Abstract
Previous studies have demonstrated that learning-related cerebellar plasticity and stimulus-elicited neuronal activity emerge ontogenetically in parallel with delay eyeblink conditioning in rats. The present study examined cerebellar interpositus field potentials and multiunit neuronal activity evoked by microstimulation of the inferior olive in Postnatal Day 17 and 24 rats. The slope and amplitude of the excitatory postsynaptic potential and the number of evoked multiunit spikes increased with age, whereas the inhibitory postsynaptic potential caused by Purkinje cell input remained stable. These results are consistent with the notion that the postsynaptic depolarization of cerebellar interpositus neurons caused by cerebellar afferents (e.g., the climbing fibers of the inferior olive) is a critical factor contributing to the ontogeny of delay eyeblink conditioning in rats.
Characterizing the development of synaptic interactions within maturing neural circuits may provide valuable insight into the conditions that are necessary for the induction of neural plasticity underlying certain learned behaviors (Carew, 1989; Carew, Menzel, & Shatz, 1998; Freeman & Nicholson, 2001, 2004). Delay eyeblink conditioning, in which repeated pairings of a conditioned stimulus (CS; e.g., a tone) and an unconditioned stimulus (US; e.g., an airpuff) promote the acquisition of a precisely timed conditioned response (CR), depends critically on brainstem–cerebellum circuitry (Christian & Thompson, 2003; Medina, Repa, Mauk, & LeDoux, 2002). The ontogeny of the delay eyeblink CR in rats occurs between Postnatal Day (P) 17 and 24 and is characterized by myriad anatomical and neurophysiological changes that likely have functional consequences on network activity within the brainstem–cerebellum eyeblink circuitry (Freeman & Nicholson, 2004). Two major brain regions that interact extensively during eyeblink conditioning are the cerebellar interpositus nucleus, where the critical cerebellar plasticity is formed and stored (Christian & Thompson, 2003; Kleim et al., 2002; Lavond, Kim, & Thompson, 1993), and the dorsal accessory olive (DAO), which supplies the cerebellum with the necessary and sufficient information about the US to support eyeblink conditioning (Christian & Thompson, 2003; Kim, Krupa, & Thompson, 1998; Medina, Nores, & Mauk, 2002).
In a previous study, Freeman and Nicholson (2000) found that cerebellar interpositus neurons from P17 rats exhibited weaker stimulus-elicited responses than interpositus neurons from P24 rats. Neurons in the deep cerebellar nuclei receive climbing fiber collaterals from the DAO, indicating that the same axons that produce complex spikes in Purkinje cells also innervate, for example, interpositus neurons (Eccles, Ito, & Szentagothai, 1967; Shinoda, Sugihara, Wu, & Sugiuchi, 2000; Sugihara, Wu, & Shinoda, 1999, 2001). Moreover, climbing fiber excitation of interpositus neurons exhibits summation (i.e., it is not all-or-none like Purkinje cell complex spikes) in proportion to the number of recruited climbing fiber synapses (Armstrong & Rawson, 1979; Eccles, Sabah, & Taborikova, 1974). Field potentials reflect the summed activity of a population of neurons and are mainly generated by postsynaptic potentials, rather than by action potentials (Leung, 1990). Therefore, interpositus field potentials, evoked by olivary microstimulation, are an informative index of synaptic maturity within the olivonuclear connection.
In field potential recordings, the monosynaptic climbing fiber activation of the deep cerebellar nuclei elicits a short latency (∼3 ms) excitatory postsynaptic potential (EPSP) and a longer latency (∼5 ms) disynaptic inhibitory postsynaptic potential (IPSP) caused by activated inhibitory Purkinje cells (see Figures 1a–1f; Delgado-Garcia & Gruart, 1995; Gould, Sears, & Steinmetz, 1993; Gruart, Blazquez, Pastor, & Delgado-Garcia, 1994; Ito, Yoshida, Obata, Kawai, & Udo, 1970; Kitai, McCrea, Preston, & Bishop, 1977). If the climbing fiber synapses on interpositus neurons are still maturing in P17 rats, then there should be a developmental difference in the EPSP elicited by climbing fiber input in the interpositus nucleus. Olivonuclear field potentials also might provide insight into developmental differences in corticonuclear synaptic connectivity, in that Purkinje cell inhibition of the interpositus neurons can be detected by means of their IPSP. The present study examined these two possible sources of developmental change in synaptic connectivity within the interpositus nucleus.
Figure 1.
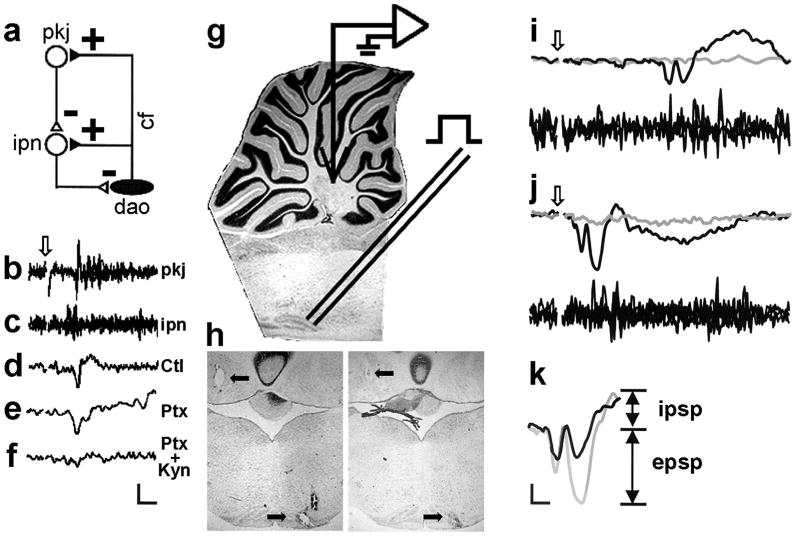
The neurophysiology of the olivocerebellar trisynaptic circuit. a: Diagram of the olivocerebellar circuit showing that climbing fibers (cf) from the dorsal accessory olive (dao) excite cerebellar interpositus neurons (ipn), and that complex spikes in Purkinje cells (pkj) inhibit cerebellar interpositus neurons milliseconds later. b: Four overlaid Purkinje cell complex spikes elicited by the climbing fiber inputs that also activate cerebellar interpositus neurons. c: Four overlaid multiunit sweeps from the interpositus nucleus. d–f: The field potential in the cerebellar interpositus nucleus evoked by DAO microstimulation under control (Ctl) conditions (d) and after picrotoxin (Ptx; e) and picrotoxin plus kynurenic acid (Ptx + Kyn; f) infusion into the cerebellar interpositus nucleus. Note that the field potential after picrotoxin infusion does not have an inhibitory postsynaptic potential (IPSP) and that the field potential after picrotoxin plus kynurenic acid infusion has neither an IPSP nor an excitatory postsynaptic potential (EPSP). Arrow in b indicates stimulus onset in b–f. g: Diagram of the experimental design, with a recording electrode in the cerebellar interpositus nucleus and a stimulating electrode in the DAO. h: Two representative brain sections showing marking lesions in the cerebellar interpositus nucleus and DAO (arrows). i: Evoked interpositus field potentials (upper traces; gray line = subthreshold, black line = threshold +100 μA) and multiunit activity (lower traces; three trials overlaid) from a Postnatal Day (P) 17 rat after microstimulation in the DAO. j: Evoked interpositus field potentials (upper traces) and multiunit activity (lower traces) from a P24 rat after microstimulation in the DAO. Arrows in i and j indicate stimulus onset. k: Illustration of EPSPs and IPSPs for each rat superimposed on each other (black line = P17 rat; gray line = P24 rat). Scale bar in f = 2 ms/600 μV. Scale bar in k = 1 ms/500 μV.
Method
Subjects
The subjects were 9 P17–18 and 9 P24–25 Long–Evans rat pups. Sex was balanced.
Surgery
Surgical methods were similar to those in a previous study (Nicholson & Freeman, 2003b). Briefly, rats were anesthetized with an intraperitoneal injection of urethane (1.4 g/kg) and given atropine (2.5 mg/kg) to reduce excess respiratory tract secretions during anesthesia. Proper hydration was maintained with an initial prophylactic injection of 0.5–0.8 ml of lactated Ringer's solution that was supplemented every hour with 0.1-ml injections. Rectal temperature was kept within 35–37 °C with a heating pad or a heat lamp.
Upon onset of anesthesia, an incision was made down the midline of the head, the tissue was reflected back, and the skull was cleaned with cotton-tipped applicators saturated with hydrogen peroxide. Rats were then placed in a stereotaxic headholder with their head oriented such that yaw, roll, and pitch were 0°. A stainless steel skull hook was affixed to the skull ∼1 mm anterior to lambda with dental acrylic (Dentsply International, York, PA). A bipolar stimulating electrode made of two insulated 50-μm stainless steel wires twisted and glued together (intertip distance = 75–100 μm) was then inserted at a 20° angle (rotated 30° axially away from center to avoid rupture of the midsagittal sinus) into a hole drilled above the contralateral DAO (P17: AP = +0.8, ML = +2.0, DV = −9.6; P24: AP = +0.6, ML = +2.0, DV = −10.0) and affixed to the skull with dental acrylic. The rat's head was then rotated such that pitch was 45° nose-down. A hole was drilled in the occipital bone, and a 33-gauge infusion cannula was implanted into the caudal portions of the left cerebellar anterior interpositus nucleus and affixed to the skull with dental acrylic (P17: AP = −7.1, ML = −2.0, DV = −6.5; P24: AP = −7.1, ML = −2.0, DV = −6.7). The rat's head was then rotated to 0° pitch, and a dental acrylic dam was constructed around the region of the skull immediately over the left cerebellar interpositus nucleus. A 1–2-mm diameter hole was drilled for insertion of the recording electrode into the left anterior interpositus nucleus (AP = −2.3, ML = −2.0, DV = −4.8). The dam was then filled with warm mineral oil. A small hole was made in the dura immediately before recording began to allow insertion of the recording electrode under the guidance of a mounted dissecting microscope.
Data Acquisition
All data were acquired with an Xcell-3+ Isolated Microelectrode Amplifier customized with an impedance check module, audio monitor, adjustable stimulus artifact suppressor, and DC output (FHC, Bowdoinham, ME). Activity was amplified (gain = 10,000), bandpass filtered, digitized at 10 kHz, written to computer disk, and analyzed offline with pClamp 6 (Axon Instruments, Foster City, CA). Recordings were made with a 25-μm stainless steel electrode insulated except at the flush-cut tip (resistance = 200–500 kΩ; gain = 10,000). Field potentials were recorded by setting the bandpass filter settings to 0.1–1,000 Hz; multiunit activity was recorded by setting the bandpass filter settings to 500–5000 Hz.
After the dura was pierced, the recording electrode was slowly lowered with a micromanipulator (World Precision Instruments, Sarasota, FL) and the neuronal activity at the electrode tip was monitored continuously. As the electrode passed through the cerebellar cortex and entered the white matter above the interpositus nucleus, the DAO was stimulated through the implanted bipolar stimulating electrode (single 50-μs pulse) to help locate the interpositus nucleus. When DAO stimulation consistently elicited action potentials (constant latency less than 3–4 ms for P24 rats; less than 5–7 ms for P17 rats), as assessed by overlaying successive sweeps on the oscilloscope (Tektronix, Beaverton, OR), recording began. The latency of climbing fiber excitation in the cerebellum is longer in younger rats because of ongoing myelination (Lang & Rosenbluth, 2003; Nicholson & Freeman, 2003b). Each recording session consisted of 264 trials. For field potentials, 15 pulses at each of 14 current levels (10–500 μA) were presented. For multiunit activity, 6 pulses at 9 of the current levels were presented. A particular current level was considered to be at threshold when it elicited multiunit activity and a reliable EPSP and IPSP at consistent latencies (see Figures 1b–1k). Short-latency activity (1–2 ms before the EPSP) was considered to be produced by antidromic invasion of the axon terminals of cerebellar projection neurons in the DAO, but was not required for consistent and robust evoked field potentials or multiunit activity and was therefore not examined extensively (see also Gruart et al., 1994).
After olivonuclear field potentials were recorded under control conditions, the same stimulation protocol was given after infusion of picrotoxin (pH = 7.3; 1.0 μl, 1.0 mM at a rate of 5 μl/hr) and kynurenic acid (pH = 7.3; 1.0 μl, 2.0 mM at a rate of 5 μl/hr) dissolved in artificial cerebrospinal fluid. Picrotoxin is a GABAA receptor antagonist and should selectively block IPSPs. Kynurenic acid is a broad-spectrum ionotropic glutamate receptor antagonist and should selectively block EPSPs.
As Figures 1i–1k illustrate, the EPSP and IPSP could be differentiated on the bases of polarity and latency. The amplitude and slope of the EPSP and IPSP were calculated by using the peak of the EPSP or IPSP relative to the prestimulation baseline. Latencies were measured for the peaks of the EPSP and IPSP. Evoked multiunit activity was assessed by counting the number of spikes summed across each of the six trials at each current level within three 4-ms time windows after olivary stimulation (P17: starting at 5 ms; P24: starting at 2 ms). When analysis of variance yielded significant main effects or interactions, significant differences were evaluated with Tukey's honestly significant difference test (p < .05). All measurements were made at threshold current levels (T), and at 50 μA (T + 50), 100 μA (T + 100), and 150 μA (T + 150) above threshold.
At the end of recording, 500 μA of constant current was passed through the recording and stimulating electrodes for 10 s to mark the location of each electrode. A wire was inserted into the infusion cannula, and a marking lesion was made ∼500 μm from the tip. After marking lesions were made, the rats were killed with a lethal injection of sodium pentobarbital (90 mg/kg) and transcardially perfused with 100 ml physiological saline followed by 300 ml of 3% Formalin. After perfusion, the brains were postfixed in the same fixative for a minimum of 96 hr and subsequently sectioned at 50 μm with a sliding microtome. Sections were then stained with cresyl violet. The locations of the recording electrodes were confirmed by examining serial sections.
Results
Of the 18 rats that had accurate recording electrode and stimulating electrode placements, 3 P17 rats and 4 P24 rats also had accurate infusion cannula placements that exhibited pharmacological blockade of the EPSP and IPSP (e.g., Figures 1d–1f). The EPSPs and IPSPs under control conditions in these rats were essentially identical to those recorded in the other 11 rats that had only correct recording and stimulating electrode placements. Representative marking lesions from 2 of the rats are shown in Figure 1h.
Figures 1i–1k show cerebellar interpositus field potentials and multiunit activity evoked by microstimulation in the inferior olive from a P17 (Figure 1i) and a P24 (Figure 1j) rat. Note that three main differences are apparent. First, there is a dramatic developmental increase in the climbing fiber EPSP in the absence of any notable change in the IPSP. Second, there is a substantial age-related decrease in the latencies of both the EPSP and the IPSP. And third, there is more elicited multiunit interpositus neuronal activity (bottom traces in Figures 1i and 1j) in older rats.
The group data for EPSP and IPSP amplitude, slope (ΔV/Δt), and latency are presented in Figures 2a–2c. Figure 2d shows the group data for evoked multiunit activity. All data are plotted as a function of current level (T, T + 50, T + 100, T + 150). As can be seen, there were substantial developmental increases in the amplitude and slope of the climbing fiber EPSP, with no change in the IPSP (Figures 2a and 2b)—amplitude: Age × EPSP/IPSP interaction, F(1, 128) = 10.889, p < .01; slope: Age × EPSP/IPSP interaction, F(1, 128) = 4.865, p < .05. There was also a developmental change in the latency of both the EPSP and the IPSP: main effect of age, F(1, 128) = 923.780, p < .01, in addition to an age-related decrease in the temporal separation between the EPSP and IPSP peaks (Figure 2c): main effect of age, F(1, 64) = 26.315, p < .01. Finally, the number of evoked multiunit spikes in the cerebellar interpositus nucleus within the first two 4-ms time windows after DAO microstimulation increased with age (Figure 2d): Age × Time Window interaction, F(2, 128) = 13.220, p < .01. Planned comparisons (all ps < .05) revealed that the developmental differences in the second time window disappeared at higher current levels (Figure 2d), which may indicate that strong input can compensate partially for immature climbing fiber afferents. Note, however, that the developmental difference remained in the EPSP at these current levels (Figures 2a and 2b), suggesting that other mechanisms of spike generation (e.g., rebound depolarization) may be responsible (Aizenman & Linden, 1999). In summary, climbing fiber input to the cerebellar interpositus nucleus exhibited substantial maturation between P17 and P24, whereas inhibition from Purkinje cells remained stable over this same developmental time period.
Figure 2.
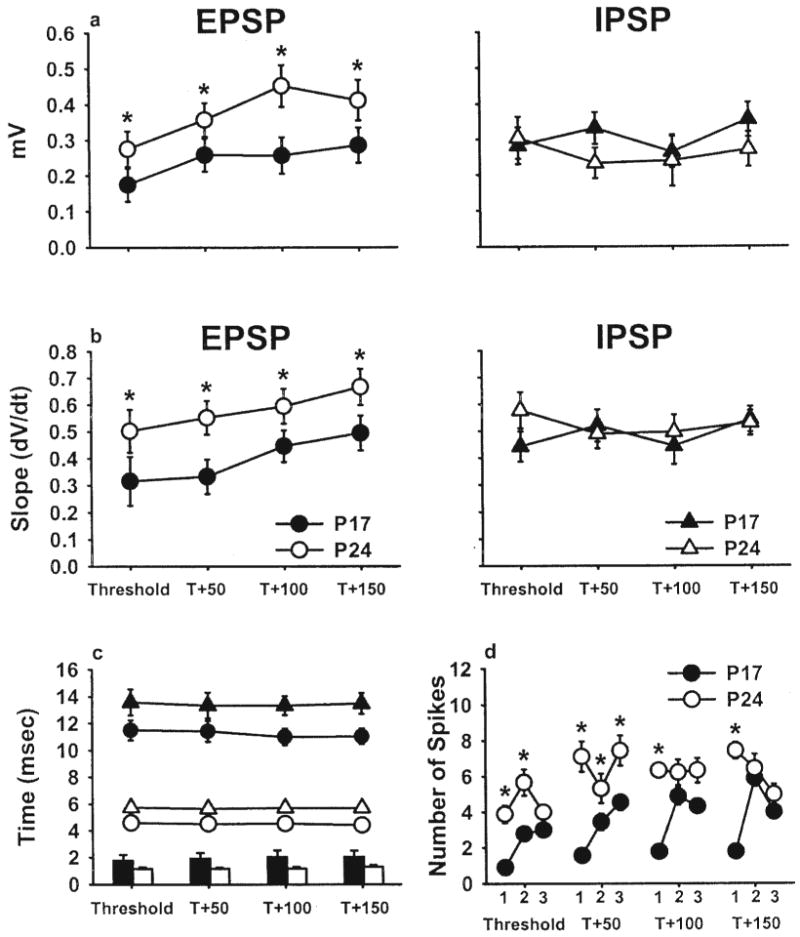
Selective developmental increase in the climbing fiber excitatory postsynaptic potential (EPSP). a: Amplitude of the EPSP and inhibitory postsynaptic potential (IPSP) for each of four current levels in Postnatal Day (P) 17 (solid) and P24 (open) rats. b: Slope of the EPSP and IPSP for each of four different current levels. c: Peak latencies for the EPSP (circles) and IPSP (triangles). Plotted along the x-axis is the time between each peak at each of the four current levels for P17 (solid) and P24 (open) rats. d: Total number of multiunit spikes evoked in the six trials at each current level for three 4-ms time windows after microstimulation of the dorsal accessory olive in P17 and P24 rats. All data are means (± SEM). T = thresholds. Asterisks indicate statistically significant differences (p < .05).
Discussion
Evoked cerebellar interpositus nucleus field potentials and multiunit neuronal recordings showed that the EPSP elicited by climbing fiber input exhibits a selective developmental increase between P17 and P24. In contrast, the IPSP from Purkinje cell synaptic input, which was examined simultaneously, showed no reliable developmental change. These findings provide additional evidence that the interconnections between the cerebellar interpositus nucleus and the DAO undergo substantial maturation during the developmental period in which the eyeblink CR emerges.
Freeman and Nicholson (2000) first discussed the possibility of maturation within the olivonuclear input on the basis of developmental increases in US-elicited single-unit activity in the cerebellar interpositus nucleus. The present study provides further evidence that the developmental increase in the climbing fiber EPSP may be attributable to an addition of excitatory climbing fiber synapses or an increase in the size or efficacy of climbing fiber synapses. The age-related increase in EPSP amplitude and slope, in the absence of any change in IPSP parameters, was accompanied by increases in the average number of spikes evoked in the multiunit activity. This latter finding suggests that olivary microstimulation at P24 was more effective in depolarizing interpositus neurons beyond their spike threshold.
Although P17 rats show poor eyeblink conditioning and virtually absent in vivo cerebellar plasticity (Freeman & Nicholson, 2000; Nicholson & Freeman, 2004), recent in vitro studies have used cerebellar tissue from rats younger than P17 to demonstrate various modifications in the synaptic efficacy and postsynaptic excitability of cerebellar neurons (Aizenman, Huang, & Linden, 2003; Aizenman, Huang, Manis, & Linden, 2000; Zhang & Linden, 2003). The increases in excitability depend on postsynaptic depolarization (and Ca2+ in flux), which indicates that the cellular cascades involved in at least these forms of neuronal plasticity are functionally mature before P17. These studies, combined with the results of the present experiment, suggest that the ability of cerebellar afferents (e.g., climbing fibers) to sufficiently depolarize cerebellar interpositus neurons may be a critical factor influencing the induction of cerebellar plasticity. The developmental increases in cerebellar interpositus EPSP amplitude and slope may contribute to the ontogeny of cerebellar plasticity and eyeblink conditioning by producing levels of postsynaptic depolarization sufficient to initiate cellular cascades at P24, but not at P17 (Freeman & Nicholson, 2001).
Consistent with this supposition is the observation that, although olivary neurons are more difficult to activate in P24 rats (Nicholson & Freeman, 2000, 2003a, 2003b), the olivary action potentials lead to robust EPSPs in the deep cerebellar nuclei and are highly effective in driving cerebellar interpositus neurons beyond their spike threshold. The balance between inhibition and excitation that emerges in the DAO by P24 (Nicholson & Freeman, 2003a) might act as a filter, such that the rare but robust input from individual olivary neurons is particularly proficient in relaying temporally and somatotopically specific information to the cerebellar nuclei. The “less is more” nature of olivary input to the deep cerebellar nuclei is amplified during eyeblink conditioning (Delgado-Garcia & Gruart, 1995; Gruart et al., 1994). Specifically, in cats, the climbing fiber EPSP recorded in the deep cerebellar nuclei is more than doubled when olivary microstimulation is preceded (< 40 ms) by either the peripheral CS during conditioning trials or by microstimulation in the pontine nuclei (the necessary and sufficient source of CS input to the cerebellum). It is possible that the augmentation of olivonuclear synaptic input that results from concurrent pontine stimulation enables interpositus neurons to function as coincidence detectors. This idea, however, remains to be tested because, although computer models can acquire simulated eyeblink CRs without olivonuclear input (Mauk & Donegan, 1997; Medina & Mauk, 1999; Medina, Nores, & Mauk, 2002; Medina, Repa, et al., 2002), there is no direct empirical evidence for or against the role of climbing fiber inputs to the deep cerebellar nuclei during eyeblink conditioning. It is important to note, however, that animals with aspiration lesions of the cerebellar cortex can still acquire eyeblink CRs, possibly indicating that interpositus neurons alone can form the eyeblink CR memory (Freeman, Carter, & Stanton, 1995; Lavond & Steinmetz, 1989; but see Garcia, Steele, & Mauk, 1999).
There are at least three possible mechanisms of structural synaptic growth between P17 and P24 that could contribute to increases in the climbing fiber EPSP. First, it is possible that there is a net addition of excitatory synapses between climbing fiber collaterals and cerebellar interpositus neurons. Second, there may be an increase in the size of climbing fiber–interpositus neuron synapses. Increases in postsynaptic density size occur in the synapses within CA1 stratum radiatum of rabbits trained in trace eyeblink conditioning (Geinisman et al., 2000), which is consistent with the training-related potentiation of the CA1 dendritic field potential (Power, Thompson, Moyer, & Disterhoft, 1997). A third possible structural substrate of increased olivonuclear synaptic efficacy is the proliferation of multiple synapse boutons, which has been reported in the visual cortex (Jones, Klintsova, Kilman, Sirevaag, & Greenough, 1997) and cerebellar cortex (Federmeier, Kleim, & Greenough, 2002) of rats, and in the hippocampus of rabbits (Geinisman, Berry, Disterhoft, Power, & Van der Zee, 2001) after learning. These three mechanisms of synaptic structural change are not mutually exclusive and may occur in combination. Such a circumstance has been reported previously, in that a developmental addition of inhibitory multiple synapse boutons occurs simultaneously with, and could be the mechanism underlying, an increase in the number of inhibitory synapses within the DAO of developing rats (Nicholson & Freeman, 2003a). The widespread involvement of changes in synapse number, postsynaptic density dimensions, and production of multiple synapse boutons during development and learning indicates that alterations in these parameters may be major mechanisms contributing to the selective developmental increase in the climbing fiber EPSP.
In summary, the results of the present experiment indicate that climbing fiber input to cerebellar interpositus neurons in rats is significantly weaker at P17 than at P24. These results, combined with previous work, contribute to an emerging view regarding the ontogeny of delay eyeblink conditioning. During delay eyeblink conditioning, immature US input may fail to sufficiently depolarize cerebellar interpositus neurons at P17, obstructing the induction of cerebellar plasticity. The weak synaptic plasticity underlying the cerebellar memory trace in P17 rats (Freeman & Nicholson, 2000; Nicholson & Freeman, 2004), subject to the chronic inability to regulate climbing fiber activity through inhibitory cerebellar feedback (Nicholson & Freeman 2003a) and the prevalence of spatiotemporally diffuse US information (Nicholson & Freeman, 2003b), which experimental studies and computer simulations indicate interfere with the formation and preservation of cerebellar plasticity (Kim et al., 1998; Medina & Mauk, 1999; Medina, Nores, & Mauk, 2002; Medina, Repa, et al., 2002; Raymond & Lisberger, 1998), may be incrementally reversed between conditioning trials and sessions. The results of the present experiment are consistent with the notion that elaboration of olivocerebellar synaptic interactions is a critical prerequisite for the induction and maintenance of persistent, robust, and specific cerebellar plasticity in developing rats.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS38890 to John H. Freeman Jr. and Predoctoral Fellowship NS41713 to Daniel A. Nicholson. Preparation of this manuscript was supported in part by Postdoctoral Fellowship T32 AG20506 from the National Institute on Aging. We thank Adam Muckler and Christine Rabinak for help with data analysis.
References
- Aizenman CD, Huang EJ, Linden DJ. Morphological correlates of intrinsic electrical excitability in neurons of the deep cerebellar nuclei. Journal of Neurophysiology. 2003;89:1738–1747. doi: 10.1152/jn.01043.2002. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Huang EJ, Manis PB, Linden DJ. Use-dependent changes in synaptic strength at the Purkinje cell to deep nuclear synapse. Progress in Brain Research. 2000;124:247–273. doi: 10.1016/s0079-6123(00)24022-3. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slice of rat cerebellum. Journal of Neurophysiology. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Rawson JA. Responses of neurones in nucleus interpositus of the cerebellum to cutaneous nerve volleys in the awake cat. Journal of Physiology (London) 1979;289:403–423. doi: 10.1113/jphysiol.1979.sp012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ. Developmental assembly of learning in Aplysia. Trends in Neurosciences. 1989;12:389–394. doi: 10.1016/0166-2236(89)90078-7. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Menzel R, Shatz CJ. Mechanistic relationships between development and learning. New York: Wiley; 1998. [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: Acquisition and retention. Learning & Memory. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia JM, Gruart A. Signalling properties of deep cerebellar nuclei neurones. In: Ferrel WR, Proske U, editors. Neural control of movement. New York: Plenum Press; 1995. pp. 225–232. [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The cerebellum as a neuronal machine. Heidelberg, Germany: Springer; 1967. [Google Scholar]
- Eccles JC, Sabah NH, Taborikova H. The pathways responsible for excitation and inhibition of fastigial neurons. Experimental Brain Research. 1974;19:78–99. doi: 10.1007/BF00233396. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kleim JA, Greenough WT. Learning-induced multiple synapse formation in rat cerebellar cortex. Neuroscience Letters. 2002;332:180–184. doi: 10.1016/s0304-3940(02)00759-0. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Carter CS, Stanton ME. Early cerebellar lesions impair eyeblink conditioning in developing rats: Differential effects of unilateral lesions on postnatal day 10 or 20. Behavioral Neuroscience. 1995;109:893–902. doi: 10.1037//0735-7044.109.5.893. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Developmental changes in eye-blink conditioning and neuronal activity in the cerebellar interpositus nucleus. Journal of Neuroscience. 2000;20:813–819. doi: 10.1523/JNEUROSCI.20-02-00813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Ontogenetic changes in the neural mechanisms of eyeblink conditioning. Integrative, Physiological, and Behavioral Science. 2001;36:15–35. doi: 10.1007/BF02733945. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behavioral and Cognitive Neuroscience Reviews. 2004;3:3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. Journal of Neuroscience. 1999;19:10940–10947. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. Journal of Neuroscience. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Disterhoft JF, Gundersen HJ, McEchron MD, Persina IS, Power JM, et al. Remodeling of hippocampal synapses after hippocampus-dependent associative learning. Journal of Comparative Neurology. 2000;417:49–59. [PubMed] [Google Scholar]
- Gould TJ, Sears LL, Steinmetz JE. Possible CS and US pathways for rabbit classical eyelid conditioning: Electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behavioral and Neural Biology. 1993;60:172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- Gruart A, Blazquez P, Pastor AM, Delgado-Garcia JM. Very short-term potentiation of climbing fiber effects on deep cerebellar nuclei neurons by conditioning stimulation of mossy fiber afferents. Experimental Brain Research. 1994;101:173–177. doi: 10.1007/BF00243229. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K, Kawai N, Udo M. Inhibitory control of intracerebellar nuclei by the Purkinje cell axons. Experimental Brain Research. 1970;10:64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiology of Learning and Memory. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science. 1998 January 23;279:570–573. doi: 10.1126/science.279.5350.570. [DOI] [PubMed] [Google Scholar]
- Kitai ST, McCrea RA, Preston RJ, Bishop GA. Electrophysiological and horseradish peroxidase studies of precerebellar afferents to the nucleus interpositus anterior: I. Climbing fiber system. Brain Research. 1977;122:197–214. doi: 10.1016/0006-8993(77)90289-x. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Freeman JH, Jr, Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D. Synapse formation is associated with memory storage in the cerebellum. Proceedings of the National Academy of Sciences, USA. 2002;99:13228–13231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Rosenbluth J. Role of myelination in the development of a uniform olivocerebellar conduction time. Journal of Neurophysiology. 2003;89:2259–2270. doi: 10.1152/jn.00922.2002. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Annual Review of Psychology. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behavioural Brain Research. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Leung LWS. Field potentials in the central nervous system. In: Boulton AA, Baker GB, Vanderwolf CH, editors. Neuromethods: Vol 15 Neurophysiological techniques: Applications to neural systems. Clifton, NJ: Humana Press; 1990. pp. 277–312. [Google Scholar]
- Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learning & Memory. 1997;4:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Simulations of cerebellar motor learning: Computational analysis of plasticity at the mossy fiber to deep nucleus synapse. Journal of Neuroscience. 1999;19:7140–7151. doi: 10.1523/JNEUROSCI.19-16-07140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002 March 21;416:330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- Medina JF, Repa CJ, Mauk MD, LeDoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nature Reviews Neuroscience. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Developmental changes in eyeblink conditioning and neuronal activity in the inferior olive. Journal of Neuroscience. 2000;20:8218–8226. doi: 10.1523/JNEUROSCI.20-21-08218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Addition of inhibition in the olivocerebellar system and the ontogeny of a motor memory. Nature Neuroscience. 2003a;6:532–537. doi: 10.1038/nn1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Developmental changes in evoked Purkinje cell complex spike responses. Journal of Neurophysiology. 2003b;90:2349–2357. doi: 10.1152/jn.00481.2003. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Developmental changes in eyeblink conditioning and simple spike activity in the cerebellar cortex. Developmental Psychobiology. 2004;44:45–57. doi: 10.1002/dev.10149. [DOI] [PubMed] [Google Scholar]
- Power JM, Thompson LT, Moyer JR, Jr, Disterhoft JF. Enhanced synaptic transmission in CA1 hippocampus after eyeblink conditioning. Journal of Neurophysiology. 1997;78:1184–1187. doi: 10.1152/jn.1997.78.2.1184. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. Journal of Neuroscience. 1998;18:9112–9129. doi: 10.1523/JNEUROSCI.18-21-09112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Sugihara I, Wu HS, Sugiuchi Y. The entire trajectory of single climbing and mossy fibers in the cerebellar nuclei and cortex. Progress in Brain Research. 2000;124:173–186. doi: 10.1016/S0079-6123(00)24015-6. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Wu HS, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. Journal of Comparative Neurology. 1999;414:131–148. [PubMed] [Google Scholar]
- Sugihara I, Wu HS, Shinoda Y. The entire trajectories of single olivocerebellar axons in the cerebellar cortex and their contribution to cerebellar compartmentalization. Journal of Neuroscience. 2001;21:7715–7723. doi: 10.1523/JNEUROSCI.21-19-07715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nature Reviews Neuroscience. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]


