Abstract
Gas3/PMP22 plays a crucial role in regulating myelin formation and maintenance, and different genetic alterations in gas3/PMP22 are responsible for a set of human peripheral neuropathies. We have previously demonstrated that Gas3/PMP22 could regulate susceptibility to apoptosis in NIH3T3 cells but not in REF 52 cells. In this report we demonstrate that when the apoptotic response triggered by gas3/PMP22 was counteracted by Bcl-2 coexpression, morphological changes were observed. Time-lapse analysis confirmed that Gas3/PMP22 can modulate cell spreading, and this effect was strengthened after inhibition of phosphoinositide 3-kinase. Using the active form of the small GTPase RhoA, we have been able to dissect the different Gas3/PMP22 biological activities. RhoA counteracted the Gas3/PMP22-dependent morphological response but was unable to neutralize the apoptotic response. Treatment of NIH3T3 cells with cytotoxic necrotizing factor 1, which activates endogenous Rho, also counteracted Gas3/PMP22-mediated cell shape and spreading changes. Treatment of REF 52 cells, which are unresponsive to Gas3/PMP22 overexpression, with the C3 exoenzyme, inhibiting Rho activity, renders REF 52 cells responsive to Gas3/PMP22 overexpression for cell shape and spreading changes. Finally, assembly of stress fibers and focal adhesions complexes, in response to lysophosphatidic acid–induced endogenous Rho activation, was impaired in Gas3/PMP22-overexpressing cells. We hypothesize that cell shape and spreading regulated by Gas3/PMP22 through the Rho GTPase might have an important role during Schwann cells differentiation and myelinization.
INTRODUCTION
Gas3/PMP22, a member of an extended family of tetraspan membrane proteins (Marvin et al., 1995; Taylor et al., 1995; Bolin et al., 1997; Magyar et al., 1997), is highly expressed in myelinating Schwann cells, where it represents 2–5% of total myelin proteins, largely confined to compact myelin (Spreyer et al., 1991; Welcher et al., 1991; Snipes et al., 1992; Suter and Snipes, 1995). Gas3/PMP22 expression is also induced during growth arrest and apoptosis in fibroblasts (Manfioletti et al., 1990; Brancolini et al., 1997), and it has been detected during mouse development and in adulthood in different neural and non-neural tissues (Baechner et al., 1995; Fabbretti et al., 1995).
Genetic studies (for review, see Patel and Lupski, 1994; Suter and Snipes, 1995) and the generation of gas3/PMP22-deficient mice (Adlkofer et al., 1995) have clearly established that gas3/PMP22 is responsible for a set of inherited peripheral neuropathies in mice and humans. Charcot–Marie–Tooth type 1 (CMT1) disease is a peripheral neuropathy characterized by progressive distal muscle weakness and atrophy and impaired sensation of the limbs. The most common form of CMT1 disease, CMT1A, is characterized by genomic duplication on 17p11.2-p12 containing the gas3/PMP22 locus (Lupski et al., 1991; Matsunami et al., 1992; Patel et al., 1992; Timmerman et al., 1992; Valentijn et al., 1992b). Deletion of the same region is responsible for hereditary neuropathy with liability to pressure palsies (Chance et al., 1993). Further detection of point mutations in the gas3/PMP22 gene in nonduplication CMT1A families (Valentijn et al., 1992a; Roa et al., 1993b) and in the related severe congenital hypertrophic neuropathy Dejerine–Sottas syndrome (Roa et al., 1993a) confirmed gas3/PMP22 as disease gene.
If genetic studies hallmark a critical role of gas3/PMP22 in the formation and maintenance of the myelin envelope, its widespread expression suggests a more general biological function (Suter et al., 1994).
Data from culture overexpression studies indicate a role of gas3/PMP22 in regulating cell growth. Retroviral gas3/PMP22 transfer in cultured Schwann cells underlined a growth-suppressive function (Zoidl et al., 1995), and ectopic expression in NIH3T3 fibroblasts was associated with an apoptotic response (Fabbretti et al., 1995; Zoidl et al., 1997). Interestingly, in the last case when gas3/PMP22 point mutants associated with CMT1A were similarly overexpressed the apoptotic response was reduced and behaved as dominant negatives when coexpressed with the wild-type gas3/PMP22 (Fabbretti et al., 1995).
In this report we have analyzed the apoptotic response triggered by gas3/PMP22 overexpression, and we have observed that when the apoptotic response was counteracted, overexpression of gas3/PMP22 could still induce alterations of cell shape and spreading, which are counteracted by the small GTPase RhoA (for review, see Van Aelst and D’Souza-Schorey, 1997; Hall, 1998). These results confirm the role of Gas3/PMP22 in triggering cell death and unveil a second function in controlling cell morphology, possibly trough the modulation of Rho small GTPase.
MATERIALS AND METHODS
Culture Conditions
NIH3T3 and Swiss 3T3 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% FCS, penicillin (100 U/ml), and streptomycin (100 μg/ml).
For serum starvation, medium was changed to 0.1% FCS when cells were subconfluent; cells were then left in this medium for 36 h. Lysophosphatidic acid (LPA; Sigma, St. Louis, MO) was used at a final concentration of 70 μM in medium containing 0.1% FCS.
Rat Schwann cells were prepared from the sciatic nerves of neonatal Wistar rats (Brockes et al., 1979). Purified Schwann cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% FCS and 2 μM forskolin for a limited period.
For microinjection assays, cells were grown on coverslips in 35-mm Petri dishes containing 8 × 104 cells per dish. After a 24-h incubation at 37°C in 5% CO2 atmosphere, cells were microinjected with the appropriate expression plasmid.
Cytotoxic necrotizing factor 1 (CNF1; 1 μg/ml final concentration), purified as previously described (Fiorentini et al., 1997) was added to the culture medium after microinjection. z-Val-Ala-Asp.fluoromethylketone was obtained from Bachem (Bubendorf, Switzerland). Stock solutions in DMSO were stored at −80°C and used at 100 μM final concentration. Wortmannin (WT) and LY294002 were used, respectively, at 1.33 and 13.3 μM final concentrations.
Microinjection and Time Lapse
Nuclear microinjection was performed using the Automated Injection System (Zeiss, Oberkochen, Germany) as previously described (Fabbretti et al., 1995). Nuclei of the cells were injected with the different expression vectors for 0.5 s at the constant pressure of 150 hectopascal. For time-lapse analysis cells were directly plated on the Petri dishes on which squares of ∼4 mm2 were marked. After microinjection of pGDSV7gas3/PMP22 or pGDSV7bax in the nucleus of growing NIH3T3 cells, the coordinates of the injected cells were stored on a computer disk (Brancolini et al., 1995). Microinjected cells were grown for a further 10 h at 37°C in 5% CO2 atmosphere and time-lapse series were collected for 22 h at 1-h intervals. During intervals cells were incubated at 37°C in 5% CO2 atmosphere.
Immunofluorescence Microscopy
For indirect immunofluorescence microscopy, microinjected NIH3T3 cells were fixed with 3% paraformaldehyde in PBS for 20 min at room temperature. Fixed cells were washed with PBS and 0.1 M glycine, pH 7.5, and then permeabilized with 0.1% Triton X-100 in PBS for 5 min. The coverslips were treated with the different first antibodies: anti-hGAS3/PMP22 (Fabbretti et al., 1995), anti-Gas2 (Brancolini et al., 1995), anti-hTR OKT-9, anti-FLAG (Sigma), and anti-P0 (Archelos et al., 1993), diluted in PBS and 3% BSA for 1 h in a moist chamber at 37°C. They were then washed with PBS three times, followed by incubation with the relative secondary antibodies: FITC-conjugated anti-mouse (Sigma), TRITC-conjugated anti-mouse (Southern Biotechnology, Birmingham, AL), TRITC-conjugated anti-rabbit (Dako, Glostrup, Denmark), and FITC-conjugated anti-rabbit (Sigma), for 1 h at 37°C. Cells were examined by epifluorescence with a Zeiss Axiovert 35 microscope or a Zeiss laser scan microscope (LSM 410) equipped with a 488 λ argon laser and a 543 λ helium neon laser
Survival Assay
The effect on cell survival of different genes was analyzed using an automated injection system. For each experiment an established number of cells (200) was microinjected with the gene of interest and a reporter gene. Cell survival was calculated as the number of recovered cells expressing the reporter gene. All cDNAs were cloned in the same expression vector (Brancolini et al., 1995) and microinjected in NIH3T3 cells. When the pGDSV7-h-TR was used as reporter, it was injected at the concentration of 25 ng/μl.
Statistical significance was determined for all data by using one-way analysis of variance (F test); in the case of multiple comparisons the Student–Neuman–Keuls test (q test) was then applied.
Plasmid Construction
Human gas3/PMP22 cDNA (Edomi et al., 1993) was subcloned in frame with a C-terminal FLAG (Gas3/PMP22-FLAG) in pGDSV7-FLAG. The hgas3/PMP22 cDNA was amplified by PCR using a sense primer (5′-GAGTGAATTCAACTCCGCTGAGCAGAACTT-3′) containing an EcoRI site and a reverse primer containing a HindIII site (5′-CGCAAGCTTTTCGCGTTTCCGCAAGATCA-3′).
The pGDSV7-FLAG vector was created by inserting an oligonucleotide cassette for the FLAG peptide (DYKDDDDK) in the pGDSV7 vector (Brancolini et al., 1995).
All constructs generated were sequenced using an automated laser fluorescence system to check for the translating fidelity of the inserted PCR fragments.
The crmA cDNA was excised from plasmid pDX10 (Xue and Horvitz, 1995) and cloned as NotI–HindIII fragment into pGDSV7. The bax cDNA (Oltvai et al., 1993) was excised from pSK as an EcoRI fragment and subcloned into the EcoRI site of pGDSV7. The cDNAs encoding Bcl-2 (Brancolini et al., 1995), the P0 protein (Lemke et al., 1988), and adenylate kinase (AK) were subcloned into the EcoRI site of the pEXV vector (Ridley et al., 1992).
RESULTS
Apoptosis and Morphological Changes Induced by gas3/PMP22 Overexpression in NIH3T3 Cells
To dissect the apoptotic response triggered by overexpression of gas3/PMP22 in NIH3T3 cells we decided to study whether well-characterized apoptotic antagonists such as the proto-oncogene bcl-2 (Reed, 1997) and the cowpox virus gene crmA (cytokine response modifier A), which encodes a caspase inhibitor (Cohen, 1997), were able to block cell death as elicited by gas3/PMP22.
Apoptosis can be easily monitored and assessed by analyzing cell survival. We used a microinjection-based assay to score cell survival in response to both overexpression of apoptotic genes and the presence of apoptotic stimuli (see MATERIALS AND METHODS).
We first analyzed the antiapoptotic activity of bcl-2 and crmA in NIH3T3 cells deprived of serum. bcl-2, crmA, and two different controls, gas2 and human placental alkaline phosphatase (hPLAP) (100 ng/μl), were coexpressed with the human transferrin receptor (h-TR) as reporter gene (25 ng/μl). Six hours after microinjection apoptosis was induced by serum deprivation, and 16 h later cells were fixed for immunofluorescence analysis. Cell survival was scored as number of recovered cells positive for h-TR.
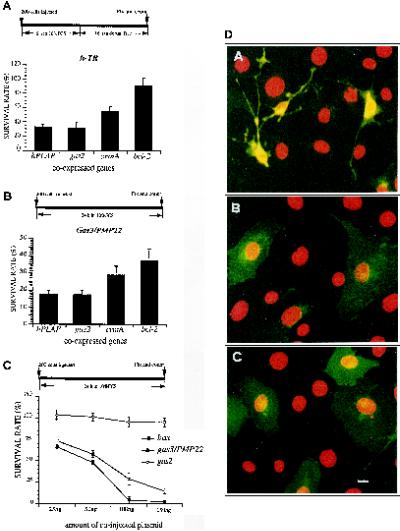
In cells coexpressing the control genes gas2 and hPLAP, recovery of h-TR-positive cells after induction of apoptosis by complete serum deprivation was impaired with a survival rate of 31 ± 9–33 ± 5% (Figure 1A). In contrast, survival was dramatically increased in cells coexpressing bcl-2/h-TR with 90 ± 15% of survival rate (p < 0.05). Coexpression of crmA less efficiently counteracted apoptosis with respect to bcl-2; in fact survival rate was 55 ± 6.5% (p < 0.05).
Figure 1.
bcl-2 counteracts apoptosis induced by gas3/PMP22. (A) bcl-2, crmA, gas2, and hPLAP were coexpressed with h-TR in NIH3T3 cells. Six hours after microinjection apoptosis was induced by serum deprivation. Sixteen hours later cells were fixed and processed for immunofluorescence to detect h-TR. Survival was scored as described in the text. Data represent arithmetic means ± SD for five independent experiments (p < 0.001). (B) bcl-2, crmA, gas2, and hPLAP were coexpressed with gas3/PMP22 in NIH3T3 cells. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect Gas3/PMP22. Survival was scored as described in the text. Data represent arithmetic means ± SD for five independent experiments (p < 0.001). (C) gas3/PMP22 and bax killer activity. Different amounts of pGDSV7S containing gas3/PMP22, bax, or gas2 were comicroinjected with pGDSV7h-TR (25 ng/μl) in the nuclei of NIH3T3 cells. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect h-TR. Survival was scored as described in the text. Datarepresent arithmetic means ± SD for three independent experiments. (D) gas3/PMP22-dependent morphological changes: confocal generated overlay showing cellular and nuclear phenotypes in NIH3T3 cells coexpressing Gas3/PMP22 and Bcl-2 (A) or h-Tr and Bcl-2 (B) or P0 (C). NIH3T3 cells 24 h after seeding were microinjected with pGDSV3-hTR (100 ng/μl) and pGDSV7-bcl-2 (50 ng/μl), with pGDSV7-gas3/PMP22 (100 ng/μl) and pGDSV7-bcl-2 (50 ng/μl), or with pEXV-P0 (100 ng/μl). After 24 h cells were fixed and processed for immunofluorescence analysis to visualize Gas3/PMP22 (dA), h-TR (dB), or P0 (dC) (green) using the specific antibodies. Propidium iodide was used to visualize nuclei (red). Images were overlayered using a Zeiss confocal microscope and are displayed in pseudocolor. Bar, 5 μm.
Having demonstrated the antiapoptotic activity of Bcl-2 and CrmA in our cellular system we next assessed the protective effects of both crmA and bcl-2 on cells overexpressing gas3/PMP22.
gas3/PMP22 was coexpressed with bcl-2, crmA, or different control genes by nuclear microinjection. Cells were grown for further 24 h in 10% FCS and then scored for Gas3/PMP22 expression using anti-Gas3/PMP22 antibody, by immunofluorescence analysis.
The survival rate of microinjected cells coexpressing gas3/PMP22 and the different control genes was 17 ± 2.5%. Coexpression of either bcl-2 or crmA significantly added to the number of Gas3/PMP22 expressing cells recovered, thus increasing the survival rate, respectively, to 37 ± 6 or 28.5 ± 5% (p < 0.05) (Figure 1B). Similar increased survival rates (27 ± 5%) in cells overexpressing Gas3/PMP22 were also obtained when a broad-spectrum cell-permeable caspase inhibitor, the benzyloxycarbonyl-Val-Ala-Asp.fluoromethylketone (McCarthy et al., 1997), was used (our unpublished results).
Having demonstrated that gas3/PMP22 overexpression leads to an apoptotic response inhibited by Bcl-2, the next step was to compare the Gas3/PMP22 apoptotic activity with that of a well-known killer gene such as bax. Bax is a proapoptotic member of the Bcl-2 protein family (Oltvai et al., 1993; Green and Kroemer, 1998; Silke and Vaux, 1998).
NIH3T3 fibroblasts were injected with h-TR, as reporter gene, and with increasing amounts of gas3/PMP22 or the killer gene bax (Figure 1C). bax reduced survival rates to 66 ± 1% when coexpressed at a concentration of 25 ng/μl, and full killer activity was detected at 100 ng/μl (survival rate, 4 ± 1.5%).
gas3/PMP22 when coexpressed at concentration of 25 ng/μl reduced the survival rate to 74.5 ± 3%, and its killer activity constantly increased at higher coexpression dosages, reaching a survival rate of 13.5 ± 2% at a concentration of 150 ng/μl. When h-TR was coexpressed with an increasing amount of the control gene gas2, survival rates were ∼100% (Figure 1C).
In summary, even though gas3/PMP22 and bax showed an overall similar dose-dependent killer activity, when measured as survival rates, bax was more active in triggering cell death than gas3/PMP22.
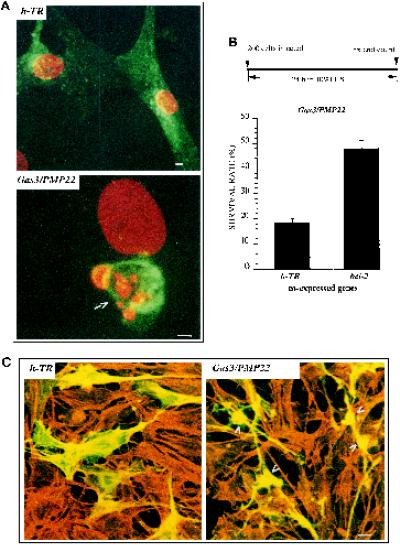
When a detailed analysis of the cell morphology was performed in Gas3/PMP22- and Bcl-2-coexpressing cells, it was evident that relative to the increased recovery of Gas3/PMP22-overexpressing cells a significant fraction of them showed peculiar morphological changes (see the next sections for the quantitative analysis). As shown in Figure 1D, B, NIH3T3 cells coexpressing h-TR and Bcl-2 presented a normal phenotype, cells appearing well spread and nuclei regular. On the contrary, cells coexpressing Gas3/PMP22 and Bcl-2 showed a peculiar phenotype. In these cells spreading was apparently reduced, some thin cellular processes could be observed, and nuclei maintained their overall normal morphology without evident markers of fragmentation (Figure 1D, A).
Several mutations in the P0 gene, which encodes for another component of the compact myelin, have been found, and the resulting phenotypes were clinically classified as CMT1B or Dejerine–Sottas syndrome (for review, see Suter and Snipes, 1995). Therefore, we analyzed whether similar morphological changes were induced in NIH3T3 cells after overexpression of P0. Despite the mutual involvement of gas3/PMP22 and P0 in generating peripheral neuropathies, overexpression of P0 was unable to induce morphological changes in NIH3T3 cells (Figure 1D, C).
The described altered phenotype could be dependent on a delayed apoptotic response or on a second biological activity of Gas3/PMP22, which could become more evident in the presence of Bcl-2. This observation prompted us to investigate whether Gas3/PMP22 could play a role also in regulating morphological changes.
Overexpression of Gas3/PMP22 in Schwann Cells Triggers Apoptosis and Cell Shape Changes
Gas3/PMP22 is abundantly expressed in myelinating Schwann cells, and different genetic alterations involving Gas3/PMP22 are responsible for different peripheral neuropathies. Having demonstrated a role of Gas3/PMP22 in regulating apoptosis and possibly morphological changes in NIH3T3 fibroblasts, we next analyzed whether similar biological activities could be observed also after its overexpression in highly purified rat Schwann cells (Zoidl et al., 1995).
Schwann cells were microinjected with pGDSV7-hgas3/PMP22, fixed 24 h later, and processed for immunofluorescence analysis to detect Gas3/PMP22. A reduced number of Schwann cells overexpressing Gas3/PMP22 were recovered with respect to h-TR-overexpressing cells used as a control, thus suggesting a reduced cell survival (our unpublished results). In addition, some Gas3/PMP22-overexpressing cells showed apoptotic features such as a collapsed cellular body and nuclear fragmentation, whereas h-TR-overexpressing Schwann cells showed a normal phenotype (Figure 2A).
Figure 2.
gas3/PMP22-dependent biological activities in Schwann cells. (A) Confocal generated overlay showing nuclear morphology in rat Schwann cells overexpressing Gas3/PMP22 or h-TR. Schwann cells 24 h after seeding were microinjected with pGDSV7-gas3/PMP22 (100 ng/μl) or with pGDSV3-hTR (100 ng/μl); after 24 h cells were fixed and processed for immunofluorescence analysis to visualize Gas3/PMP22 or h-TR using the specific antibodies (green). Propidium iodide was used to visualize nuclei (red). Images were overlayered using a Zeiss confocal microscope and are displayed in pseudocolor. The arrow indicates a Gas3/PMP22-overexpressing cell. Bar, 5 μm. (B) bcl-2 and h-TR were coexpressed with gas3/PMP22 in Schwann cells. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect Gas3/PMP22. Survival was scored as described in the text. Data represent arithmetic means ± SD for five independent experiments (p < 0.001). (C) Confocal generated overlay showing actin architecture and cell morphology in Schwann cells cells coexpressing Gas3/PMP22 Bcl-2 and Gas2 or h-Tr Bcl-2 and Gas2. Schwann cells 24 h after seeding were microinjected with pGDSV3-hTR (50 ng/μl), pGDSV7-bcl-2 (50 ng/μl), and pGDSV7-gas2 (25 ng/μl) or with pGDSV7-gas3/PMP22 (50 ng/μl), pGDSV7-bcl-2 (50 ng/μl), and pGDSV7-gas2 (25 ng/μl). After 24 h cells were fixed and processed for immunofluorescence analysis to visualize Gas2 (green), using the specific antibody and actin filaments (red) and rhodamine-phalloidin. Images were overlayered using a Zeiss confocal microscope and are displayed in pseudocolor. Bar, 15 μm.
To confirm the induction of an apoptotic phenotype after Gas3/PMP22 overexpression in Schwann cells, gas3/PMP22 was coexpressed with bcl-2 or with h-TR as control gene. Cells were grown for further 24 h in 10% FCS and then scored for Gas3/PMP22 expression using anti-Gas3/PMP22 antibody.
The survival rate of the microinjected cells coexpressing gas3/PMP22 and the h-TR was ∼18 ± 1.7%. In contrast, survival was increased in Schwann cells coexpressing gas3/PMP22 and bcl-2, with ∼48 ± 3.3% of survival rate (Figure 2B).
We next analyzed cell morphology of Schwann cells coexpressing Gas3/PMP22 and Bcl-2. For this purpose gas3/PMP22 (50 ng/μl), bcl-2 (50 ng/μl), and gas2 (25 ng/μl), used as reporters, were comicroinjected in Schwann cells, and as a control h-TR (50 ng/μl), bcl-2 (50 ng/μl), and gas2 (25 ng/μl) were coexpressed. Double immunofluorescence was performed to visualize Gas2 and actin filaments. As shown in Figure 2C, Schwann cells coexpressing, h-TR Bcl-2, and Gas2 presented a normal phenotype; on the contrary, Schwann cells coexpressing Gas3/PMP22 Bcl-2 and Gas2 showed an altered phenotype. As described above for NIH3T3 fibroblasts, cell spreading was apparently reduced, and some thin cellular processes could be observed (Figure 2C, arrowheads).
In summary, Schwann cells respond to Gas3/PMP22 overexpression, showing a reduced survival and a specific morphological response, as reported above for NIH3T3 fibroblasts. These results differ from a previously reported growth-suppressing function of Gas3/PMP22 in cultured Schwann cells using retroviral-mediated gene transfer (Zoidl et al., 1995). This discrepancy probably reflects the distinct methodological approaches applied in these studies, which lead to different levels of Gas3/PMP22 expression and different times of analysis.
Time-Lapse Observation of the Morphological Changes in Living gas3/PMP22-overexpressing Cells
From the previous analysis it was clear that primary Schwann cells and NIH3T3 fibroblasts respond similarly to Gas3/PMP22 overexpression in terms of both cell death and morphological changes. To observe the appearance, development, and dynamics of gas3/PMP22-induced morphological changes in living cells and to discriminate between the apoptotic and the morphological responses, we decided to perform a time-lapse analysis. Because Schwann cells and NIH3T3 fibroblasts respond similarly to Gas3/PMP22 overexpression, we decided to use NIH3T3 cells for this analysis.
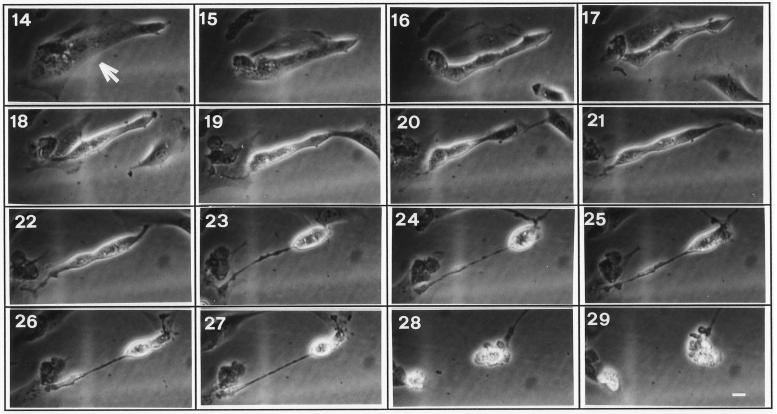
Figure 3 shows views of a typical cell overexpressing gas3/PMP22 (arrow). Fifteen hours after microinjection some changes on cell morphology can be observed. A gas3/PMP22-overexpressing cell reduced spreading on the substratum, and this process slowly evolved until 22 h after microinjection. Between 22 and 23 h the cell became rounded, and a thin process reminiscent of the adhesion area was detectable. After this time the cell maintained this morphology, with a slight decrease in size, until 28 h when membrane blebbing was evident, indicating a switch to apoptosis.
Figure 3.
Time-lapse images of a NIH3T3 cell overexpressing gas3/PMP22. Representative cell (arrow) injected with pGDSV7-gas3/PMP22 (100 ng/μl). Pictures at selected times after microinjection (as indicated) show the morphological changes from 17 h and the appearance of membrane blebbing at 28 h. Bar, 20 μm.
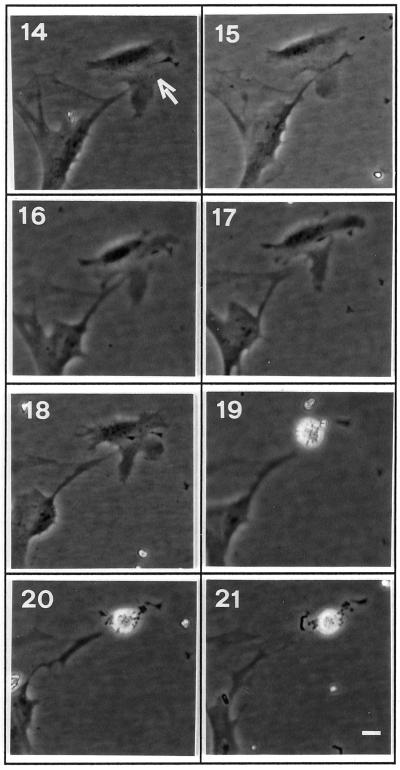
When the same analysis was performed for a bax-overexpressing cell, the emerging picture was different. As shown in Figure 4, a bax-overexpressing cell (arrow) showed a normal phenotype until 18 h from microinjection. At 19 h membrane blebbing was evident, indicating activation of the apoptotic process. This behavior is in aggreement with a previously reported analysis in fibroblastic cells (McCarthy et al., 1997) in which apoptosis, in terms of morphological events, was described as a process that typically takes between 30 and 60 min. Therefore, in the bax-overexpressing cells no specific morphological changes anticipating the appearance of membrane blebbing, as in the case of gas3/PMP22, were observed.
Figure 4.
Time-lapse images of an NIH3T3 cell overexpressing bax. Representative cell (arrow) injected with pGDSV7-bax (50 ng/μl). Pictures at selected times after microinjection (as indicated) show the appearance of membrane blebbing at 19 h. Bar 20 μm.
This analysis suggested that Gas3/PMP22 overexpression in NIH3T3 cells leads to both drastic alteration of cell morphology characterized by reduced spreading and retraction from adhesion area, and cell death by apoptosis.
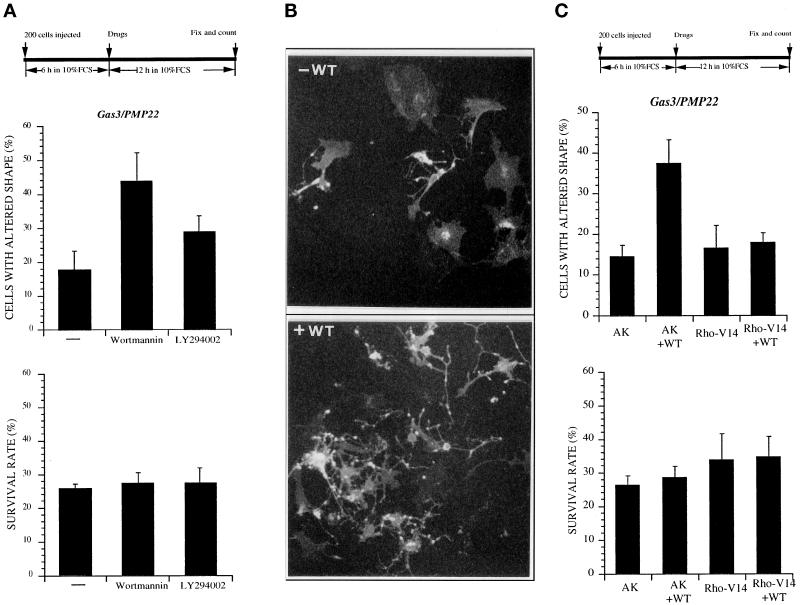
Morphological Changes but Not Apoptosis Can Be Counteracted by Overexpression of Active RhoA
The Rho family of GTP-binding proteins have been implicated in different biological activities (for review, see Van Aelst and D’Souza-Schorey, 1997; Hall, 1998) and more specifically the organization of the actin cytoskeleton and the formation/maintenance of focal adhesion, the sites where stress fibers are linked via integrins to the extracellular matrix (Ridley and Hall, 1992; Machesky and Hall, 1996). Having identified a putative function of Gas3/PMP22 in regulating cell shape and spreading, we decided to analyze whether the constitutively active form of RhoA (RhoA-V14) was able to interfere with the morphological changes induced by gas3/PMP22 overexpression.
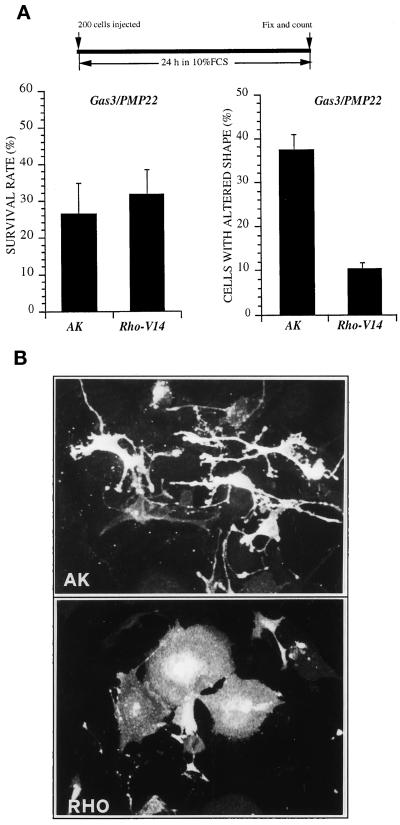
gas3/PMP22FLAG was coexpressed by nuclear microinjection in NIH3T3 cells together with rhoA-V14 or with AK as control gene. All the cDNAs were cloned in the same expression vector and microinjected, respectively, at the concentrations of 100 ng/μl (gas3/PMP22FLAG) and 25 ng/μl (AK and rhoA-V14). Cells were grown for further 24 h in 10% FCS and then scored both for Gas3/PMP22 expression and for morphological changes by immunofluorescence analysis.
To analyze the Gas3/PMP22-specific morphological changes, therefore excluding cells in apoptosis, only cells that presented reduced spreading sometimes with thin elongated processes (Figure 5B), but without apoptotic markers such as membrane blebbing and evident nuclear fragmentation, were considered for this analysis.
Figure 5.
Coexpression of Gas3/PMP22 and RhoA-V14 in NIH3T3 cells. (A) AK and rhoA-V14 were coexpressed with gas3/PMP22 in NIH3T3 cells. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG. Survival and morphological changes were scored as described in the text. Data represent arithmetic means ± SD for seven independent experiments (survival rate, p = 0.225; altered shape, p < 0.001). (B) Immunofluorescence analysis of NIH3T3 cells coexpressing gas3/PMP22 and AK or gas3/PMP22 and rhoA-V14 or gas3/PMP22. NIH3T3 cells 24 h after seeding were microinjected with pEXV-gas3/PMP22FLAG (100 ng/μl) and pEXV-AK (25 ng/μl) or with pEXV-gas3/PMP22FLAG (100 ng/μl) and pEXV-RhoA-V14 (25 ng/μl). After 24 h cells were fixed and processed for immunofluorescence to visualize Gas3/PMP22FLAG. Bar, 5 μm.
When compared with the survival rates reported above (Figure 1B) overexpression of gas3/PMP22FLAG showed reduced killer activity (∼27 ± 8% survival rate compared with the ∼17 ± 2.5% of the previous experiments). Such a discrepancy was dependent on the different detection system used for the immunofluorescence analysis. In fact, the antibody against the FLAG epitope allowed us also to score cells expressing lower levels of Gas3/PMP22, which were undetectable using the antibody against Gas3/PMP22. This effect has been clearly demonstrated by performing a double immunofluorescence analysis in cells overexpressing Gas3/PMP22FLAG using both antibodies against the FLAG or Gas3/PMP22 (our unpublished results).
Cell survival was not appreciably increased in cells coexpressing rhoAV14; in fact, the survival rate was ∼32 ± 7%, similar to the survival rate of ∼27 ± 8% observed after coexpression of the control gene AK (Figure 5A).
Instead, a marked difference was observed for the number of cells showing altered shape. Coexpression of rhoA-V14 induced a clear suppression of the Gas3/PMP22-dependent cell shape and spreading changes described above, with 10 ± 1.4% of the cells showing altered morphology in comparison with 37 ± 3.5% of the cells when the control gene was coexpressed (Figure 5A). In Figure 5B representative fields of cells coexpressing gas3/PMP22FLAG and AK or rhoA-V14 as detected after immunofluorescence analysis are reported.
Apoptosis and Morphological Changes Triggered by Gas3/PMP22 Are Differentially Counterbalanced by bcl-2 and RhoA
Previous experiments, in which Bcl-2 and gas3/PMP22 were coexpressed, suggested that although apoptosis was efficiently counteracted, cell shape and spreading changes were still detectable. In this context, Bcl-2 and RhoA could be useful tools to dissect the different biological activities of Gas3/PMP22. With this aim, we next decided to perform a quantitative analysis on the differential effects of RhoA and Bcl-2 on the Gas3/PMP22-induced phenotypes.
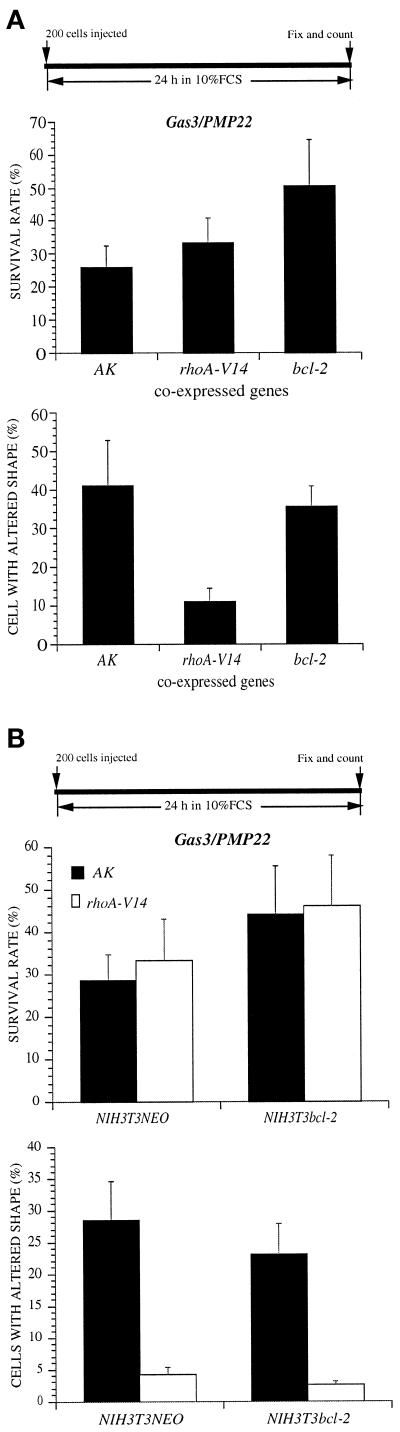
gas3/PMP22FLAG was coexpressed by nuclear microinjection in NIH3T3 cells together with rhoA-V14, bcl-2, or AK as a control gene. All the cDNAs were cloned in the same expression vector and microinjected, respectively, at the concentrations of 100 ng/μl (gas3/PMP22FLAG) and 25 ng/μl (AK, bcl-2, and rhoA-V14). Cells were grown for a further 24 h in 10% FCS and then scored both for Gas3/PMP22 expression and for morphological changes by immunofluorescence analysis.
As described above, coexpression of bcl-2 increased the survival rate of cells expressing Gas3/PMP22 to 50.5 ± 14% compared with 26 ± 6.3% when AK was coexpressed (p < 0.05). Here again, rhoA only slightly increased cell survival, 33 ± 7.5% survival rate compared with 26 ± 6.3% (Figure 6A). An opposite result was obtained when we analyzed cell shape and spreading changes induced by gas3/PMP22 overexpression. Here again, RhoA-V14 efficiently counteracted Gas3/PMP22-dependent cell shape and spreading changes; 11 ± 3.3% of cells presented morphological alterations compared with the 41 ± 11.8% of the control (p < 0.05) (Figure 6A). On the contrary, coexpression of bcl-2 only modestly interfered with the appearance of cell shape and spreading changes (35 ± 5.5% of the cells affected).
Figure 6.
Coexpression of Gas3/PMP22, RhoA-V14 and Bcl-2 in NIH3T3 cells. (A) AK, rhoA-V14, and bcl-2 were coexpressed with gas3/PMP22 in NIH3T3 cells. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG. Survival and morphological changes were scored as described in the text. Data represent arithmetic means ± SD for seven independent experiments (p < 0.001). (B) gas3/PMP22 and rhoA-V14 were coexpressed in NIH3T3NEO and in NIH3T3bcl-2 cells by nuclear microinjection. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG. Survival and morphological changes were scored as described in the text. Data represent arithmetic means ± SD for three independent experiments.
To further confirm the differential effect of Bcl-2 and RhoA-V14 on Gas3/PMP22-dependent changes in cell shape and survival, we decided to comicroinject gas3/PMP22 with rhoA-V14 or with AK in an NIH3T3 cell line stably expressing bcl-2 (Brancolini et al., 1995). As reported in Figure 6B, when gas3/PMP22 was coexpressed with rhoA-V14 in NIH3T3 bcl-2 cells, survival was increased, and cell shape and spreading alterations were reduced. Therefore, the rescue of the phenotype induced by gas3/PMP22 overexpression in NIH3T3 cells was possible only when both Bcl-2 and RhoA-V14 were simultaneously present.
Gas3/PMP22-dependent Changes in Cell Shape and Spreading Can Be Modulated by Bacterial Toxin-regulating Rho Activity
CNF1 from Escherichia coli, a toxin that activates Rho small GTPase, and the Clostridium botulinum C3 exotransferase, which inhibits Rho, have been widely used to study the role of this GTPase in different cellular processes (Fiorentini et al., 1998). Therefore, they represent useful tools to confirm the role of this GTPase in regulating Gas3/PMP22 cell shape and spreading changes.
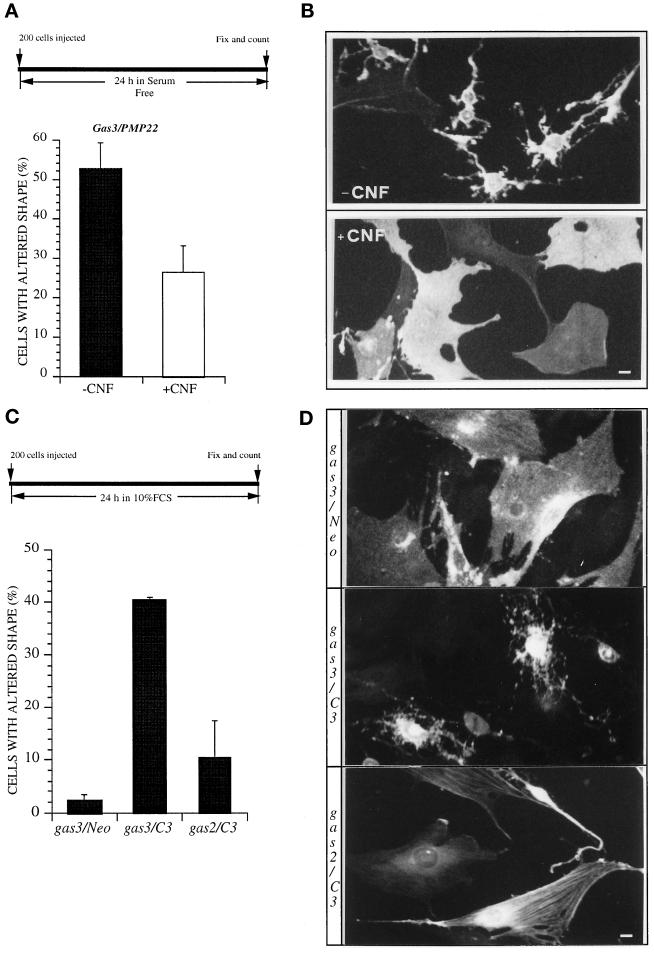
NIH3T3 cells were microinjected with 100 ng/μl pGDSV7gas3/PMP22-FLAG and grown for 24 in serum-free medium in the presence or absence of CNF1. In the control experiments >50 ± 7% of the Gas3/PMP22-overexpressing cells showed cell shape and spreading changes (Figure 7A). CNF1 treatment reduced to ∼26 ± 6% the percentage of cells showing a Gas3/PMP22 phenotype, thus suggesting that endogenous Rho activity is sufficient to counteract the Gas3/PMP22-dependent effect on cell shape and spreading. Representative fields of cells expressing gas3/PMP22FLAG treated or not with CNF-1, as detected after immunofluorescence analysis, are reported in Figure 7B.
Figure 7.
Gas3/PMP22 effect on cell shape and spreading can be modulated by bacterial toxin regulating Rho activity. (A) gas3/PMP22 FLAG was overexpressed in NIH3T3 cells by nuclear microinjection. After microinjection cells were treated or not with CNF-1 in serum-free medium; 24 h later cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG. Morphological changes were scored as described in the text. Data represent arithmetic means ± SD for four independent experiments (p = 0.002). (B) Immunofluorescence
C3 exoenzyme is an ADP-ribosyltransferase that inhibits Rho by covalently linking ADP-ribose to Rho asparagine 48 (Fiorentini et al., 1998). REF 52 cells are unresponsive to Gas3/PMP22 overexpression, thus representing the ideal system to confirm whether Rho is the critical player that mediates the Gas3/PMP22-dependent alterations in cell shape and spreading.
pGDSV7gas3/PMP22FLAG (100 ng/μl) was coexpressed by nuclear microinjection in REF 52 cells together with pcDNAC3 (25 ng/μl) or pcDNANEO (25 ng/μl). pGDSV7gas2 (100 ng/μl) together with pcDNAC3 (25 ng/μl) was used as control.
Overexpression of Gas3/PMP22 was unable to induce morphological changes in REF 52 cells, as previously described (Figure 7C). Coexpression of Gas2 with low amounts of C3 exoenzyme (25 ng/μl) induced morphological changes in ∼10 ± 7% of the injected cells. However, when the same amount of C3 was coexpressed with Gas3/PMP22, REF 52 cells were made fully responsive, in terms of alterations of cell shape and spreading, to Gas3/PMP22 overexpression. In fact, after 24 h from microinjection ∼40 ± 0.5% (p < 0.05) of the cells coexpressing Gas3/PMP22 and C3 cells showed a Gas3/PMP22 phenotype.
Representative fields of cells expressing gas3/PMP22FLAG and Neo, gas3/PMP22FLAG and C3, or Gas2 and C3, as detected after immunofluorescence analysis, are reported in Figure 7D.
Overexpression of gas3/PMP22 CMT1A Point Mutations Shows a Significant Reduced Apoptotic Response and Was Unable to Trigger Morphological Changes
Different point mutations in the human gas3/PMP22 gene have been described in CMT1A patients (Patel and Lupski, 1994; Suter and Snipes, 1995). Therefore, we assessed whether the Gas3/PMP22 point mutants responsible for the CMT1A were able to modulate changes in cell shape and spreading.
We used the Gas3/PMP22 point mutants as found in human CMT1A (L16P and S79C) and one mutation carried by mice with the Trembler phenotype (G150D) (Fabbretti et al., 1995). These mutations code for a single amino acid substitution within one of the four putative transmembrane domains of Gas3/PMP22.
gas3/PMP22FLAG and the different point mutations were overexpressed by nuclear microinjection in NIH3T3 cells. All the cDNAs were cloned in the same expression vector and microinjected at the concentration of 100 ng/μl. Cells were grown for further 24 h in 10% FCS and then scored both for Gas3/PMP22 expression and for morphological changes by immunofluorescence analysis.
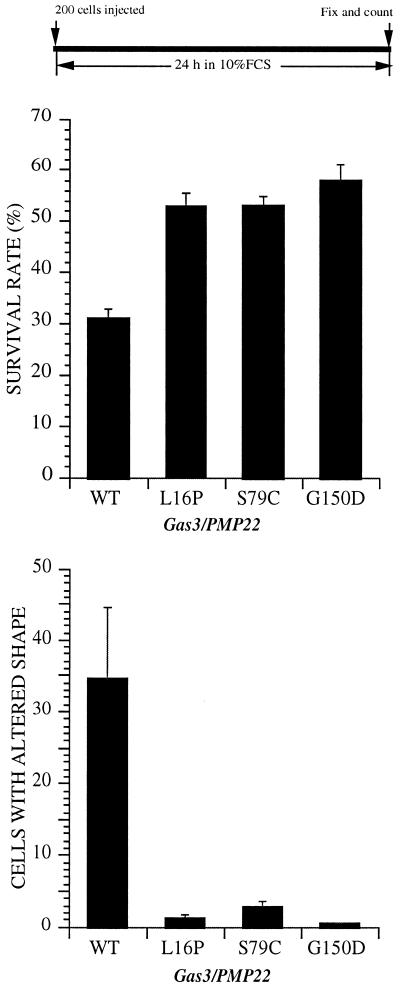
Cell survival was appreciably increased in cells expressing the mutants, ∼50% survival rate compared with the Gas3/PMP22 wild type (∼30 ± 1% survival rate) (Figure 8), thus indicating that the apoptotic response was significantly reduced in the case of the Gas3/PMP22 point mutants as previously described (Fabbretti et al., 1995).
Figure 8.
Overexpression of Gas3/PMP22 CMT1A point mutants in NIH3T3 cells. gas3/PMP22wt-FLAG, gas3/PMP22L16P-FLAG, gas3/PMP22S79C-FLAG, and gas3/PMP22G150D-FLAG were overexpressed in NIH3T3 cells by nuclear microinjection. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG. Survival and morphological changes were scored as described in the text. Data represent arithmetic means ± SD for five independent experiments (p < 0.001).analysis of NIH3T3 cells expressing gas3/PMP22 in the presence of CNF-1. NIH3T3 cells 24 h after seeding were microinjected with pGDSV7-gas3/PMP22FLAG (100 ng/μl). After microinjection medium was changed to serum free, and cells were treated or not with CNF-1. Twenty-four hours later cells were fixed and processed for immunofluorescence to visualize Gas3/PMP22FLAG. Bar, 25 μm. (C) C3 exoenxyme was coexpressed with gas3/PMP22 or gas2 in REF 52 cells. After 24 h from microinjection cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG or Gas2. Morphological changes were scored as described in the text. Data represent arithmetic means ± SD for four independent experiments (p < 0.001). (D) Immunofluorescence analysis of REF 52 cells expressing gas3/PMP22. REF 52 cells 24 h after seeding were microinjected with pGDSV7-gas3/PMP22FLAG (100 ng/μl) and pcDNA3NEO (25 ng/μl), with pGDSV7-gas3/PMP22FLAG (100 ng/μl) and pcDNA3C3 (25 ng/μl), or with pGDSV7-gas2 (100 ng/μl) and pcDNA3C3 (25 ng/μl). Twenty-four hours later cells were fixed and processed for immunofluorescence to visualize Gas3/PMP22FLAG or Gas2. Bar, 25 μm.
An even more marked difference was observed for the number of cells showing altered shape. As shown in Figure 8, overexpression of the different Gas3/PMP22 point mutants analyzed was unable to trigger morphological changes.
Wortmannin Can Augment the Morphological Changes Induced by Gas3/PMP22 Overexpression in NIH3T3 Cells
Phosphatidylinositol lipids have been implicated in regulating the Rho family of small GTPases. Phosphoinositide 3-kinase (PI3K) might act upstream of the small GTPases in controlling cytoskeletal changes in response to extracellular factors (Kotani et al., 1994; Wennstrom et al., 1994; Van Aelst and D’Souza-Schorey, 1997).
To gain further insights on the mechanisms regulating the Gas3/PMP22-dependent morphological response, we analyzed whether two well-characterized PI3K inhibitors such as WT and LY294002 were able to agument such morphological response.
NIH3T3 cells were microinjected with 50 ng/μl pGDSV7gas3/PMP22-FLAG, and 6 h after microinjection they were treated with the different inhibitors. To discriminate a positive effect on the Gas3/PMP22-dependent modifications of cell shape and spreading, cells were fixed at an early time after microinjection (18 h).
After this period overexpression of Gas3/PMP22 induced cell shape and spreading alterations in ∼17 ± 6% of injected cells (Figure 9A). WT treatment dramatically increased the percentage (∼44 ± 9%; p < 0.05), of Gas3/PMP22-overexpressing cells showing altered morphology (Figure 9, A and B). Under the same experimental conditions, LY294002 less efficiently raised the percentage of Gas3/PMP22-overexpressing cells showing altered morphology, reaching ∼29 ± 5% of the injected cells (p < 0.05). The effect of Gas3/PMP22 on cell survival was unaffected after both WT and LY294002 treatments (Figure 9A). The reduced effect of LY294002 compared with WT could be dependent on a different half-life of inhibitors in our experimental system. In fact, when MAPK activation was assessed in our experimental system as marker of PI3K activity (Goruppi et al., 1997), WT was a more potent inhibitor than LY294002 (our unpublished results). In Figure 9B representative fields of cells expressing gas3/PMP22FLAG treated or not with WT, as detected after immunofluorescence analysis, are reported.
Figure 9.
Wortmannin (WT) can agument changes of cell shape and spreading induced by Gas3/PMP22. (A) gas3/PMP22 FLAG was overexpressed in NIH3T3 cells by nuclear microinjection. After 6 h cells were treated or not with WT and LY294002; 12 h later cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG. Survival and morphological changes were scored as described in the text. Data represent arithmetic means ± SD for five independent experiments (altered shape, p < 0.001). (B) Immunofluorescence analysis of NIH3T3 cells expressing gas3/PMP22 in the presence of WT. NIH3T3 cells 24 h after seeding were microinjected with pEXV-gas3/PMP22FLAG (50 ng/μl). After 6 h cells were treated or not with WT; 12 h later cells were fixed and processed for immunofluorescence to visualize Gas3/PMP22FLAG. Bar, 25 μm. (C) AK and rhoA-V14 were coexpressed with gas3/PMP22 in NIH3T3 cells. After 6 h cells were treated or not with WT; 12 h later cells were fixed and processed for immunofluorescence to detect Gas3/PMP22FLAG. Survival and morphological changes were scored as described in the text. Data represent arithmetic means ± SD for five independent experiments (altered shape, p < 0.001).
Having demonstrated a compelling effect of WT on Gas3/PMP22 morphological activity, we next analyzed whether RhoA-V14 was able to counteract such an effect.
gas3/PMP22FLAG was coexpressed in NIH3T3 cells together with rhoA-V14 or with AK as a control gene. Six hours after microinjection cells were treated with WT, and 12 h later they were fixed and processed for immnuofluorescence.
Under these experimental conditions coexpression of Gas3/PMP22 and of the control gene AK induced modification of cell shape and spreading in ∼15 ± 3% of the injected cells (Figure 9C). Here again WT treatment dramatically increased, up to 35 ± 6% (p < 0.05), the percentage of Gas3/PMP22-overexpressing cells showing altered cell shape and spreading. On the contrary, in cells coexpressing Gas3/PMP22 and RhoA-V14, WT treatment was unable to exhibit any clear effect on cell morphology (Figure 9C).
The effect of Gas3/PMP22 on cell survival was unaffected by WT treatment.
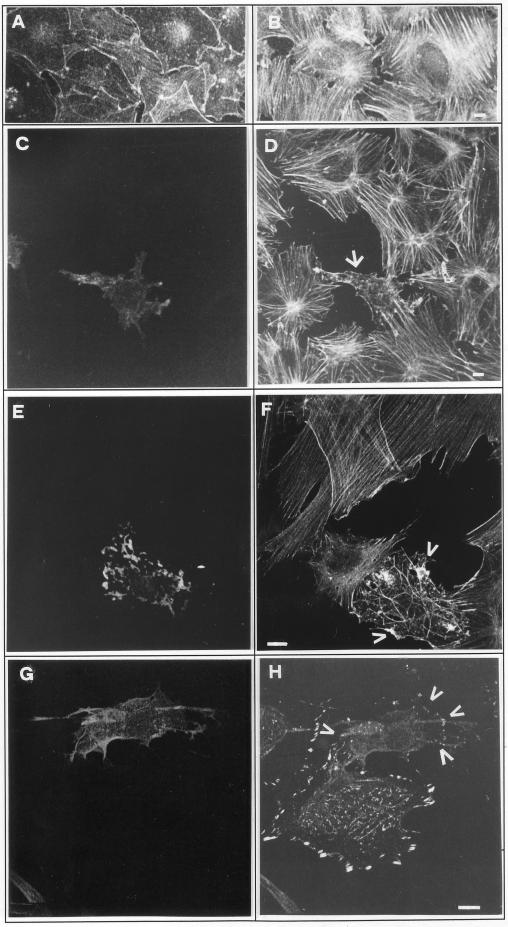
Gas3/PMP22 Overexpression Can Interfere with LPA-dependent Stress Fiber Formation
Having demonstrated an interference of RhoA on Gas3/PMP22-dependent changes of cell shape and spreading, we next attempted to understand whether Gas3/PMP33 could interfere with a Rho activity
Lysophosphatidic acid (LPA) stimulates, in a Rho-dependent manner, the assembly of stress fibers and the formation of focal adhesion complexes in fibroblasts (Ridley and Hall, 1992; Machesky and Hall, 1996). Therefore, we analyzed the response to LPA, in terms of stress fibers formation, in cells overexpressing Gas3/PMP22.
Stress fibers extending throughout the cell body can be induced in quiescent, serum-starved Swiss 3T3 cells by addition of LPA (Figure 10, A and B). In cells overexpressing Gas3/PMP22 and showing the above-described morphological changes, the assembly of stress fibers was impaired. In a typical cell overexpressing Gas3/PMP22 (Figure 10, C and D, arrow), stress fibers were barely detectable; instead actin staining can be observed as punctuate pattern throughout the cell. An alteration in stress fiber formation in response to LPA was also observed in cells overexpressing lower levels of Gas3/PMP22; therefore, the cells do not showing dramatic signs of shape and spreading changes. A representative cell is shown in Figure 9, E and F; here few stress fibers were observed in response to LPA, and they rarely traversed the cell body; on the contrary, patches of actin filaments were present (Figure 10, E and F, arrowheads).
Figure 10.
Gas3/PMP22 overexpression and stress fiber formation. Swiss 3T3 grown for 48 h in 0.1% FCS (A) and then treated for 30 min with LPA (B) were stained to visualize microfilaments. Serum-starved Swiss 3T3 cells were microinjected with pEXVhGas3/PMP22 (100 ng/μl). After 15 h from microinjection, LPA was added, and 30 min later cells were fixed and processed for immunofluorescence analysis to visualize actin filaments (D and F), Gas3/PMP22 (C, E, and G) and talin (H). Arrows indicate microinjected cells. Bar, 10 μm.
To visualize focal adhesion complexes, Gas3/PMP22-overexpressing and LPA-treated cells were immunofluorescently stained for the focal adhesion protein talin. As shown in Figure 10, G and H (arrowheads), also focal adhesion complex assembly in response to LPA was reduced in cells overexpressing Gas3/PMP22 compared with the uninjected cells.
In summary, the Rho-dependent changes of the microfilament system in response to LPA were impaired in Gas3/PMP22-overexpressing cells.
DISCUSSION
Different elegant in vivo studies on Gas3/PMP22 have been fundamental in confirming the crucial role of this protein in regulating myelin formation and stability and its involvement in different human peripheral neuropathies (Adlkofer et al., 1995, Huxley et al., 1996; Magyar et al., 1996; Sereda et al., 1996, D’Urso et al., 1997). On the other hand, they have been unable to unveil the biological mechanisms regulated by Gas3/PMP22. Furthermore, the discovery of Gas3/PMP22-related proteins also expressed in myelinating Schwann cells (Kaprielian et al., 1995; Schaeren-Wiemers et al., 1995; Gillen et al., 1996; Taylor and Suter, 1996; Bolin et al., 1997) adds additional complexity to the problem of the in vivo studies, in which compensatory mechanisms might also exist. In this regard, our strategy, based on a transient overexpression in cultured cells, could represent a useful approach to define the functional roles of Gas3/PMP22.
In this report we have unveiled a role of gas3/PMP22 in regulating cell morphology, as evidenced in terms of reduced cell spreading, which can be separated from the previously reported apoptotic response.
The Gas3/PMP22-dependent morphological response was easily detectable when the apoptotic response was counteracted by antiapoptotic genes such as bcl-2 and crmA. In our experiments Bcl-2 was consistently more efficient in counteracting apoptosis triggered by Gas3/PMP22 with respect to CrmA. Bcl-2 (Reed, 1997; Green and Kroemer, 1998; Silke and Vaux, 1998) is a broad inhibitor of caspases, a family of cysteine-protease, which plays a critical role in the execution of the apoptotic program (for review, see Cohen, 1997; Crynes and Yuan, 1998), whereas CrmA, a 38-kDa serpin from cowpox virus, is a potent inhibitor of some caspases, such as caspase-1 and 8, but a weak inhibitor for others such as caspase-3 and -2 (Ray et al., 1992; Nicholson et al., 1995; Sirinivasula et al., 1996; Cohen, 1997). Therefore, the relative pattern of caspase inhibition reflects the differential effect of Bcl-2 and CrmA on Gas3/PMP22 apoptotic response. In addition, these data indicate that Gas3/PMP22 can trigger a “classical” apoptotic response, characterized by caspase activation.
The time-lapse analysis confirmed that overexpression of gas3/PMP22 induces, precedent or parallel to the apoptotic response, morphological changes that can be observed as a reduction in cell spreading.
This phenotype was specific for Gas3/PMP22 because overexpression of P0, another component of compact myelin, was unable to induce changes in cell spreading. Moreover, the gas3/PMP22 point mutations associated with CMT1A and Trembler failed to induce changes in cell spreading when similarly overexpressed in NIH3T3 cells. This evidence indicates that the effect of Gas3/PMP22 on cell spreading is strictly linked to the disease and therefore to the process of myelinization in vivo.
The effect of Gas3/PMP22 on cell morphology and spreading involved the small GTPase Rho (for review, see Machesky and Hall, 1996; Van Aelst and D’Souza-Schorey, 1997; Hall, 1998). Different pieces of evidence support this conclusion: 1) coexpression of an active form of RhoA can efficiently counteract the morphological changes induced by Gas3/PMP22; 2) the bacterial toxin CNF1 from E. coli, which activates Rho, can also counteract the Gas3/PMP22 phenotype; and 3) the C. botulinum exoenzymes C3, which inhibits Rho activity, can transform the REF 52 cells, normally refractory to Gas3/PMP22 overexpression in a highly responsive cell line.
Our results obtained with the bacterial toxins, and in particular the recovery of a Gas3/PMP22 response in REF 52 cells when endogenous Rho was inhibited by C3 exoenzyme, strongly support the idea that the endogenous Rho is the critical switch to regulate cell shape and spreading changes in response to Gas3/PMP22.
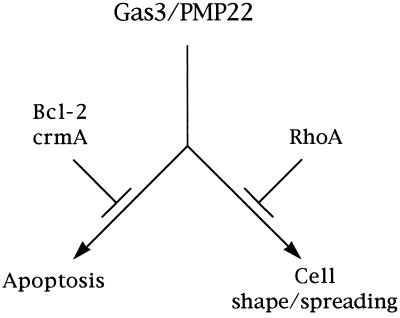
Interestingly if an active Rho specifically suppressed the morphological response triggered by Gas3/PMP22, it was unable to efficiently counteract Gas3/PMP22-dependent apoptosis. Conversely, Bcl-2 specifically interfered with the apoptotic response, but it was unable to interfere with the morphological response. This clearly indicates that apoptosis and cell shape and spreading changes represent two distinct responses primed by Gas3/PMP22, thus suggesting that such signal should be branching at some point (Figure 11).
Figure 11.
Schematic representation of Gas3/PMP22 biological activities. Gas3/PMP22 could regulate both susceptibility to apoptosis and morphological changes. Bcl-2 counteracts the apoptotic response, whereas Rho specifically counteracts morphological changes.
Rho GTPase is required for many actin-dependent cellular processes, such as platelet aggregation, lymphocyte and fibroblast adhesion, cell motility, contraction, and cytokinesis (Ridley and Hall, 1992; Narumiya et al., 1997; Hall, 1998). Several direct targets of Rho have been identified (for review, see Van Aelst and D’Souza-Schorey, 1997). Among them the Rho-associated kinases seem to mediate RhoA effects on actin cytoskeleton (Leung et al., 1995; Ishizaki et al., 1996; Amano et al., 1997). These kinases can regulate cell contractility by directly phosphorylating myosin light chain or indirectly by phosphorylating and inhibiting myosin light chain phosphatase (Amano et al., 1996; Kimura et al., 1996). More recently also members of the ezrin/radixin/moesin family of proteins, which are required to mediate the Rho-dependent stress fiber assembly and focal complex formation (Mackay et al., 1997), seem to be modulated by Rho kinases (Matsui et al., 1998). Regulation of phosphatidylinositol-4,5-biphosphate synthesis is another key event orchestrating the actin cytoskeleton, but it is still unclear whether phosphatidylinositol 4-phosphate 5-kinase interacts with Rho directly (Van Aelst and D’Souza-Schorey, 1997; Hall, 1998). Within this complex scenario it would be of great interest to determine which Rho-specific target is necessary to modulate Gas3/PMP22 activity on cell shape and spreading.
The Gas3/PMP22 dependent alteration of cell shape and spreading was dramatically increased by WT and to a lesser extent by LY294002, thus possibly suggesting an involvement of a PI3K in antagonizing such activity. WT and LY294002 are commonly used as inhibitors of PI3K; however, at micromolar concentrations they can also inhibit a phosphatidylinositol 4-kinase (Nakanishi et al., 1995; Carpenter and Cantley, 1996); therefore, further studies will be necessary to confirm an involvement of PI3K in regulating Gas3/PMP22 morphological signaling. Nevertheless, it is interesting to note that Cdc42, Rac, and Rho can operate in a hierarchical cascade wherein CDC42 activates Rac, which in turn activates Rho (Nobes and Hall, 1995). Experiments using WT suggest that PI3K might act upstream of Rac (Kotani et al., 1994; Wennstrom et al., 1994; Nobes et al., 1995; Van Aelst and D’Souza-Schorey, 1997), and a constitutively active PI3K kinase mutant was able to trigger membrane ruffles and stress fibers in a Rac- and Rho-dependent manner (Reif et al., 1996). We noticed that the WT reinforcement of the Gas3/PMP22 phenotype was efficiently suppressed by an active RhoA. Therefore, it is possible that the effect of WT on Gas3/PMP22-triggered morphological changes could be dependent on the PI3K-regulated Rho activity.
How could the effect of Gas3/PMP22 on cell shape and spreading, possibly by acting on Rho GTPase, contribute to myelin formation and stability? Studies made in melanocytes, astrocytes, and neuronal cells have shown that changes in microfilament organization and cell shape, such as dendrite and neurite outgrowth, which are linked to a differentiative response, require Rho inhibition (Gebbink et al., 1997; Jin and Strittmatter, 1997; Busca et al., 1998; Ramakers and Moolenaar, 1998). Altogether these reports support a model in which inactivation of Rho is the necessary requirement to promote specific cytoskeletal and morphological changes characterizing the differentiated phenotype. In this regard we can speculate that changes in cell shape and actin architecture occurring during differentiation of the Schwann cells, which lead to myelinization, require Rho inhibition. In this scenario there are two possible explanations for the observed functional link between Gas3/PMP22 and Rho. Gas3/PMP22-dependent regulation of cell shape and spreading could only be possible after Rho inactivation, which might occur independently of Gas3/PMP22. Alternatively, Gas3/PMP22 might regulate Rho signaling within the cells, thus leading to changes in cell shape and spreading necessary for myelinization. We favor the second possibility, and some evidence supports our speculation. Gas3/PMP22 can interfere with the LPA-triggered assembly of stress fibers and focal adhesion complexes. LPA-mediated activation of Rho and attachment of integrins to the extracellular matrix are both required for cell spreading and formation of focal complexes, whereas Rho-dependent assembly of stress fibers is not dependent from extracellular matrix (Hotchin and Hall, 1995). Because in cells overexpressing Gas3/PMP22 stress fiber assembly in response to LPA was impaired, it is therefore possible that Gas3/PMP22 regulates, in a still undefined manner, Rho activity within the cell. In addition, it is important to note that at early stages of myelinogenesis, PMP22-deficient peripheral nerves are retarded in myelin formation, and it has been suggested that Gas3/PMP22 could be a component of the complex mechanism contributing to the driving force for turning the Schwann cell loops around the axon (Adlkofer et al., 1995).
Gas3/PMP22 should also be involved in regulating myelin thickness and stability, because PMP22-deficient mice show redundant myelin loops with normal spacing (tomacula), together with signs of myelin degeneration at a more advanced stage (Adlkofer et al., 1995). Indeed, a specific organization of the actin cytoskeleton associated with the myelin envelope has been proposed based on localization studies of the actin capping and severing protein gelsolin (Tanaka and Sobue, 1994).
In conclusion, we have demonstrated that two different biological functions can be ascribed to Gas3/PMP22, and we have explored the correlation between a tetraspan protein and the small GTPase Rho. How this relates to the differentiation and myelinization of the Schwann cells and to the peripheral neuropathies is an intriguing question that can now be investigated.
ACKNOWLEDGMENTS
We are grateful to Alan Hall (Medical Research Council Laboratory University College, London, United Kingdom) for the pEXV-rhoAV14 construct, Craig B. Thompson (University of Chicago, Chicago, IL) for bax cDNA, Licio Collavin (Laboratorio Nazionale Consorzio Interuniversitario Biotecnologie) for AK cDNA, Ding Xue (Massachusetts Institute of Technology, Boston, MA) for crmA cDNA, and Donatella D’Urso (University of Dusseldorf, Dusseldorf, Germany) for P0 cDNA. We thank Guido Tarone (University of Torino, Torino, Italy) for fibronectin and useful comments and Juan J. Archelos (University of Wurzburg, Wurzburg, Germany) for anti-P0 monoclonal antibody. We are indebted to Lawrence Wrabetz (Department of Biological and Technological Research, Milan, Italy) for very useful comments and suggestions on the manuscript. This work was supported by Telethon-Progetto grant 770 (to C.S.).
Abbreviations used:
- AK
adenylate kinase
- CMT
Charcot–Marie–Tooth
- CNF
cytotoxic necrotizing factor
- h-TR
human transferrin receptor
- LPA
lysophosphatidic acid
- PI3K
phosphoinositide 3-kinase
- WT
wortmannin
REFERENCES
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in PMP22-deficient mice. Nat Genet. 1995;11:274–280. doi: 10.1038/ng1195-274. [DOI] [PubMed] [Google Scholar]
- Amano M, Chiahara K, Kimura K, Fukuta Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions is enhanced by Rho kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Archelos JJ, Roggenbuck K, Schneider-Schaulies J, Linington C, Toyka KV, Hartung HP. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J Neurosci Res. 1993;35:46–53. doi: 10.1002/jnr.490350107. [DOI] [PubMed] [Google Scholar]
- Baechner D, Liehr T, Hameister H, Altenberger H, Grehl H, Suter U, Rautenstrauss B. Widespread expression of the peripheral myelin protein-22 gene (pmp22) in neural and nonneural tissue during murine development. J Neurosci Res. 1995;42:733–741. doi: 10.1002/jnr.490420602. [DOI] [PubMed] [Google Scholar]
- Bolin LM, McNeil T, Lucian LA, DeVaux B, Franz-Bacon K, Gorman DM, Zurawski S, Murray R, McClanahan TK. HNMP-1: a novel hematopoietic and neural membrane protein differentially regulated in neural development and injury. J Neurosci. 1997;17:5493–5502. doi: 10.1523/JNEUROSCI.17-14-05493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C, Benedetti M, Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 1995;14:5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C, Marzinotto S, Schneider C. Susceptibility to p53 dependent apoptosis correlates with increased levels of Gas2 and Gas3 proteins. Cell Death Differ. 1997;4:247–253. doi: 10.1038/sj.cdd.4400232. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Busca R, Bertolotto C, Abbe P, Englaro W, Ishizaki T, Narumiya S, Boquet P, Ortonne J-P, Ballotti R. Inhibition of Rho is required for cAMP-induced melanoma cell differentiation. Mol Biol Cell. 1998;9:1367–1378. doi: 10.1091/mbc.9.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide kinase. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- Chance PF, Alderson MK, Leppig KA, Lensch MW, Matsunami N, Smith B, Swanson PD, Odelberg SJ, Disteche CM, Bird TD. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72:143–151. doi: 10.1016/0092-8674(93)90058-x. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executionerof apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crynes V, Yuan J. Proteases to die for. Genes & Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- D’Urso D, Schmalenbach C, Zoidl G, Prior R, Muller HW. Studies on the effect of altered PMP22 expression during myelination in vitro. J Nerosci Res. 1997;48:31–42. doi: 10.1002/(sici)1097-4547(19970401)48:1<31::aid-jnr3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Edomi P, Martinotti A, Colombo MP, Schneider C. Sequence of human gas3/PMP22 full-length cDNA. Gene. 1993;126:289–290. doi: 10.1016/0378-1119(93)90384-f. [DOI] [PubMed] [Google Scholar]
- Fabbretti E, Edomi P, Brancolini C, Schneider C. Apoptotic phenotype induced by overexpression of the wilde type gas3/PMP22: its relation to the demyelinating peripheral neuropathy CMT1A. Genes & Dev. 1995;9:1846–1856. doi: 10.1101/gad.9.15.1846. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Fabbri A, Flatau G, Donelli G, Matarrese P, Lemichez E, Falzano L, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a Toxin that activates the Rho GTPase. J Biol Chem. 1997;272:19532–19537. doi: 10.1074/jbc.272.31.19532. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Gauthier M, Donelli G, Bouquet P. Bacterial toxins and the Rho GTP-binding protein:what microbes teach us about cell regulation. Cell Death Differ. 1998;5:720–728. doi: 10.1038/sj.cdd.4400412. [DOI] [PubMed] [Google Scholar]
- Gebbink M, Kranenburg O, Poland M, van Horck F, Houssa B, Moolenaar WH. Identification of a novel putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen C, Gleichmann M, Greiner-Petter R, Zoidl G, Kupfer S, Bosse F, Auer J, Muller HW. Full-length cloning, expression and cellular localization of rat plasmolipin mRNA a proteolipid of PNS and CNS. Eur J Neurosci. 1996;8:405–414. doi: 10.1111/j.1460-9568.1996.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphotidylinositol 3-kinase-dependent pathway and src for Gas6-Axl mitogenic and survival activities in NIH3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–4453. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPase. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C, Passage E, Manson A, Putzu G, Figarella-Branger D, Pellissier JF, Fontes M. Construction of a mouse model of Charcot-Marie-Tooth disease type 1A by pronuclear injection of human YAC DNA. Hum Mol Genet. 1996;5:563–569. doi: 10.1093/hmg/5.5.563. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Th protein kinase homologus to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac-1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprielian Z, Cho KO, Hadjiargyrou M, Patterson PH. CD9, a major platelet cell surface glycoprotein, is a ROCA antigen and is expressed in the nervous system. J Neurosci. 1995;15:574–583. doi: 10.1523/JNEUROSCI.15-01-00562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kotani K, et al. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the ras-related RhoA GTPase which translocates to the peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Lemke G, Lamar E, Patterson J. Isolation and analysis of the gene encoding peripheral myelin myelin protein zero. Cell. 1988;40:501–508. doi: 10.1016/0896-6273(88)90211-5. [DOI] [PubMed] [Google Scholar]
- Lupski JR, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219–232. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: a connection between membrane receptor signaling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Mackay DJG, Esch F, Furthmayr H, Hall A. Rho- and Rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar JP, Ebensperger N, Schaeren-Wiemers N, Suter U. Myelin and lymphocyte protein (MAL/MVP17/VP17) and plasmolipin are members of an extended gene family. Gene. 1997;189:269–275. doi: 10.1016/s0378-1119(96)00861-x. [DOI] [PubMed] [Google Scholar]
- Magyar JP, Martini R, Ruelicke T, Aguzzi A, Adlkofer K, Dembic Z, Zielasek J, Toyka KV, Suter U. Impaired differentiation of Schwann cells in transgenic mice with increased PMP22 gene dosage. J Neurosci. 1996;16:5351–53060. doi: 10.1523/JNEUROSCI.16-17-05351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfioletti G, Ruaro ME, Del Sal G, Philipson L, Schneider C. A growth arrest-specific (gas) gene codes for a membrane protein. Mol Cell Biol. 1990;10:2924–2930. doi: 10.1128/mcb.10.6.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin KW, Fujimoto W, Jetten AM. Identification and characterization of a novel squamous cell-associated gene related to PMP22. J Biol Chem. 1995;270:28910–28916. doi: 10.1074/jbc.270.48.28910. [DOI] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Dori Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami N, et al. Peripheral myelin protein-22 gene maps in the duplication in chromosome 17p11.2 associated with Charcot-Marie-Tooth type 1A. Nat Genet. 1992;1:176–179. doi: 10.1038/ng0692-176. [DOI] [PubMed] [Google Scholar]
- McCarthy NJ, Whyte MKB, Gilber CS, Evan GI. Inhibition of ced-3/ICE related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 hommologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, et al. Identification and inhibition of the ICE/CED3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, cdc42 GTPase regulate the assembly of multimolecular focal complexes associated with actin stress fiber, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterdimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Patel IP, Lupski JR. Charcot-Marie-Tooth disease: a new paradigm for the mechanism of inherited disease. Trends Genet. 1994;10:128–133. doi: 10.1016/0168-9525(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Patel IP, et al. The gene for the peripheral myelin protein PMP-22 is a candidate for Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992;1:159–165. doi: 10.1038/ng0692-159. [DOI] [PubMed] [Google Scholar]
- Ramakers GJA, Moolenaar WH. Regulation of astrocyte morphology by RhoA and lysophosphatidic acid. Exp Cell Res. 1998;245:252–262. doi: 10.1006/excr.1998.4224. [DOI] [PubMed] [Google Scholar]
- Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3-kinase signals activates a selective subset of Rac/Rho effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Roa BB, Dyck PJ, Marks HG, Chance PF, Lupski JR. Dejerine-Sottas syndrome associated with point mutation in the peripheral myelin protein 22 (PMP22) gene. Nat Genet. 1993a;5:269–273. doi: 10.1038/ng1193-269. [DOI] [PubMed] [Google Scholar]
- Roa BB, Garcia CA, Suter U, Kulpa DA, Wise CA, Müller J, Welcher AA, Snipes GJ, Shooter EM, Patel P. Charcot-Marie-Tooth disease type 1A. Association with a spontaneous point mutation in the PMP22 gene. N Engl J Med. 1993b;329:96–101. doi: 10.1056/NEJM199307083290205. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Valenzuela DM, Frank M, Schwab ME. Characterization of the rat gene, rMAL, encoding a protein with four hydrophobic domains in central and peripheral myelin. J Neurosci. 1995;15:5753–5764. doi: 10.1523/JNEUROSCI.15-08-05753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereda M, et al. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron. 1996;16:1049–1060. doi: 10.1016/s0896-6273(00)80128-2. [DOI] [PubMed] [Google Scholar]
- Silke J, Vaux DL. Cell death: shadow baxing. Curr Biol. 1998;8:528–531. doi: 10.1016/s0960-9822(07)00339-9. [DOI] [PubMed] [Google Scholar]
- Sirinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a crmA-inhibitable protease that activates multiple ced-3/ICE-like cystein proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes GJ, Suter U, Welcher AA, Shooter EM. Characterization of a novel peripheral nervous system myelin protein (PMP22/SR13) J Cell Biol. 1992;117:225–238. doi: 10.1083/jcb.117.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreyer P, Kuhn G, Hanemann CO, Gillen C, Schaal H, Kuhn R, Lemke G, Müller HW. Axon-regulated expression of a Schwann cell transcript that is homologous to a “growth arrest-specific” gene. EMBO J. 1991;10:3661–3668. doi: 10.1002/j.1460-2075.1991.tb04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U, Snipes JC. Biology and genetics of hereditary motor and sensory neuropathies. Annu Rev Neurosci. 1995;18:45–75. doi: 10.1146/annurev.ne.18.030195.000401. [DOI] [PubMed] [Google Scholar]
- Suter U, Snipes GJ, Schoener-Scott R, Welcher AA, Pareek S, Lupski JR, Murphy RA, Shooter EM, Patel PI. Regulation of tissue-specific expression of alternative peripheral myelin protein-22 (PMP22) gene transcripts by two promoter. J Biol Chem. 1994;269:25795–25808. [PubMed] [Google Scholar]
- Tanaka J, Sobue K. Localization and charcaterization of gelsolin in nervous tissues: gelsolin is specifically enriched in myelin-forming cells. J Neurosci. 1994;14:1038–1052. doi: 10.1523/JNEUROSCI.14-03-01038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V, Suter U. Epithelial membrane protein-2 and epithelial membrane protein-3: Two novel members of the peripheral myelin protein 22 gene family. Gene. 1996;175:115–120. doi: 10.1016/0378-1119(96)00134-5. [DOI] [PubMed] [Google Scholar]
- Taylor V, Welcher AA, Program AE, Suter U. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J Biol Chem. 1995;270:28824–28833. doi: 10.1074/jbc.270.48.28824. [DOI] [PubMed] [Google Scholar]
- Timmerman V, et al. The peripheral myelin protein gene PMP22 is contained within the Charcot-Marie-Tooth disease type 1A duplication. Nat Genet. 1992;1:171–175. doi: 10.1038/ng0692-171. [DOI] [PubMed] [Google Scholar]
- Valentijn LJ, Baas F, Wolterman RA, Hoogendijk JE, van den Bosch NHA, Zorn I, Gabreels-Festen WM, de Visser M, Bolhuis PA. Identical point mutations of PMP-22 in Trembler-J mouse and Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992a;2:288–291. doi: 10.1038/ng1292-288. [DOI] [PubMed] [Google Scholar]
- Valentijn LJ, et al. The peripheral myelin gene PMP-22/GAS-3 is duplicated in Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992b;1:166–170. doi: 10.1038/ng0692-166. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPase and signaling network. Genes & Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Welcher AA, Suter U, De Leon M, Snipes GJ, Shooter EM. A myelin protein is encoded by the homologue of a growth arrest-specific gene. Proc Natl Acad Sci USA. 1991;88:7195–7199. doi: 10.1073/pnas.88.16.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson WL, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Xue D, Horvitz RH. Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature. 1995;377:248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Blass-Kampann S, D’Urso D, Schmalenbach C, Muller HW. Retroviral-mediated gene transfer of the peripheral myelin protein PMP22 in Schwann cells: modulation of cell growth. EMBO J. 1995;14:1122–1128. doi: 10.1002/j.1460-2075.1995.tb07095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoidl G, D’Urso D, Blass-Kampamann S, Schmalenbach C, Kuhn R, Muller HW. Influence of elevated expression of rat wild-type PMP22 and its mutant PMP22Trembler on cell growth of NIH3T3 fibroblasts. Cell Tissue Res. 1997;287:459–470. doi: 10.1007/s004410050770. [DOI] [PubMed] [Google Scholar]