luciferase reactivation compared to parental A1–5 cells suggesting that a process other than the heat-activated stress response was compromised. Curiously, ALTR15 cells had the least capacity for activation of the MTV-GRE reporter (Figure 4B). Hence, it may be that a chaperone not induced by Hsf1 is needed for transcriptional activation of GR is defective in ALTR15s.
Hsf1 is a major regulator of the heat shock response, which is implicated in protecting organisms from a broad range of stresses [16]. It has long been noted that HSP levels increase in a wide range of tumor types [17]. Many of the signaling pathways and transcription factors that are frequently mutated in cancers display a striking dependence on the chaperone machinery, especially Hsp90 [18]. Recently, Hsf1 expression was found to be elevated in human prostate carcinoma cell lines [19] and eliminating Hsf1 protects mice from tumors induced by the Ras oncogene or a mutant p53 [20]. However, the role of Hsf1 in mammalian oncogenesis is unknown. These studies show that Hsf1 is essential for regulating p53 nuclear transport, demonstrating that Hsf1 may have a role in cancer by regulating p53 activation.
Acknowledgments
The authors thank S. Subramani for providing pSa244 plasmid, R. Voellmy for wild type Hsf1 and constitutively activated Hsf1 mutant, Roger L. Miesfeld for pMMfluc plasmid, and Bert Vogelstein for the pWWP-Luc plasmid.
Footnotes
This work was supported by National Institutes of Health Grant CA090776.
References
- 1.Komarova EA, Zelnick CR, Chin D, Zeremski M, Gleiberman AS, Bacus SS, Gudkov AV. Intracellular localization of p53 tumor suppressor protein in gamma-irradiated cells is cell cycle regulated and determined by the nucleus. Cancer Res. 1997;57:5217–5220. [PubMed] [Google Scholar]
- 2.Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritsche M, Haessler C, Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993;8:307–318. [Erratum appears in Oncogene 1993 8 (9), 2605] [PubMed] [Google Scholar]
- 4.Shaulsky G, Goldfinger N, Ben-Ze'ev A, Rotter V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol. 1990;10:565–6577. doi: 10.1128/mcb.10.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang SH, Clarke MF. A bipartite nuclear localization signal is required for p53 nuclear import regulated by a carboxyl-terminal domain. J Biol Chem. 1999;274:32699–32703. doi: 10.1074/jbc.274.46.32699. [DOI] [PubMed] [Google Scholar]
- 6.Martinez J, Georgoff I, Martinez J, Levine AJ. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991;5:151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Falsey RR, Gaitonde S, Sotello V, Kislin K, Martinez JD. Genetic analysis of p53 nuclear importation. Oncogene. 2007;26:7885–7893. doi: 10.1038/sj.onc.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayelzadeh F, Martinez JD. DNA binding and selective gene induction by different forms of the p53 protein. Oncogene. 2007;26:2955–2963. doi: 10.1038/sj.onc.1210110. [DOI] [PubMed] [Google Scholar]
- 9.Gaitonde SV, Riley JR, Qiao D, Martinez JD. Conformational phenotype of p53 is linked to nuclear translocation. Oncogene. 2000;19:4042–4049. doi: 10.1038/sj.onc.1203756. [DOI] [PubMed] [Google Scholar]
- 10.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 12.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 13.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 14.Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Kawai K, Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992;12:3490–3498. doi: 10.1128/mcb.12.8.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [Erratum appears in Mol Cell Biol 1993 13 (6), 3838–3839] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 17.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 19.Tang D, Khaleque MA, Jones EL, Theriault JR, Li C, Wong WH, Stevenson MA, Calderwood SK. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10:46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]