Abstract
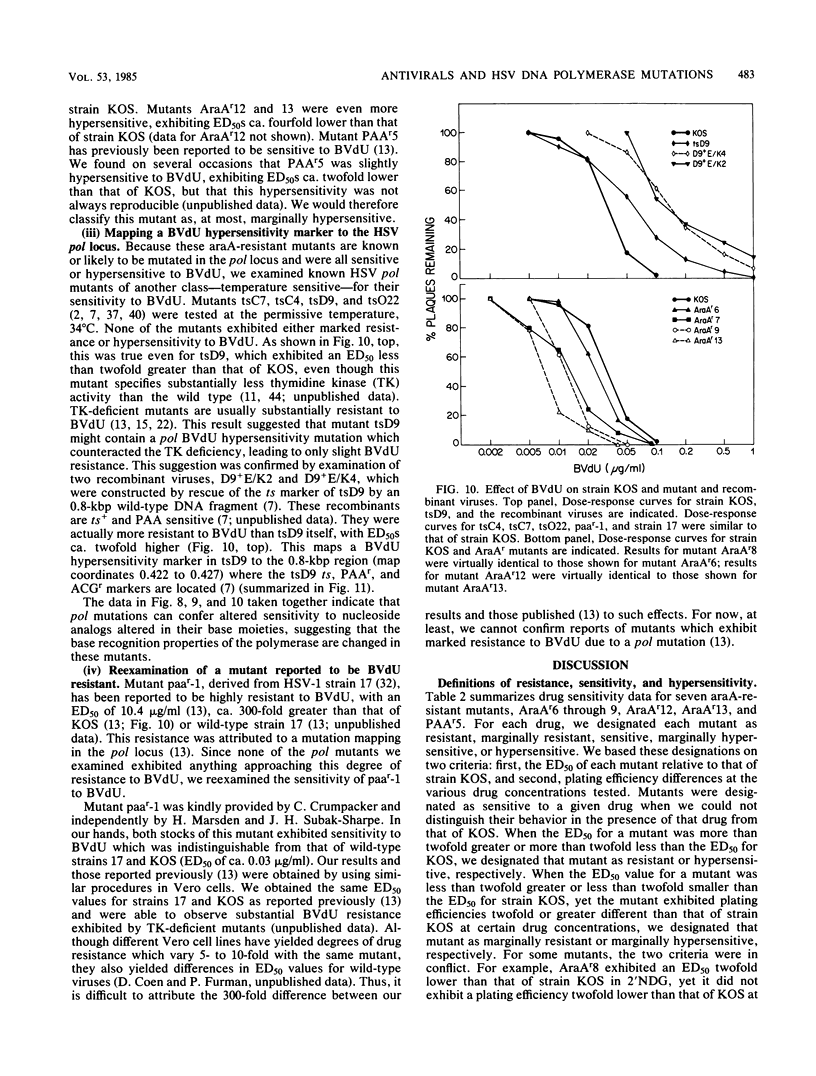
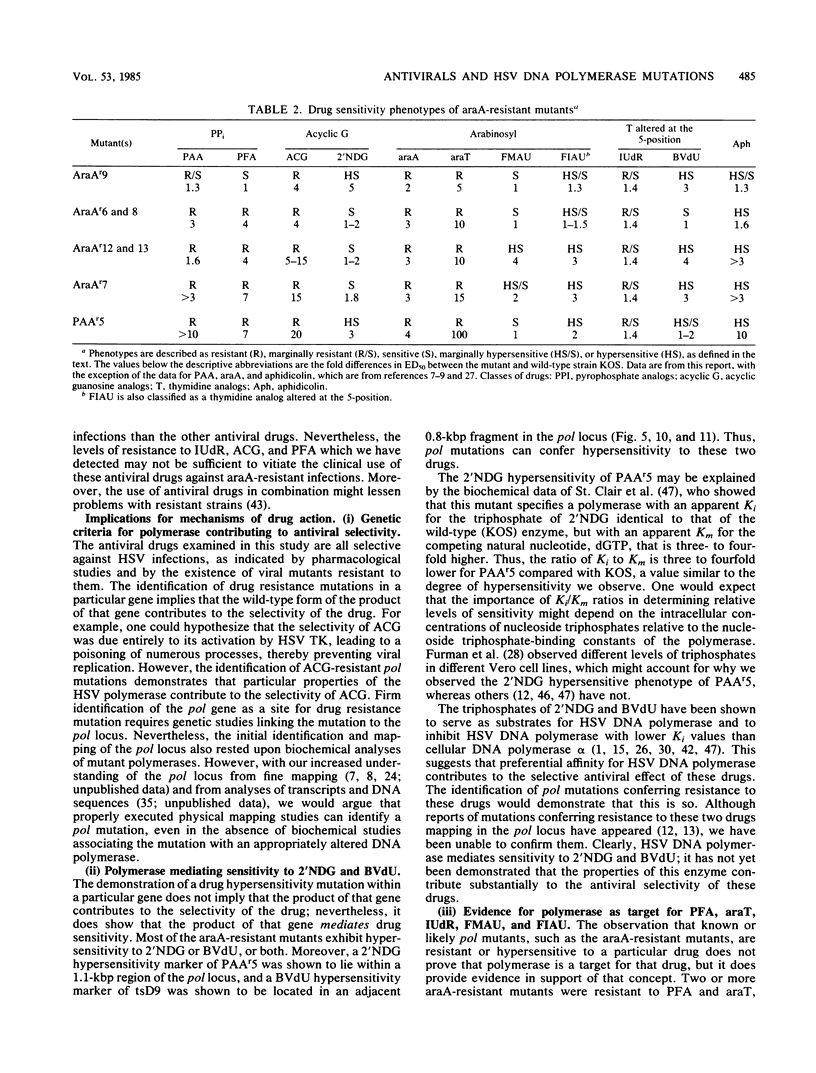
Seven herpes simplex virus mutants which have been previously shown to be resistant to arabinosyladenine were examined for their sensitivities to four types of antiviral drugs. These drugs were a pyrophosphate analog, four nucleoside analogs altered in their sugar moieties, two nucleoside analogs altered in their base moieties, and one altered in both. The seven mutants exhibited five distinct phenotypes based on their sensitivities to the drugs relative to wild-type strain KOS. All mutants exhibited resistance to acyclovir and arabinosylthymine, as well as marginal resistance to iododeoxyuridine, whereas all but one exhibited resistance to phosphonoformic acid. The mutants exhibited either sensitivity or hypersensitivity to other drugs tested--2'-nor-deoxyguanosine, 5-methyl-2'-fluoroarauracil, 5-iodo-2'-fluoroarauracil, and bromovinyldeoxyuridine--some of which differed only slightly from drugs to which the mutants were resistant. These results suggest ways to detect and treat arabinosyladenine-resistant isolates in the clinic. Antiviral hypersensitivity was a common phenotype. Mutations conferring hypersensitivity to 2'-nor-deoxyguanosine in mutant PAAr5 and to bromovinyldeoxyridine in mutant tsD9 were mapped to nonoverlapping regions of 1.1 and 0.8 kilobase pairs, respectively, within the herpes simplex virus DNA polymerase locus. Thus, viral DNA polymerase mediates sensitivity to these two drugs. However, we could not confirm reports of mutations in the DNA polymerase locus conferring resistance to these two drugs. All of the mutants exhibited altered sensitivity to two or more types of drugs, suggesting that single mutations affect recognition of the base, sugar, and triphosphate moieties of nucleoside triphosphates by viral polymerase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Kozarich J. W., Bertino J. R., De Clercq E. On the mechanism of selective inhibition of herpesvirus replication by (E)-5-(2-bromovinyl)-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1981 May;78(5):2698–2702. doi: 10.1073/pnas.78.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow K. F., Derse D. D., Cheng Y. C. Susceptibility of phosphonoformic acid-resistant herpes simplex virus variants to arabinosylnucleosides and aphidicolin. Antimicrob Agents Chemother. 1983 Jun;23(6):914–917. doi: 10.1128/aac.23.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand P., Crumpacker C. S., Schaffer P. A., Wilkie N. M. Physical and genetic analysis of the herpes simplex virus DNA polymerase locus. Virology. 1980 Jun;103(2):311–326. doi: 10.1016/0042-6822(80)90190-7. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Stow N. D., Timbury M. C., Wilkie N. M. Physical mapping of paar mutations of herpes simplex virus type 1 and type 2 by intertypic marker rescue. J Virol. 1979 Aug;31(2):265–276. doi: 10.1128/jvi.31.2.265-276.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Aschman D. P., Gelep P. T., Retondo M. J., Weller S. K., Schaffer P. A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984 Jan;49(1):236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Gelep P. T., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene can confer resistance to 9-beta-D-arabinofuranosyladenine. J Virol. 1982 Mar;41(3):909–918. doi: 10.1128/jvi.41.3.909-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Chartrand P., Subak-Sharpe J. H., Wilkie N. M. Resistance of herpes simplex virus to acycloguanosine--genetic and physical analysis. Virology. 1980 Aug;105(1):171–184. doi: 10.1016/0042-6822(80)90165-8. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., Kowalsky P. N., Oliver S. A., Schnipper L. E., Field A. K. Resistance of herpes simplex virus to 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine: physical mapping of drug synergism within the viral DNA polymerase locus. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1556–1560. doi: 10.1073/pnas.81.5.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Kowalsky P. N., Sherman D. M. Resistance of herpes simplex virus to adenine arabinoside and E-5-(2-bromovinyl)-2'-deoxyuridine: a physical analysis. J Infect Dis. 1982 Aug;146(2):167–172. doi: 10.1093/infdis/146.2.167. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Zaia J. A., Levin M. J. Growth inhibition by acycloguanosine of herpesviruses isolated from human infections. Antimicrob Agents Chemother. 1979 May;15(5):642–645. doi: 10.1128/aac.15.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby G., Churcher M. J., Larder B. A. Cooperative effects between two acyclovir resistance loci in herpes simplex virus. J Virol. 1984 Jun;50(3):838–846. doi: 10.1128/jvi.50.3.838-846.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesman G. R., Benyesh-Melnick M. Spectrum of human cytomegalovirus complement-fixing antigens. J Immunol. 1967 Dec;99(6):1106–1114. [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B., Oberg B. Characteristics of herpesvirus mutants resistant to phosphonoformate and phosphonoacetate. Antimicrob Agents Chemother. 1979 Jun;15(6):758–762. doi: 10.1128/aac.15.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H., McMillan A., Darby G. The sensitivity of acyclovir-resistant mutants of herpes simplex virus to other antiviral drugs. J Infect Dis. 1981 Feb;143(2):281–285. doi: 10.1093/infdis/143.2.281. [DOI] [PubMed] [Google Scholar]
- Fischer P. H., Chen M. S., Prusoff W. H. The incorporation of 5-iodo-5'-amino-2',5-dideoxyuridine and 5-iodo-2'-deoxyuridine into herpes simplex virus DNA. Relationship between antiviral activity and effects on DNA structure. Biochim Biophys Acta. 1980 Feb 29;606(2):236–245. doi: 10.1016/0005-2787(80)90033-7. [DOI] [PubMed] [Google Scholar]
- Fleming H. E., Jr, Coen D. M. Herpes simplex virus mutants resistant to arabinosyladenine in the presence of deoxycoformycin. Antimicrob Agents Chemother. 1984 Sep;26(3):382–387. doi: 10.1128/aac.26.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Chiou J. F., Cheng Y. C. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984 Feb 10;259(3):1566–1569. [PubMed] [Google Scholar]
- Furman P. A., Coen D. M., St Clair M. H., Schaffer P. A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981 Dec;40(3):936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., de Miranda P., St Clair M. H., Elion G. B. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother. 1981 Oct;20(4):518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germershausen J., Bostedor R., Field A. K., Perry H., Liou R., Bull H., Tolman R. L., Karkas J. D. A comparison of the antiviral agents 2'-nor-2'-deoxyguanosine and acyclovir: uptake and phosphorylation in tissue culture and kinetics of in vitro inhibition of viral and cellular DNA polymerases by their respective triphosphates. Biochem Biophys Res Commun. 1983 Oct 31;116(2):360–367. doi: 10.1016/0006-291x(83)90530-2. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Coen D. M., Fisher B. L., Weisslitz M., Randall S., Almy R. E., Gelep P. T., Schaffer P. A. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology. 1984 Jan 15;132(1):26–37. doi: 10.1016/0042-6822(84)90088-6. [DOI] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Helgstrand E., Eriksson B., Johansson N. G., Lannerö B., Larsson A., Misiorny A., Norén J. O., Sjöberg B., Stenberg K., Stening G. Trisodium phosphonoformate, a new antiviral compound. Science. 1978 Sep 1;201(4358):819–821. doi: 10.1126/science.210500. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Schooley R. T. Drug therapy. Treatment of herpesvirus infections. N Engl J Med. 1983 Oct 27;309(17):1034–1039. doi: 10.1056/NEJM198310273091706. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Sandri-Goldin R. M., Goldin A. L., Glorioso J. C., Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984 Mar;49(3):947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Purifoy D. J., Young D., Gopal R., Cammack N., O'Hare P. Single mutations at many sites within the DNA polymerase locus of herpes simplex viruses can confer hypersensitivity to aphidicolin and resistance to phosphonoacetic acid. J Gen Virol. 1984 Jan;65(Pt 1):1–17. doi: 10.1099/0022-1317-65-1-1. [DOI] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Reinke C. M., Drach J. C., Shipman C., Jr, Weissbach A. Differential inhibition of mammalian DNA polymerases alpha, beta and gamma and herpes simplex virus-induced DNA polymerase by the 5'-triphosphates of arabinosyladenine and arabinosylcytosine. IARC Sci Publ. 1978;(24 Pt 2):999–1005. [PubMed] [Google Scholar]
- Ruth J. L., Cheng Y. C. Nucleoside analogues with clinical potential in antivirus chemotherapy. The effect of several thymidine and 2'-deoxycytidine analogue 5'-triphosphates on purified human (alpha, beta) and herpes simplex virus (types 1, 2) DNA polymerases. Mol Pharmacol. 1981 Sep;20(2):415–422. [PubMed] [Google Scholar]
- Schinazi R. F., Peters J., Williams C. C., Chance D., Nahmias A. J. Effect of combinations of acyclovir with vidarabine or its 5'-monophosphate on herpes simplex viruses in cell culture and in mice. Antimicrob Agents Chemother. 1982 Sep;22(3):499–507. doi: 10.1128/aac.22.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D. F., Martin J. C., Verheyden J. P., Matthews T. R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983 May;23(5):676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Galloway K. S., Kennell W. L., Ogilvie K. K., Radatus B. K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982 Jul;22(1):55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H., Miller W. H., Miller R. L., Lambe C. U., Furman P. A. Inhibition of cellular alpha DNA polymerase and herpes simplex virus-induced DNA polymerases by the triphosphate of BW759U. Antimicrob Agents Chemother. 1984 Feb;25(2):191–194. doi: 10.1128/aac.25.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]


