Abstract
Background
Cancer mortality rates among American Indians (AIs) in the Northern Plains are among the highest in the nation. Reasons for this disparity are unclear but are probably due to multiple barriers. AIs appear to experience more intense side effects from therapeutic radiation compared with other populations. This differential response to treatment, a disparity in itself, might be overcome if the molecular reasons were better understood.
Methods
The National Cancer Institute developed the Cancer Disparity Research Partnership to address these inequities. This initiative, known as the Walking Forward program, attempts to lower cancer mortality rates for AIs by increasing access to innovative clinical trials, behavioral research, patient navigation, and the ataxia telangiectasia mutated (ATM) gene study. The ATM component of the project was initiated to determine if there is a molecular basis for this apparent differential response to therapeutic radiation. Successful implementation of the genetic study relied on achieving a trusting partnership with AIs since a lack of trust has historically been a barrier to performing research in this population. The authors detail the nature of building partnerships and trust by utilizing lessons learned.
Results
Establishing a trusting partnership between a community hospital and AIs in South Dakota resulted in successful recruitment to this ATM clinical trial. To date, 26 AIs and 40 non-AIs have consented to participate in this ATM analysis. Their shared human desire to assist others, especially family and community members, and their demonstrated responsiveness to community priorities by academic researchers are the primary reasons for participant eagerness to enroll on this study.
Conclusions
The relatively rapid approval of the ATM genetic study by multiple tribal organizations and the successful accrual of AIs on this study reflect the trusting partnerships achieved at the patient and community levels.
Introduction
American Indians (AIs) are among several groups in the United States who experience health care disparities. Although AIs from regions of the United States have lower cancer incidence rates compared with other racial/ethnic groups, this is not the case for AIs living in the Northern Plains (South Dakota, North Dakota, and Nebraska) and Alaska. AIs from all regions have the poorest 5-year relative survival from cancer for 7 of the 10 most common cancer sites. The cancer mortality rate of AIs served by the 10-state Billings, Aberdeen, and Bemidji Service Areas of the Indian Health Service (IHS) is approximately 40% higher than that of the overall US population.1
The Rapid City Regional Hospital is a community hospital located in a town with approximately 70,000 residents. It serves all of western South Dakota and parts of adjacent states, including four large Indian reservations in western South Dakota and other smaller AI communities in the region. Its “service basin” incorporates a geographic area of approximately 250,000 square miles, with a population base of 350,000. The reservations served include Cheyenne River, Rosebud, and Pine Ridge.
This article describes the process of establishing a trusting partnership with AI communities in South Dakota that ultimately led to the successful implementation of the Walking Forward program. The program is designed to address cancer disparities in the AI population through clinical trials, behavioral research, a genetic protocol, patient navigator (PN) services, and the provision of culturally appropriate community education efforts with members of three western South Dakota tribes.2–4 This paper describes the lessons learned and applied to secure approval of the study protocols by the tribal organizations and AI communities.
Historical Distrust of Research Protocols
AI populations have valid reasons for distrust of researchers.5 Christopher et al6 documented multiple examples of AIs’ distrust of researchers based on numerous historical events. Unethical research or care protocols have historical relevance to government policies designed to annihilate AIs in the 19th century and the first half of the 20th century; others are more recent and include sterilization of AI women without informed consent in the 1970s.5 The Havasupais recently initiated a lawsuit against Arizona State University researchers for misusing blood samples taken from nearly every tribal member. Such misuse creates mistrust throughout “Indian Country” and hinders the ability to develop successful community-academic partnerships for the purpose of research. Appreciating and understanding the multiple reasons for this lack of trust are critical in order for researchers to pursue areas of investigation in this population.
AI communities are resistant to participating in research studies for many reasons. Among these reasons, (1) AIs do not want to be “guinea pigs,” (2) the study findings are rarely shared with the AI communities that participated in the study, (3) the study findings rarely improve local services for the AI community, (4) the promised study benefits rarely reach the AI community, and (5) access to resources to allow community members to participate in the study (eg, transportation) is insufficient.7
Distrust of genetic studies among AI communities is also well documented and based on projects such as the Human Genome Diversity Project.8,9 A summary of historical events resulting in AI communities requiring careful and rigorous study review for all genetic studies has been summarized,10 and examples of genetic policy language suggested by AI communities are described elsewhere.5,10
Existing Treatment Challenges
Distance is a treatment barrier as AIs travel a median of 140 miles one way to the cancer center in Rapid City, South Dakota. This is challenging for both the patient, who is likely to not be feeling well, and the accompanying family members. The Walking Forward study introduced treatment protocols that address these and other common barriers that interfere with AIs taking part in clinical trials and other research studies.4 This is accomplished through the use of advanced radiation delivery systems such as tomotherapy and brachytherapy, which reduce the overall treatment time from 8 to 4 weeks or, in some circumstances, from 1 week to 1 day.11,12
Variation in Treatment Response
Patients diagnosed with the same stage and histologic grade of cancer react differently to the same cancer treatments. Individual variability in normal tissue response to fractionated radiotherapy has been observed in clinical and laboratory studies.13–15 It has been the observation of radiation oncologists at Rapid City Regional Hospital that AIs appear to be more sensitive to the effects of radiation therapy compared to the non-AI population. In a retrospective analysis of 61 AIs undergoing definitive radiotherapy at the cancer center in Rapid City, 50% experienced grade 2 toxicities and 17% experienced grade 3 toxicities. The majority of these toxicities were skin reactions in patients with breast and colorectal cancer.3,4 These sensitivities are the foundation that led to the ataxia telangiectasia mutated (ATM) genetic arm of the Walking Forward study. Thus, a possible explanation for the observed increase in toxicity is an underlying genetic susceptibility.16 This phenomenon has been observed in other conditions, perhaps most dramatically in patients with homozygous mutations of the ataxia telangiectasia (AT) gene.17 Approximately 1% to 5% of the general population is heterozygous for the AT gene. A recent report positively correlated ATM heterozygosity with increased late subcutaneous toxicities for breast patients undergoing adjuvant radiotherapy.18
The following describes the efforts of the Walking Forward research team to create and maintain relationships to successfully recruit AIs into Walking Forward projects, including the ATM genetic study.
Methods
The process of initiating, nurturing, and establishing a trusting partnership with any underserved community must occur at multiple levels, beginning with shared participation in the planning processes, continued consultation through project implementation, and shared responsibilities for data analyses and writing and disseminating study findings. Establishing a trusting partnership that can carry out these steps takes time and can be damaged or even destroyed by a single action.
Results
Several principles and recommendations emerged that affected the implementation, success, and expansion of the Walking Forward program. Below are 10 lessons learned while developing successful partnerships among participating tribal nations, urban representatives of tribal nations, and the Rapid City Regional Hospital’s research and administrative staff. Documenting the process of obtaining trust from this community is critical in order to expand the success of the Walking Forward project to other disparate populations.
Lesson #1. Do Not Let the Original Grant Application Drive the Partnership Process
This lesson encompasses several issues that address trust, relevance, communications, and partnerships.
Build trust prior to grant submission
The clinical impression of radiation oncologists at the Rapid City Regional Hospital was that AI cancer patients appeared more sensitive to the effects of therapeutic radiation, as discussed above. Upon arrival to the area in 1999, the Principal Investigator (PI) invited meetings with AI community leaders and IHS physicians to discuss the elevated radiation side effects and the availability of advanced radiation oncology treatment options. These meetings were initiated 2 years prior to applying for federal support. State-of-the-art technologies included 3-D conformal radiotherapy (3D-CRT), permanent seed implants for prostate cancer patients, and high-dose-rate brachytherapy for a variety of malignancies. The importance and potential benefits to patients using these new services were emphasized. For example, prostate cancer patients who underwent a permanent seed implant would complete their radiation procedure in 1 day compared to 8 weeks for external-beam radiation.
Identify a Request for Application relevant to both community and academic priorities and ensure both partners share interests
In 2001, the National Cancer Institute (NCI) released a Request for Application requesting planning grant applications from communities that experience health disparities. The PI (D.G.P.) coordinated additional meetings with both the tribal leadership and the IHS to introduce the general principles of cancer treatment and cancer research studies. He also provided updated information relating to the new technologies available in Rapid City and including photographs of the technology to help the community leaders and members gain familiarity with and understand the purpose of the equipment. He was available to answer questions from people in attendance at the meetings. At the end of the cancer treatment research presentation, members of the Tribal Council were asked for their opinions. The sentinel issues raised were trust and levels of commitment from physicians and researchers, high cancer death rates, and potential resolutions. After much discussion, the Tribal Councils approached were in favor of partnering with Rapid City Regional Hospital on the research proposal. They prepared and passed a tribal resolution in support of the study and included letters of support.
Initiate and maintain communication between community and academic partners following application submission and while waiting for NIH grant decisions
The PI (D.G.P.) continued the dialogue with the tribes in the event that the grant was funded. This dialogue consisted of cancer treatment updates and the potential research opportunity. Most of the communication was by telephone and e-mail. It was essential to maintain these emerging trust relationships.
Visit the community frequently
The PI spent time and effort to meet with tribal leadership in their home communities rather than assuming they were interested in driving to Rapid City. This demonstrated both commitment and respect to the AI communities. Academicians and researchers might assume they are busier than their community partners and that community partners welcome a visit to an academic setting. However, sharing the hosting of meetings and being sensitive to the costs of time and travel need to be considered carefully. In AI communities, members of the tribal leadership have responsibilities that allow little time for additional commitments. Also, in South Dakota, the leadership wanted the research team to experience the long drives and the driving conditions between the partner locations to enhance their understanding about the rigors of travel in rural areas and particularly the challenges for cancer patients to make these trips.
Ensure that the grant award is made to both academic and community partners
The PI received a competitive score and a notice of grant award early in 2002. He traveled to all three South Dakota tribal reservations and the urban Indian center that later became the four named community partners in the Walking Forward program. The division of funds was based on the original grant application and budget. Each reservation received one full-time employee: a community research representative (CRR) to work within that community. Each CRR was allocated funds for travel and education.
Be aware of changing tribal leadership and the need to maintain and establish partnerships and commitments
Each tribal nation has its own governance policies. Some tribal Nations in South Dakota vote on new leadership and other key positions every 2 years. When Rapid City was awarded the grant in 2002, the PI implemented another round of talks with the tribal health leaders and IHS medical staff to discuss implementation of the project on the three reservations and with the urban Indian program in Rapid City. At that time, the current tribal leaders were different than the leaders the PI had initially met with. It was necessary to re-explain the origins of the project and how previous tribal boards had endorsed the study. Many of the new tribal members were unaware of the project despite the tribal resolutions passed in previous settings approving the study. Appreciating the need for continuing conversations between academic and community partners, the Walking Forward staff provided quarterly updates to Tribal Councils representing each of the three reservations. This continued effort of keeping the tribal leadership engaged and informed helped to maintain and build trust as numerous letters of support and/or resolutions were required for each study protocol (discussed below).
Lesson #2. Consider How Oncology Consultations and Secure Study Consents Are Planned and Structured
Protocols and choices described by the provider (radiation oncologist)
The oncology consultation for a new patient requires 60 to 90 minutes. At least two-thirds of the consultation takes place in the physician’s office, where a detailed explanation of the treatment options is provided. For prostate cancer patients, numerous radiation options are presented using pictures on PowerPoint slides. A general written outline of treatment options is given to the patient, with additional handouts describing each treatment approach in detail. This process of describing various treatment options takes 30 to 45 minutes. Clinical trial options are discussed with patients only after the foundation of treatment has been presented. Patients and their family members are strongly encouraged to ask questions. Importantly, the health care provider avoids asking dichotomous questions that can be responded to with a nodding or shaking of the head (eg, “Do you understand?”). Each patient is asked questions that use interrogative pronouns such as “who, how, where, and when.” For example, “How would you or your family members travel to Rapid City for treatments if they were scheduled several days each week for six weeks?” or “If your brother called you tonight, how would you explain to him what these treatments include?” This question structure stimulates interactive communication between the provider and the patient. It is important to note that “why” questions are avoided because they can stimulate a more defensive response from a patient and/or imply that the patient’s actions were inappropriate (eg, “Why did you do that?” “Why did you think that was important?”). Concerns about coercion by the provider were addressed by excluding opportunities for enrollment until a second visit, as well as building in the PN component during which patients were invited to further consider what participation in a trial could require and how those needs would be met.
Patients are not asked to choose a treatment option during the initial consultation. If the patient expresses interest in a clinical trial, the written consent is taken home to be further reviewed and discussed with family members. Patients are not asked to sign a consent form during the initial consultation with the provider because there is too much information to process and some could simply sign without realizing what they are agreeing to. After having time to think about, reread, and talk about the treatment choices with others, the patient is invited to return to the hospital to meet again with the provider. Patients who choose a radiation option are invited to meet with the radiation oncologist to further discuss their treatment choice and what treatment will require. Consents are signed at the second appointment. Two consents are possible: one for radiation and one for the clinical trial, if the patient is eligible and chooses to participate. Initially, the PI was the only clinician who performed the above consent process. This process is based on his experience and guidance from the CRRs and other AIs in the health care profession. A second radiation oncologist is now involved in meeting, evaluating, and consenting AI patients for treatment and potentially for clinical trial participation.
Incorporate opportunities to address cultural needs and poverty-related barriers
AI cancer patients are invited to meet with a PN at the time of the initial consultation. The Walking Forward PN is an AI and also a nurse. The PN assesses each patient’s social support system and cultural beliefs and also identifies and suggests ways to address specific needs in order to complete the radiation treatment plan in a timely fashion. This consultation occurs regardless of whether the patient is interested in clinical trial participation. Logistical issues related to meals, transportation, lodging, and insurance are evaluated, and a plan for addressing these is established.
Coercion vs informed choice and respect for logistical challenges
Some federal committees challenge the validity of the clinical trial PI implementing the informed consent process. This challenge is based on fears of coercion by the provider. However, by not allowing patients to enroll in a clinical trial during their initial consult, the provider eliminates many aspects of possible coercion. Claims of coercion are also inappropriate because the PN works with the patients to address logistical factors regardless of their choice to participate or not participate in a clinical trial. PNs typically do not know if the patient chooses to enroll in a clinical trial. Thus, the protocols for addressing the patients’ logistical factors are unaffected by their clinical trial choice. Recognition and respect for the logistical barriers affecting cancer patients living in poverty (whether in rural reservations or in Rapid City) contribute to greater trust.
Lesson #3. The Number, Type, and Order in Which Approvals Are Secured Is Important
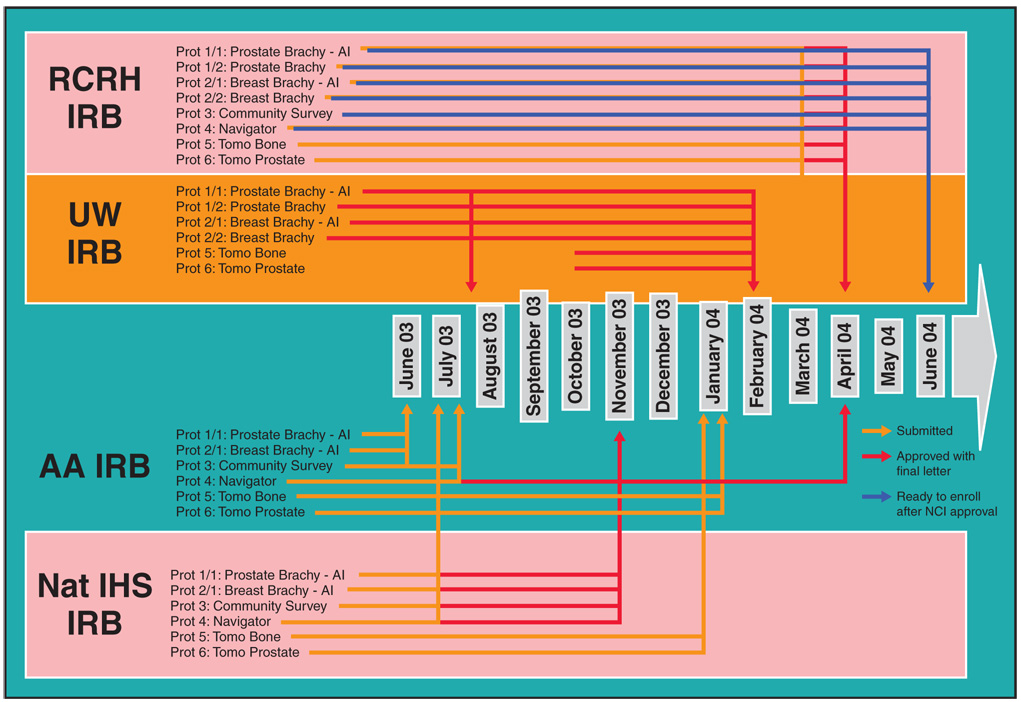
IRB approval was required from the Aberdeen Area IHS, the National IHS, the Rapid City Regional Hospital, the University of Wisconsin, the Mayo Clinic (ATM study only), and the NCI. The Aberdeen Area IHS IRB reviewed the protocol only after the scientific validity was reviewed and approved by the University of Wisconsin IRB and only after letters of support and/or tribal resolutions were obtained from the service unit director of the IHS, Tribal Councils from each reservation, as well as a letter of support from the Aberdeen Area Tribal Chairmen’s Health Board. Thus, each protocol required 7 letters of support and/or resolutions. With 9 distinct protocols, 63 letters of support were received. Due to the rigorous and necessary peer-review process for our project, a minimum of 12 months was needed to activate the first study (Figure). Each consent was written in a culturally appropriate manner with guidance, direct feedback, and input from the AI community. The process of achieving cultural competency is reviewed elsewhere.19
Figure.
Walking Forward Institutional Review Board (IRB) approval processes. From Burhansstipanov L, Bemis LT, Petereit DG. Native American communities perspective and genetics. In: Monsen R, ed. Genetics and Ethics in Health Care: New Questions in the Age of Genomic Health. Silver Spring, MD: American Nurses Publishing; 2008. In press. Reprinted with permission. RCRH = Rapid City Regional Hospital, UW = University of Wisconsin, AA = Aberdeen Area, Nat IHS = National Indian Health Service, AI = American Indian, Tomo = tomotherapy, Brachy = brachytherapy, Prot = protocol.
Due to the complexity of our project and also because some of the protocols were studies involving invasive cancer treatment, several meetings took place with members of the Aberdeen Area IHS IRB to not only explain the consent process for a study but also demonstrate how patients are consented to receive radiation and/or chemotherapy irrespective of study participation. These IRB members became more comfortable with the overall process once the formal consultation was explained in detail. This diligence and willingness to re-explain and/or illustrate the protocols via Power-Point slides contributed to tribal IRBs and tribal leadership feeling more comfortable that patients from their community were not being considered or treated as “guinea pigs”. Also, an open discussion about this concern was necessary between the Walking Forward research team, the PI, and the PN to alleviate uneasiness and improve trust on the staff level.
Lesson #4. Discontinue “Helicopter Research” Approaches
Initially, many tribal members and leaders suspected that our project would be another “fly by night” operation where we would come in, abstract the data, leave, and publish the results without any feedback or continuity (commonly referred to as “helicopter research” by many AI communities). By committing to 2 years of visits and discussions with community partners prior to the release of a relevant Request for Application, then initiating meetings during and following the notice of the grant award, and then participating in and ultimately securing the extensive IRB approvals necessary, the research team demonstrated their diligence, persistence, and ultimately their commitment to conducting the project and to addressing health disparities with their community partners.
By 2004, the genetic ATM protocol was refined and ready for the approval process. Because of the trusting partnership already established, this new protocol, which could have been considered by many as the most controversial component of the Walking Forward program, received approvals from the IRB and the tribal research committee in only 10 months vs the 12 to 18 month timelines necessary for the initial protocols. Initially, there was concern that offering a genetic test to a disparate population would never be approved and therefore we should consider abandoning this effort. These concerns were also due to cultural resistance and overall distrust of genetic research by many tribal Nations throughout Indian country. However, within the first year of implementation, 26 patients have enrolled, and none have withdrawn from the study. Only 1 AI patient declined to participate based on concerns related to sending blood for analysis.
Lesson #5. Share Preliminary and Final Research Findings With Tribal Leadership and the Community Along the Way
The PI and the PN shared preliminary findings (those that would not bias subsequent components of the study) with each of the Tribal Health Councils. Beginning in the middle of project year 2002, these updates with the tribal leadership were conducted every 3 to 6 months. Abstracts were submitted to the Tribal Councils for approval that allowed for presentation of data at national meetings. The community and the general public were also informed of the study findings through local radio shows, newsletters, and documents distributed by the PN within each community.
The PNs and CRRs have been the critical link between our program and the tribal leadership and the community. They are part of our research staff and are employed by Walking Forward. The details of our navigation program are discussed elsewhere in this issue.20
Lesson #6. Understand the Difference Between the Patient Informed Consent Process and Tribal IRBs Charged With Protecting Community Well-Being
The above approval process demonstrates the difference between the consent process for an individual at an academic center vs consenting the individual and the entire Lakota Nation in western South Dakota. Appropriately so, the Tribal Councils and IHS IRBs are charged with protecting the well-being of tribal community members. This is particularly evident when the historical trauma of Wounded Knee and the recent sterilization of AI women remain in the collective consciousness of AIs. Patience and respect are required of the researcher in order to learn more about local research appropriateness and the cultural aspects of this community and other disparate populations where historical trauma has occurred. The tragedy of Wounded Knee occurred in 1890, but in 1990, when honoring those who died, racist violent attacks were again made against the AIs in this region. The tragedy of Wounded Knee, an armed conflict between the United States and the Lakota Sioux where hundreds of AIs were killed, occurred in 1890 and is essentially ingrained in the collective conscience of the Lakota. Thus, these and many other historical trauma events are fresh in the memory of AIs living in South Dakota. If the researcher is unaware of the sensitivity of these events and the community’s memory, then trust will evaporate quickly.
Lesson #7. Do Not Underestimate Administrative Infrastructure Needs
The administrative infrastructure at Rapid City Regional Hospital was a limiting factor to the timely implementation of the Walking Forward project since there was no institutional experience with NIH grants of this magnitude. The NCI staff also underestimated this obstacle. A culture of research and NIH grants is not present in most community hospitals. Initially, basic issues such as a functional IRB and determination of the fiscal and administrative (indirect) rates were time consuming and onerous. In addition, many community hospitals are forced into a “bottom line” perspective where the value of NCI-sponsored research is vastly underappreciated until extensive education takes place. In Rapid City, approximately 2 years passed before various mid-level administrators understood the value of the Walking Forward program. In institutions with research experience, it is obvious that centers participating in research and particularly in clinical trials provide higher levels of care to all of their patients. Thus, it is not only the study participants but also the hospital and overall community who benefit from cutting-edge research in a rural setting.
Lesson #8. Who Should Consent the Patient for the Clinical Trial?
The consent process as implemented for the Walking Forward clinical trials arm of the study is described in Lesson #2. It is the opinion of the PI (D.G.P.) and coauthor (L.B.) that the physician should follow a respectful protocol for the informed consent process such as described in #2 (ie, choices described slowly enough for the patient to conceptualize and understand the process, allowing approximately 40 minutes compared to 15 minutes allocated by some health care settings) and that the patient should not decide to participate in a clinical trial during the initial consultation and explanation of what the trial entails. Because the protocols used within Walking Forward (eg, brachytherapy, tomotherapy) are complex, and although the PN can explain the overall processes, the patient usually has medical questions that can be answered only by the provider. Thus, the authors believe that in such informed consent processes, the physician is most appropriate to implement the informed consent process. The level of trust established at the initial and subsequent consultation processes is what leads to an individual patient feeling more comfortable about the treatment choices. Likewise, because the physician has taken the time to clearly explain and show all of the treatments, patients feel the information they have received is honest and accurate. They are able to make an informed choice regarding their participation in a clinical trial. The PNs are critical in this overall process as they emphasize the validity of the Walking Forward program and also discuss the possible benefits and drawbacks of each treatment with the patients in language that is easy to understand (these benefits and drawbacks are also addressed by the provider implementing the informed consent processes). As the program has evolved, the consent process has become easier because the community is already aware of the Walking Forward program through the PNs. Trust built at the community level rapidly translates into trust at the patient level when they present for treatment options at the cancer center.
Lesson #9. Consenting Process for the ATM Study
The ATM study is not presented until patients have started radiation. This approach minimizes patient information overload. During the second to third week of radiation, the rationale of the ATM study is presented to patients by the PI or the second radiation oncologist. Patients who have an interest in participating are given information describing the ATM study rationale and a copy of the consent form. They are instructed to read the consent form only after they have read the study background information and only if they have an interest in participating. Patients are directed to read the consent form but not sign it. One week later, another discussion takes place to determine if the patient understands the study and has any questions about participation. The formal consent process occurs only after all questions are asked and answered. Patients are asked to sign four consent forms. The first two address the following: (1) Is the information provided by the PI sufficient to consent to the blood draw, or do you want to visit with a genetic counselor via telemedicine for further questions? A genetic counselor is available for anyone interested. (2) Do you want the blood destroyed at the University of Wisconsin where the gene is sequenced, or do you want the blood returned to you? The third and fourth forms are the consent to have the blood drawn and the mandatory consent by the Health Insurance Portability and Accountability Act (HIPAA). To minimize any discomfort, the blood needed for the ATM sequencing is drawn at the same time as the weekly blood check to avoid a second venipuncture and a second visit. The ATM test is not offered to terminal patients with a short life expectancy to minimize any additional burdens.
The above consent process is performed in a fashion that attempts to be culturally competent. Achieving cultural competency was recently described in detail by our group.21 Many of these cultural competency issues were identified by both the AI community and the Walking Forward staff who are predominantly AIs.
Lesson #10. Consider Using Community Research Representatives to Promote Visibility for the Project
CRRs act as local liaisons to the PI and the PN. While the PI and other physicians consent patients to clinical trials, the continued local presence of CRRs on each reservation enhances this overall process through cancer education and outreach efforts. Cancer patients routinely seek out the CRRs to ask about breast brachytherapy and tomotherapy, terms which were not part of AI vocabulary prior to the Walking Forward study. CRRs provide quarterly updates at each Tribal Council meeting to maintain and enhance program credibility and visibility. The role of the CRRs demonstrates that the research project is being carried out in a manner respectful of cultural values and traditions.
Results of the ATM Study Component
The establishment of a trusting community-academic partnership to address cancer health disparities is evidenced by increasing numbers of community-based referrals for participation, rapid approvals by tribal leadership, and successful rates of patient consent/study completion rates. Unlike the first 8 project protocols that required 12 to 18 months to obtain IRB and tribal research committee approvals, the ATM study enrolled the first patient only 10 months after it was presented to the tribal health board. To date, we have offered the ATM study to every AI patient with cancer after the individual has completed the initial phases of radiation. Twenty-six of 27 patients have agreed to participate, and none have chosen to withdraw from the study. To date, no patients have asked to see a genetic counselor upfront or to discuss pending results. One AI did not participate due to concerns about sending the specimen to a different institution for analysis. Forty of 41 non-AIs have undergone ATM testing as well. So far, the gene has been sequenced in less than half of the patients with analysis underway. The primary reasons for participant eagerness to enroll on the study are the shared human desire to assist others, especially family and community members, and the demonstrated responsiveness to community priorities by academic researchers.
Discussion
The goal of the Walking Forward program is to lower cancer mortality rates and improve quality of life for AIs in the Northern Plains region. The research plan to accomplish this includes clinical trials, behavioral research, PN services, community outreach and education, and a genetic protocol (ATM) for members of three western South Dakota reservations, as well as the urban population in Rapid City.
The era of molecular profiling both the tumor and the normal tissues in order to optimize the therapeutic ratio for the patient is here. The ATM study is important since it attempts to correlate heterozygosity of the gene to radiation side effects. Furthermore, if there is a correlation, then radiation approaches can be customized for the individual patient. This is also known as radiogenomics and is currently evolving. Experiments are underway that enable the discovery of biomarkers for personalized cancer diagnosis, therapy, and prognosis of patient response to therapy.21,22 The ultimate goal of the ATM study is to determine a potential relationship to radiation toxicities, but it could not have been initiated without establishing a foundation of trust within the AI community. Many of these potential barriers to clinical trial accrual were identified through conducting focus groups. These led to culturally appropriate interventions such as those identified in the ATM consent process.
The swift approval of the ATM study by the IHS IRBs and the rapid enrollment of AIs on the protocol reflect years of planning, building trust, and conducting the required community-based research. The development and demonstration of mutual respect among the tribal communities, the IHS facilities, and the Rapid City Regional Hospital-based research team have laid the foundation for numerous future research projects under the Walking Forward infrastructure. Plans are now underway to significantly expand the Walking Forward program. Enhancements are expected to include radiogenomics, cancer screening and education efforts, access to more innovative treatment approaches, behavioral research, and palliative care. The research team is expected to expand via the inclusion of medical anthropologists. Discussions are currently underway with tribal health boards to elicit their input, advice, and potential support.
We recently found that, compared with white patients, AIs with cancer exhibit higher medical mistrust and lower satisfaction with health care (P = .0001) (D.G.P., unpublished data, February 2008). Developing methods to overcome this mistrust and dissatisfaction was a primary goal of many of the Walking Forward interventions detailed above. We are unaware of any other previous recruitment strategies that have been attempted and published for this population. The current strategy appears to be effective.
Conclusions
The ongoing Walking Forward study has been successful in recruiting to all arms of the research project. The recruitment and retention of patients into the ATM arm of the study is primarily due to the high level of trust established by the primary author and the AI CRRs within the study communities.3
The Walking Forward program has provided AIs in the western South Dakota area access to state-of-the-art cancer interventions and clinical trials, whereas in the past they were often the last to receive these types of therapies, if at all. Most AI cancer patients have been receptive and many even eager to participate. The principal reason why patients participate is to help other patients and their relatives with the cancer experience. The ultimate goal of the Walking Forward program is to lower cancer mortality rates, but realizing this goal will likely take 5 to 10 years.
Acknowledgments
Disclosures
No significant relationship exists between the authors and the companies/organizations whose products or services may be referenced in this article.
NIH grant - RFA 1U56CA99010-01. This project has been funded by the National Cancer Institute, National Institutes of Health, under Contract No. N01-CO-12400.
Abbreviations used in this paper
- AI
American Indian
- PN
patient navigator
- IHS
Indian Health Service
- CRR
community research representative
Footnotes
Appreciation is expressed to Sarah L. Esmond, MS, at the Center for the Study of Cultural Diversity in Healthcare, University of Wisconsin School of Medicine and Public Health, Madison, WI, for editing and reviewing this manuscript.
Disclaimer
The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the IHS, National Cancer Institute, or the National Institutes of Health.
References
- 1.Espey DK, Paisano RE, Cobb N. Cancer mortality among American Indians and Alaska Natives: regional difference, 1994–1998. Rockville, MD: Indian Health Service; 2003. Oct, [Google Scholar]
- 2.Petereit DG, Rogers D, Govern F, et al. Increasing access to clinical cancer trials and emerging technologies for minority populations: the Native American Project. J Clin Oncol. 2004;22(22):4452–4455. doi: 10.1200/JCO.2004.01.119. [DOI] [PubMed] [Google Scholar]
- 3.Petereit DG, Rogers D, Burhansstipanov L, et al. Walking Forward: the South Dakota Native American Project. J Cancer Educ. 2005;20 suppl 1:65–70. doi: 10.1207/s15430154jce2001s_14. [DOI] [PubMed] [Google Scholar]
- 4.Rogers D, Petereit DG. Cancer disparities research partnership in Lakota Country: clinical trials, patient services and community education for the Oglala, Rosebud and Cheyenne River Sioux tribes. Am J Public Health. 2005;95(12):2129–2132. doi: 10.2105/AJPH.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burhansstipanov L, Bemis LT, Petereit DG. Native American communities perspective and genetics. In: Monsen R, editor. Genetics and Ethics in Health Care: New Questions in the Age of Genomic Health. Silver Spring, MD: American Nurses Publishing; 2008. In press. [Google Scholar]
- 6.Christopher S. Recommendations for conducting successful research with Native Americans. J Cancer Educ. 2005;20(1 suppl):47–51. doi: 10.1207/s15430154jce2001s_11. [DOI] [PubMed] [Google Scholar]
- 7.Burhansstipanov L, Christopher S, Schumacher SA. Lessons learned from community-based participatory research in Indian Country. Cancer Control. 2005;12 supp2:70–76. doi: 10.1177/1073274805012004s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed on April 16, 2008];US Dept of Energy Office of Science, Office of Biological and Environmental Research, Human Genome Program; Human Genome Project Information: Minorities, Race, and Genomics. 2006 http://www.ornl.gov/sci/techresources/Human_Genome/elsi/minorities.shtml.
- 9.Human Genome Diversity Project: Model Ethical Protocol for Collecting DNA Samples. North American Regional Committee: Morrison Institute for Population and Resource Studies, Stanford University; 1999. [Accessed on April 16, 2008]. http://www.stanford.edu/group/morrinst/hgdp/protocol.html#Q1. [Google Scholar]
- 10.Burhansstipanov L, Bemis L, Kaur JS, et al. Sample genetic policy language for research conducted with Native communities. J Cancer Educ. 2005;20 suppl 1:52–57. doi: 10.1207/s15430154jce2001s_12. [DOI] [PubMed] [Google Scholar]
- 11.Mackie TR, Kapatoes J, Ruchala K, et al. Image guidance for precise conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56(1):89–105. doi: 10.1016/s0360-3016(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 12.Eastmo E, Petereit DG. Accelerated partial breast irradiation: expanding options for breast preservation. Adv Imaging Oncol Admin. 2005:91–95. [Google Scholar]
- 13.Tucker SL, Turesson L, Thames HD. Evidence for individual differences in the radiosensitivity of human skin. Eur J Cancer. 1992;28(A):1783–1791. doi: 10.1016/0959-8049(92)90004-l. [DOI] [PubMed] [Google Scholar]
- 14.Russell NS, Knaken H, Bruinvis IA, et al. Quantification of patient to patient variation of skin erythema developing as a response to radiotherapy. Radiother Oncol. 1994;30(3):213–221. doi: 10.1016/0167-8140(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 15.Geara FB, Peters LJ, Ang KK, et al. Intrinsic radiosensitivity of normal human fibroblasts and lymphocytes after high- and low-dose-rate irradiation. Cancer Res. 1992;52(22):6348–6352. [PubMed] [Google Scholar]
- 16.Weichselbaum RR, Epstein J, Little JB. In vitro radiosensitivity of human diploid fibroblasts derived from patients with unusual clinical responses to radiation. Radiology. 1976;121(2):479–482. doi: 10.1148/121.2.479. [DOI] [PubMed] [Google Scholar]
- 17.Swift M, Morrell D, Massey RB, et al. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med. 1991;325(26):1831–1836. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- 18.Iannuzzi CM, Atencio DP, Green S, et al. ATM mutations in female breast cancer patients predict for an increase in radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2002;52(3):606–613. doi: 10.1016/s0360-3016(01)02684-0. [DOI] [PubMed] [Google Scholar]
- 19.Molloy K, Reiner M, Ratteree K, et al. Cultural competency in cancer care: developing and implementing a patient navigator program in American Indian communities. Assoc Community Cancer Cent. 2007;22(5) [Google Scholar]
- 20.Petereit DG, Molloy K, Reiner ML, et al. Establishing a patient navigator program to reduce cancer disparities in the American Indian communities of western South Dakota: initial observations and results. Cancer Control. 2008;15(3):254–259. doi: 10.1177/107327480801500309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svensson JP, Stalpers LJ, Esveldt-van Lange RE, et al. Analysis of gene expression using gene sets discriminates cancer patients with and without late radiation toxicity. PLoS Med. 2006;3(10):e422. doi: 10.1371/journal.pmed.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claudino WM, Quattrone A, Biganzoli L, et al. Metabolomics: available results, current research projects in breast cancer, and future applications. J Clin Oncol. 2007;25(19):2840–2846. doi: 10.1200/JCO.2006.09.7550. Epub 2007 May 14. [DOI] [PubMed] [Google Scholar]