Abstract
Thirteen Listeria monocytogenes strains were compared for the ability to survive in a dynamic gastrointestinal model. Strains displayed various degrees of susceptibility to gastric acidity; however, strain-to-strain variations became evident mainly after 90 min of exposure (pH 2.0). Cell levels transferred to the intestine depended on initial populations, while reductions during intestinal exposure were relatively small for all strains.
Strains of Listeria monocytogenes are diverse in serological and molecular features, while serotypes and genetic groups display great diversity in virulence and environmental distribution (14, 26). Variations in physiological responses of L. monocytogenes strains have also been reported (9, 10, 17) and may contribute to virulence heterogeneity, as resistance to stresses is critical for survival within the host (13).
The acidity of the stomach is considered a major defense barrier against food-borne infection (24). Subsequently, cells that survive gastric passage and reach the small intestine must withstand the presence of bile and high-osmolarity conditions (13). Various aspects of food-borne listeriosis have been examined using artificial gastrointestinal fluid broth systems (2, 11, 15, 22, 25); however, findings may not accurately reflect the specific stages of L. monocytogenes survival in the digestive tract, since those studies did not account for the changing conditions to which pathogens are subjected while in the digestive tract. In this respect, artificial gastrointestinal systems that closely simulate the dynamics of gastrointestinal transit may be valuable instruments for identification of factors affecting the gastrointestinal survival of L. monocytogenes, including strain-to-strain variations.
To our knowledge, the use of dynamic gastrointestinal systems in the study of food-borne pathogens is limited (3, 4, 16). In this study, we examined differences in gastrointestinal survival among 13 L. monocytogenes strains, representing different serotypes and three genotypic lineages (26), using a simulated model of the human stomach and small intestine.
Bacteria.
To examine the potential contribution of the alternative sigma factor σB function in the gastrointestinal survival of L. monocytogenes, tested strains (Table 1) also included 10403S and its in-frame sigB deletion mutant strain, A1-254 (27).
TABLE 1.
Listeria monocytogenes strains used in this study
| Strain | Serotypea | Lineagea | Origin (yr of isolation) | Reference |
|---|---|---|---|---|
| 558 | 1/2 | NK | Pork meat | |
| R2-500b | 4b | 1 | Food, epidemic case, North Carolina (2000) | 12 |
| R2-501b | 4b | 1 | Human, epidemic case, North Carolina (2000) | 12 |
| Scott A | 4b | 1 | Human | |
| N1-225b | 4b | 1 | Human, epidemic case, United States (1998-99) | 12 |
| N1-227b | 4b | 1 | Food, epidemic case, United States (1998-99) | 12 |
| C1-056b | 1/2a | 2 | Human, sporadic case | 12 |
| N3-031b | 1/2a | 2 | Food (hot dog), sporadic case (1989) | 12 |
| J1-101b | 1/2a | 2 | Human, sporadic case (1989) | 12 |
| 10403Sc | 1/2a | 2 | NK | 5 |
| A1-254c | ΔsigB mutant of 10403S | 27 | ||
| J1-158b | 4b | 3 | Goat | 12 |
| J1-168b | 4a | 3 | Human, sporadic case | 12 |
NK, not known. Serotype and lineage designations were provided by the donor or reference (except for strains 558 and Scott A).
Kindly provided by Martin Wiedmann (Department of Food Science, Cornell University, Ithaca NY).
Kindly provided by Kathryn J. Boor (Department of Food Science, Cornell University, Ithaca NY).
Cultures of individual strains were prepared in 100 ml tryptic soy broth without dextrose (Difco, Becton Dickinson, Sparks, MD) supplemented with 0.6% yeast extract (Acumedia, Lansing, MI) (TSBYE-G), inoculated with active cultures of each strain (6.9 to 7.2 log CFU/ml), and incubated for 4 or 16 h (30°C) to investigate potential effects of the age of cells on the survival of each strain.
Simulated gastrointestinal fluids.
An artificial saliva solution (6.2 g/liter NaCl, 2.2 g/liter KCl, 0.22 g/liter CaCl2, and 1.2 g/liter NaHCO3) (18, 19) was autoclaved and cooled to ambient temperature (25°C) before use. Gastric fluid (pH 2; 5N HCl) (20, 21) contained 0.4 g/liter glucose, 3.0 g/liter yeast extract, 1.0 g/liter Bacto peptone (Difco), 4.0 g/liter porcine mucin (Sigma-Aldrich, St. Louis, MO), 0.5 g/liter cysteine, 0.08 g/liter NaCl, 0.4 g/liter NaHCO3, 0.04 g/liter K2HPO4, 0.04 g/liter KH2PO4, 0.008 g/liter CaCl2·2H2O, 0.008 g/liter MgSO4·7H2O, 1.0 g/liter xylan (Sigma-Aldrich), 3.0 g/liter soluble starch (Sigma-Aldrich), 2.0 g/liter pectin (Sigma-Aldrich), 1 ml/liter Tween 80, and 3 g/liter pepsin from porcine stomach mucosa (Sigma-Aldrich). Intestinal fluid (16) consisted of 0.1 g/liter porcine trypsin (type IX-S; Sigma-Aldrich) and 3.5 g/liter porcine pancreatin (Sigma-Aldrich), and the solution was filtered (0.45-μm-pore-diameter cellulose filter; Millipore Corp., Bedford, MA). Biliary secretions were simulated by preparing 2% or 4% porcine bile (Sigma-Aldrich) solution.
Dynamic gastrointestinal system.
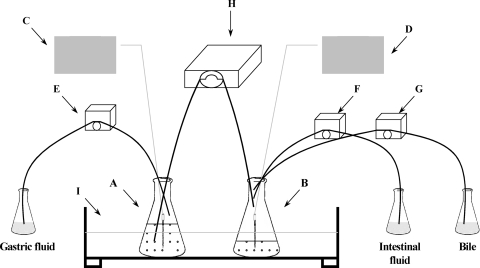
The simulated gastrointestinal tract (16) (Fig. 1) consisted of Erlenmeyer flasks (500 ml), representing the gastric (GC) and the intestinal (IC) compartments, kept in a water bath (shaking water bath model 50; Precision Scientific, Chicago, IL) at 37°C. Peristaltic pumps (variable-speed low-flow pump; Fisher Scientific) delivered gastric fluid into the GC (flow rate, 0.33 ml/min) and intestinal fluid (flow rate, 0.33 ml/min) and bile solutions (4% for the first 30 min and 2% for the remaining time; flow rate, 0.5 ml/min) into the IC. A multichannel peristaltic pump (205U; Watson-Marlow Limited, Cornwall, England) transferred the gastric contents into the IC (initiated 15 min after the beginning of the challenge) at a flow rate of 1.1 ml/min (7).
FIG. 1.
Schematic diagram of the dynamic gastrointestinal model used in this study. (A, B) 500-ml Erlenmeyer flasks representing the GC and IC; (C, D) pH meters monitoring the pH in the GC and IC; (E, F, G) peristaltic pumps delivering gastric fluid (flow rate, 0.33 ml/min) in the GC and intestinal fluid (flow rate, 0.33 ml/min) and 2 or 4% bile (flow rate, 0.5 ml/min) in the IC; (H) peristaltic pump transferring the gastric contents (flow rate, 1.1 ml/min) in the IC; (I) shaking water bath stabilized at 37°C.
Gastrointestinal passage tolerance assay.
Prior to each challenge, 10 ml of gastric fluid was added to the GC, whereas the IC contained 7 ml of 4% bile solution (19). Each 100-ml culture (4 or 16 h) was diluted (1:1 [vol/vol]) with artificial saliva. The pH of the GC was adjusted (with 5 N HCl) to 5.0, 4.0, 3.0, and 2.0 at 10, 28, 58, and 88 min, respectively, to reproduce in vivo gastric pH values corresponding to young adults after ingestion of a standard meal (8); the gastric pH then remained constant until the end of the challenge (120 min). The intestinal pH was maintained at 6.5 ± 0.3 with 0.3 M NaHCO3 (18, 19). The GC and IC pH conditions were monitored continuously (Ultra Basic; Denver Instrument, Arvada, CO). Secretion of gastrointestinal fluids continued for 120 min after the beginning of each challenge; however, the IC was maintained (statically) in the water bath for a final microbiological analysis at 240 min. L. monocytogenes populations were assessed before being mixed with saliva and at intervals during exposure to each compartment by diluting 1-ml samples with 0.1% buffered peptone water (9 ml; Difco) and plating, in duplicate, 0.1-ml portions onto tryptic soy agar (Difco) supplemented with 0.6% yeast extract (TSAYE) and Palcam agar (Difco) (30°C, 48 h).
Statistical analyses.
The study was conducted three times. Cell counts were divided by dilution factors (16) to account for the continuous addition or removal of gastrointestinal fluids. Numbers obtained were converted into log CFU/ml and analyzed using the Glimmix procedure of SAS, version 9.2 (SAS Institute Inc., Cary, NC) (23). Independent variables included strain, time and age of the culture, and their interactions. Mean differences were separated at the significance level of 95%.
L. monocytogenes data (Palcam agar) were fitted to the model of Baranyi and Roberts (1), using DMFit software (Institute of Food Research, Norwich, United Kingdom), to determine shoulder durations (lags in death) and inactivation rates (IRs) of cultures. Inactivation kinetics were analyzed with the mixed procedure of SAS (23), with strain being the independent variable; additional analyses, in which serotype or lineage was the independent variable, were conducted to identify potential serotype/lineage-related effects on gastrointestinal survival.
Gastric survival.
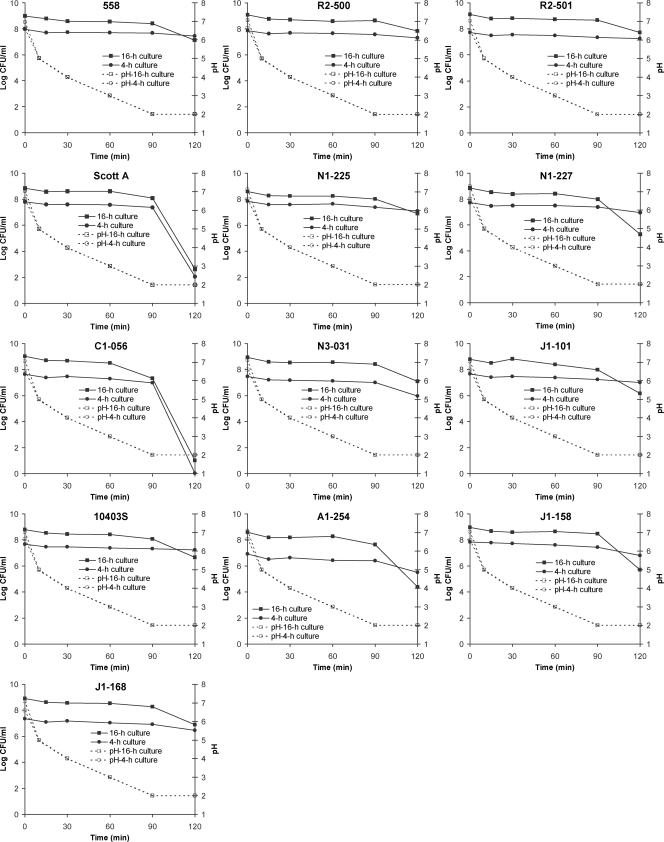
Overall, counts from Palcam agar (Fig. 2) and TSAYE (not shown) were similar (<0.3-log CFU/ml difference) throughout the challenge; thus, the reported results are for populations on Palcam agar. Although the reasons for a lack of detection of substantial sublethal injury are unclear, traits of individual strains and potential acid adaptation (due to gradual gastric acidification) might have contributed to these findings.
FIG. 2.
Survival (log CFU/ml [Palcam agar counts]) of individual L. monocytogenes strains and pH values within the GC (37°C) during a simulated gastrointestinal challenge, conducted after inoculation into 100 ml of TSBYE-G and incubation for 4 or 16 h at 30°C. Experiments were conducted in triplicate, and the results are means.
With the exception of the ΔsigB mutant strain A1-254, all cultures had reached the exponential (7.4 to 8.0 log CFU/ml) and stationary (8.6 to 9.1 log CFU/ml) phases in TSBYE-G within 4 and 16 h, respectively. Strain A1-254 exhibited no apparent changes in its population during the 4-h incubation, suggesting that σB may affect the growth potential, even at 30°C.
Cell counts of all L. monocytogenes strains remained in the range of 6.4 to 7.6 (4-h cultures) and 7.3 to 8.7 (16-h cultures) log CFU/ml during the first 90 min of gastric exposure (Fig. 2). Major (P < 0.05) reductions in populations and strain differences were observed mainly at 120 min. The highest (P < 0.05) acid sensitivity was displayed by strain C1-056, while the second most acid-sensitive strain was Scott A. This was confirmed by IR data indicating that strain C1-056 displayed the highest IR, followed (P ≥ 0.05) by Scott A (Tables 2 and 3). However, as suggested by their large shoulder durations (Tables 2 and 3), reductions in populations of these strains occurred mainly at later stages of exposure. The ΔsigB mutant, A1-254, declined faster (P ≥ 0.05) than the wild-type strain, 10403S (Table 2). It should be noted, however, that under the examined conditions a valid comparison between the gastric resistances of 4-h cultures of A1-254 and 10403S may not be feasible, as the slow growth of the former strain prevented it from being in the same growth phase as the remaining strains. On the other hand, growth experiments (data not shown) suggested that both A1-254 and 10403S had reached stationary phase within 16 h of incubation, enabling a valid comparison between these strains. As a 16-h culture, strain A1-254 was more (P < 0.05) acid sensitive than 10403S and displayed an IR similar (P ≥ 0.05) to that of Scott A (Table 3). Overall, IRs of 16-h cultures were not different (P ≥ 0.05) between pairs (outbreak sets; strains belonging to each set were genetically close) of food and human isolates (Table 1) (12). Unlike findings of previous studies (6, 15), the IRs suggested that 4-h cultures were more resistant than 16-h cultures of the respective strains. A possible explanation for this discrepancy could be that the 4-h cultures were still in the early stages of the exponential phase and thus contained a substantial portion of the original stationary-phase cells of the inoculum used to initiate the culture, which could have exhibited more acid resistance (6).
TABLE 2.
Mean (n = 3) shoulder durations and inactivation rates (± standard deviations) of individual Listeria monocytogenes strains in a simulated gastrointestinal system (GC at pH 2.0 within 88 min; IC at pH 6.5; 37°C) after inoculation (1 ml) into 100 ml of TSBYE-G and incubation for 4 h at 30°Cb
| Strain | GC
|
Maximum inactivation rate (log CFU/ml/min) in IC | |
|---|---|---|---|
| Shoulder duration (min)a | Maximum inactivation rate (log CFU/ml/min) | ||
| 558 | 0.00 ± 0.00A | 0.003 ± 0.001A | 0.008 ± 0.006A |
| R2-500 | 0.003 ± 0.003A | 0.010 ± 0.009A | |
| R2-501 | 68.66 ± 42.85BC | 0.002 ± 0.003A | 0.004 ± 0.001A |
| Scott A | 77.48 ± 0.74B | 0.209 ± 0.019B | 0.006 ± 0.002A |
| N1-225 | 40.77 ± 34.76C | 0.010 ± 0.007A | 0.009 ± 0.007A |
| N1-227 | 71.49 ± 3.31B | 0.019 ± 0.006A | 0.011 ± 0.012A |
| C1-056 | 76.88 ± 2.37B | 0.262 ± 0.022B | 0.004 ± 0.005A |
| N3-031 | 29.64 ± 51.33D | 0.029 ± 0.047A | 0.009 ± 0.011A |
| J1-101 | 0.005 ± 0.002A | 0.013 ± 0.011A | |
| 10403S | 0.002 ± 0.001A | 0.008 ± 0.007A | |
| A1-254 | 39.10 ± 32.04CD | 0.032 ± 0.018A | 0.016 ± 0.007A |
| J1-158 | 51.94 ± 19.76BC | 0.042 ± 0.029A | 0.009 ± 0.003A |
| J1-168 | 40.90 ± 31.77CD | 0.013 ± 0.009A | 0.006 ± 0.009A |
Latency to death. For blank cells, no shoulder was observed (inactivation was immediate). No shoulder was observed for the IC.
Different superior capital letters within a column show significantly different data (P < 0.05).
TABLE 3.
Mean (n = 3) shoulder durations and inactivation rates (± standard deviations) of individual Listeria monocytogenes strains in a simulated gastrointestinal system (GC at pH 2.0 within 88 min; IC at pH 6.5; 37°C) after inoculation (1 ml) into 100 ml of TSBYE-G and incubation for 16 h at 30°Cb
| Strain | GC
|
IC
|
||
|---|---|---|---|---|
| Shoulder duration (min)a | Maximum inactivation rate (log CFU/ml/min) | Shoulder duration (min)a | Maximum inactivation rate (log CFU/ml/min) | |
| 558 | 63.73 ± 16.28A | 0.043 ± 0.028AD | 0.002 ± 0.002AB | |
| R2-500 | 0.009 ± 0.001A | 0.000 ± 0.003Ac | ||
| R2-501 | 71.17 ± 1.00A | 0.023 ± 0.001A | 0.003 ± 0.005AB | |
| Scott A | 74.71 ± 1.59A | 0.197 ± 0.010B | 0.008 ± 0.005ABD | |
| N1-225 | 70.45 ± 3.30A | 0.039 ± 0.009AD | 0.003 ± 0.001AB | |
| N1-227 | 70.35 ± 2.94A | 0.092 ± 0.001BD | 0.004 ± 0.001AB | |
| C1-056 | 70.74 ± 4.99A | 0.221 ± 0.013C | 0.014 ± 0.010BC | |
| N3-031 | 86.48 ± 2.32A | 0.047 ± 0.007AD | 1.32 ± 2.29 | 0.016 ± 0.004BC |
| J1-101 | 68.15 ± 1.55A | 0.062 ± 0.010AD | 0.018 ± 0.007CD | |
| 10403S | 67.35 ± 2.77A | 0.048 ± 0.003AD | 0.010 ± 0.005BC | |
| A1-254 | 83.68 ± 22.73A | 0.188 ± 0.113B | 0.021 ± 0.013C | |
| J1-158 | 76.66 ± 1.30A | 0.103 ± 0.019BD | 0.008 ± 0.006ABD | |
| J1-168 | 65.32 ± 7.12A | 0.049 ± 0.008AD | 0.009 ± 0.006BC | |
Latency to death. For blank cells, no shoulder was observed (inactivation was immediate).
Different superior capital letters within a column show significantly different data (P < 0.05).
The slope of the survival curve was zero, indicating that no inactivation occurred.
Intestinal survival.
Populations transferred to the IC within the first 30 min of gastric emptying were correlated with the initial level of each strain and ranged from 5.4 to 8.1 log CFU/ml and 7.0 to 9.1 log CFU/ml, for 4- and 16-h cultures, respectively (data not shown).
Reductions in populations during the intestinal challenge were not as drastic as those observed in the GC, as demonstrated by the intestinal IRs (Tables 2 and 3). Differences in intestinal IRs of strains A1-254 and 10403S (16-h cultures only) suggested that the contribution of σB to intestinal survival was small (P ≥ 0.05) (Table 3). Combined isolates of serotype 1/2 and lineage 2 possessed significantly (P < 0.05) higher IRs than did combined serotype 4b and lineage 1 or 3 isolates, respectively. However, these serotype/lineage-related effects were present only in 16-h cultures. Moreover, differences among serotypes or lineages referred only to the combined observations, as the behaviors of individual strains within serotypes or lineages were not identical during intestinal exposure.
Overall, gradual acidification of the stomach contents together with gastric emptying resulted in cells being subjected to different levels of acidity. Thus, high cell numbers, even of acid-susceptible strains, survived gastric exposure while the pH was >3.0, reaching the intestine in a viable state. In their study, Dykes and Moorhead (9) reported increased acid resistance in all clinical L. monocytogenes strains, an observation that led them to remark on the importance of acid tolerance in the infection process. In our study, clinical isolates C1-056 and Scott A were the most acid susceptible among the strains examined; however, the increased survival of these isolates during the initial stages of gastric exposure may help to explain their implication in human disease, particularly if high contamination levels were involved. The fact that all strains survived the gastrointestinal passage suggested that although acid resistance is a crucial element in terms of intrahost survival, assumptions regarding the gastrointestinal survival of the pathogen might be more accurate when other gastrointestinal tract-related aspects (e.g., gastric emptying) have also been considered.
Acknowledgments
We thank Martin Wiedmann and Kathryn J. Boor (Department of Food Science, Cornell University, Ithaca, NY) for providing us with L. monocytogenes strains.
This study was supported by the National Integrated Food Safety Initiative of the United States Department of Agriculture Cooperative State Research, Education and Extension Service (agreements 2004-51110-02160 and 2005-51110-03278) and by the Colorado State University Agricultural Experiment Station.
Footnotes
Published ahead of print on 27 June 2008.
REFERENCES
- 1.Baranyi, J., and T. A. Roberts. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277-294. [DOI] [PubMed] [Google Scholar]
- 2.Begley, M., C. G. M. Gahan, and C. Hill. 2002. Bile stress response in Listeria monocytogenes L028: adaptation, cross-protection, and identification of genetic loci involved in bile resistance. Appl. Environ. Microbiol. 68:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernbom, N., T. R. Licht, P. Saadbye, F. K. Vogensen, and B. Nørrung. 2006. Lactobacillus plantarum inhibits growth of Listeria monocytogenes in an in vitro continuous flow gut model, but promotes invasion of L. monocytogenes in the gut of gnotobiotic rats. Int. J. Food Microbiol. 108:10-14. [DOI] [PubMed] [Google Scholar]
- 4.Beumer, R. R., J. de Vries, and F. M. Rombouts. 1992. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 15:153-163. [DOI] [PubMed] [Google Scholar]
- 5.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 6.Davis, M. J., P. J. Coote, and C. P. O' Byrne. 1996. Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology 142:2975-2982. [DOI] [PubMed] [Google Scholar]
- 7.Doran, S., K. L. Jones, J. M. Andrews, and M. Horowitz. 1998. Effects of meal volume and posture on gastric emptying of solids and appetite. Am. J. Physiol. 275:1712-1718. [DOI] [PubMed] [Google Scholar]
- 8.Dressman, J. B., R. R. Berardi, L. C. Dermentzoglou, T. L. Russell, S. P. Schmaltz, J. L. Barnett, and K. M. Jarvenpaa. 1990. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 7:756-761. [DOI] [PubMed] [Google Scholar]
- 9.Dykes, G. A., and S. M. Moorhead. 2000. Survival of osmotic and acid stress by Listeria monocytogenes strains of clinical or meat origin. Int. J. Food Microbiol. 56:161-166. [DOI] [PubMed] [Google Scholar]
- 10.Faleiro, M. L., P. W. Andrew, and D. Power. 2003. Stress response of Listeria monocytogenes isolated from cheese and other foods. Int. J. Food Microbiol. 84:207-216. [DOI] [PubMed] [Google Scholar]
- 11.Formato, G., I. Geornaras, I. M. Barmpalia, P. N. Skandamis, K. E. Belk, J. A. Scanga, P. A. Kendall, G. C. Smith, and J. N. Sofos. 2007. Effect of acid adaptation on growth during storage at 10°C and resistance to simulated gastric fluid of Listeria monocytogenes inoculated onto bologna formulated with or without antimicrobials. J. Food Prot. 70:65-69. [DOI] [PubMed] [Google Scholar]
- 12.Fugett, E., E. Fortes, C. Nnoka, and M. Wiedmann. 2006. International Life Sciences Institute North America Listeria monocytogenes strain collection: development of standard Listeria monocytogenes strain sets for research and validation studies. J. Food Prot. 69:2929-2938. [DOI] [PubMed] [Google Scholar]
- 13.Gahan, C. G. M., and C. Hill. 2005. Gastrointestinal phase of Listeria monocytogenes infection. J. Appl. Microbiol. 98:1345-1353. [DOI] [PubMed] [Google Scholar]
- 14.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 15.King, T., T. Ferenci, and E. A. Szabo. 2003. The effect of growth atmosphere on the ability of Listeria monocytogenes to survive exposure to acid, proteolytic enzymes and bile salts. Int. J. Food Microbiol. 84:133-143. [DOI] [PubMed] [Google Scholar]
- 16.Koo, J., D. L. Marshall, and A. DePaola. 2001. Antacid increases survival of Vibrio vulnificus and Vibrio vulnificus phage in a gastrointestinal model. Appl. Environ. Microbiol. 67:2895-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lianou, A., J. D. Stopforth, Y. Yoon, M. Wiedmann, and J. N. Sofos. 2006. Growth and stress resistance variation in culture broth among Listeria monocytogenes strains of various serotypes and origins. J. Food Prot. 69:2640-2647. [DOI] [PubMed] [Google Scholar]
- 18.Marteau, P., M. Minekus, R. Havenaar, and J. H. J. Huis In't Veld. 1997. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J. Dairy Sci. 80:1031-1037. [DOI] [PubMed] [Google Scholar]
- 19.Minekus, M., P. Marteau, R. Havenaar, and J. H. J. Huis In't Veld. 1995. A multicompartmental dynamic computer-controlled model simulating the stomach and the small intestine. Altern. Lab. Anim. 23:254-258. [Google Scholar]
- 20.Molly, K., M. V. Woestyne, I. De Smet, and W. Verstraete. 1994. Validation of the simulator of the human intestinal microbial ecosystem (SHIME) reactor using microorganisms-associated activities. Microb. Ecol. Health Dis. 7:191-200. [Google Scholar]
- 21.Naim, F., S. Messier, L. Saucier, and G. Piette. 2004. Postprocessing in vitro digestion challenge to evaluate survival of Escherichia coli O157:H7 in fermented dry sausages. Appl. Environ. Microbiol. 70:6637-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olier, M., S. Rousseaux, P. Piveteau, J.-P. Lemaitre, A. Rousset, and J. Guzzo. 2004. Screening of glutamate decarboxylase activity and bile salt resistance of human asymptomatic carriage, clinical, food, and environmental isolates of Listeria monocytogenes. Int. J. Food Microbiol. 93:87-99. [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute Inc. 2008. SAS/STAT 9.2 user's guide. http://support.sas.com/documentation/cdl/en/statug/59654/HTML/default/chap0_toc.htm.
- 24.Smith, J. L. 2003. The role of gastric acid in preventing foodborne disease and how bacteria overcome acid conditions. J. Food Prot. 66:1296-1303. [DOI] [PubMed] [Google Scholar]
- 25.Stopforth, J. D., Y. Yoon, I. M. Barmpalia, J. Samelis, P. N. Skandamis, and J. N. Sofos. 2005. Reduction of Listeria monocytogenes populations during exposure to a simulated gastric fluid following storage of inoculated frankfurters formulated and treated with preservatives. Int. J. Food Microbiol. 99:309-319. [DOI] [PubMed] [Google Scholar]
- 26.Wiedmann, M., J. L. Bruce, C. Keating, A. E. Johnson, P. L. McDonough, and C. A. Batt. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiedmann, M., T. J. Arvik, R. J. Hurley, and K. J. Boor. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]