Abstract
Transition from reversible to irreversible bacterial adhesion is a highly relevant but poorly understood step in initial biofilm formation. We hypothesize that in oral biofilm formation, irreversible adhesion is caused by bond strengthening due to specific bacterial interactions with salivary conditioning films. Here, we compared the initial adhesion of six oral bacterial strains to salivary conditioning films with their adhesion to a bovine serum albumin (BSA) coating and related their adhesion to the strengthening of the binding forces measured with bacteria-coated atomic force microscopy cantilevers. All strains adhered in higher numbers to salivary conditioning films than to BSA coatings, and specific bacterial interactions with salivary conditioning films were accompanied by stronger initial adhesion forces. Bond strengthening occurred on a time scale of several tens of seconds and was slower for actinomyces than for streptococci. Nonspecific interactions between bacteria and BSA coatings strengthened twofold faster than their specific interactions with salivary conditioning films, likely because specific interactions require a closer approach of interacting surfaces with the removal of interfacial water and a more extensive rearrangement of surface structures. After bond strengthening, bacterial adhesion forces with a salivary conditioning film remained stronger than those with BSA coatings.
Oral biofilm (“dental plaque”) formation proceeds according to a well-known sequence of events (7, 18, 19). The first step in this sequence is the adsorption of conditioning film components or, specified to the oral cavity, the adsorption of salivary components that form the acquired enamel pellicle, followed by bacterial transport to the substratum surface. Subsequently, bacteria initially (co)adhere (19) reversibly, after which a transition to an irreversible state sets in, and eventually the adhering bacteria start to grow and form a mature biofilm. The transition from reversible to irreversible adhesion is intriguing as it is largely unknown what actually happens during this transition. The transition is partly due to active bacterial processes such as anchoring through the excretion of extracellular polymeric substances (9). However, also inert polystyrene particles adhering to surfaces have demonstrated a transition from a reversible to a nearly irreversible state which has been attributed to (i) the progressive removal of interfacial water from in between the interacting surfaces, (ii) the reorientation of an adhering particle in order to face a substratum surface with its most favorable site, and (iii) conformational changes of protruding polymer chains (2, 3, 14). Using image sequence analysis (14, 15), it was found that the desorption probabilities of bacteria and polystyrene particles adhering to inert substrata decrease after their arrival at a surface within 30 to 60 s by a factor of 200 for bacteria and within 100 to 1,000 s by a factor of 100 for polystyrene particles.
The change from a reversible to irreversible state must be accompanied by an increase in the interaction forces and perhaps a decrease in repulsive forces between adhering bacteria and a substratum surface. New developments in atomic force microscopy (AFM) made it possible to hold the AFM tip at a fixed distance from a surface for periods of up to several minutes (13). Using this methodology, it has been demonstrated that Streptococcus thermophilus increases its bond strength to an inert substratum, e.g., the surface of the AFM tip, by a factor of 2.5 from 1.5 nN to 4 nN over a time span of 200 s, similar to the time required to effectively decrease its desorption probability (20).
Bond strengthening between bacteria and conditioning film-covered substrata, as occurring during initial bacterial adhesion to salivary conditioning films, has never been studied using AFM. However, since bacterial adhesion to protein-coated surfaces involves not only macroscopic physicochemical interactions but also highly specific interactions operating on a microscopic level, it can be hypothesized that bond strengthening on protein-coated substrata occurs due to specific interactions over a different time scale than to inert substrata.
Therefore, the aim of this paper was to compare the initial adhesion of six oral bacterial strains to salivary conditioning films with their adhesion to bovine serum albumin (BSA)-coated substrata in a parallel plate flow chamber and to relate their adhesion to the strengthening of the interaction forces, as measured by using AFM and bacteria-coated cantilevers. The six strains included in this study reflect the changing composition of young dental plaque over time (6, 11, 17), and the actinomyces and streptococci included, e.g., Streptococcus mitis, Streptococcus oralis, and Streptococcus sanguinis, belong to the so-called early colonizers of dental hard surfaces, whereas Streptococcus mutans and Streptococcus sobrinus are later colonizers.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and harvesting.
Six different oral bacterial strains were included in this study, as follows: four early colonizers, i.e., Actinomyces naeslundii T14V-J1, S. mitis ATCC 9811, S. oralis J22, and S. sanguinis ATCC 10556; and two later colonizers, S. mutans NS and S. sobrinus HG1025. A. naeslundii T14V-J1 was cultured in Schaedler's broth supplemented with 0.01 g/liter hemin in an anaerobic cabinet (Ruskinn Technology, West Yorkshire, United Kingdom) at 37°C, and streptococci were cultured in Todd-Hewitt broth (Oxoid, Basingstoke, United Kingdom) at 37°C in ambient air. Strains were precultured in an overnight batch culture and inoculated in a second culture which was grown for 16 h, harvested by centrifugation for 5 min at 6,500 × g, and washed twice with adhesion buffer (2 mM potassium phosphate, 50 mM potassium chloride, and 1 mM calcium chloride, pH 6.8). To break bacterial aggregates, bacteria were sonicated intermittently while being cooled in an ice-water bath for 35 s at 30 W. This procedure was found not to cause cell lysis. For experiments, bacteria were suspended in adhesion buffer to a concentration of 3 × 108 bacteria per ml.
Saliva collection and preparation.
Human whole saliva from 20 healthy volunteers of both genders was collected into ice-cooled beakers after stimulation by chewing Parafilm. The saliva was pooled, centrifuged, dialyzed against demineralized water, and lyophilized for storage. For a salivary conditioning film formation, lyophilized saliva was dissolved at a concentration of 1.5 g/liter in adhesion buffer. All volunteers gave their informed consent to donate saliva, in accordance with the rules set out by the Ethics Committee at the University Medical Center Groningen.
Deposition protocol.
The parallel plate flow chamber (dimensions: length by width by height = 7.6 by 3.8 by 0.06 cm) and the image analysis system have been described in detail previously (4). For enumeration of the adhering bacteria, the flow chamber was put on the stage of a phase contrast microscope (Olympus BH-2) equipped with a 40× ultralong working-distance objective (Olympus ULWD-CD Plan 40 PL). The numbers of adhering bacteria were observed over a surface area of 0.037 mm2 with a charge-coupled-device camera (Basler A101F; Basler AG, Germany). The images were recorded and analyzed using proprietary software based on the Matlab Image Processing Toolbox (The MathWorks, MA). Subsequently, bacteria were discriminated from the background by a single gray-value threshold yielding a binary black-and-white image, and the number of bacteria were counted and expressed per cm2.
Prior to bacterial adhesion, glass plates were coated for 16 h with reconstituted human whole saliva or 0.1% BSA (fatty acid free, ≥96%) (Sigma) in adhesion buffer. A bacterial suspension was circulated for 2 h at a flow rate of 1.4 ml/min (shear rate, 10 s−1) at room temperature. The initial increase in the number of adhering bacteria with time was expressed in a so-called initial deposition rate, j0 (cm−2 s−1), i.e., the number of bacteria adhering per unit area and time. Furthermore, the number of bacteria adhering per unit area after 2 h, n2 h (cm−2), was determined.
AFM.
Bacteria were immobilized on a tipless V-shaped silicon nitride cantilever from Veeco (Woodbury, NY). The cantilevers were first pretreated with poly-l-lysine (cell culture tested molecular weight, 70,000 to 150,000) (Sigma) for 1 min. After drying, the cantilevers were dipped into a bacterial suspension for 1 min in order to allow bacterial attachment to the cantilever and were used directly after preparation. AFM measurements were made at room temperature under adhesion buffer at pH 6.8 using an optical level microscope (Nanoscope IV; Veeco, Woodbury, NY). For each cantilever, the spring constant was experimentally determined using the thermal tune method (10). Individual force curves were collected at randomly selected locations. Retraction of the tip from the salivary conditioning film or BSA coating was done after different surface delay times, ranging from 0 to 120 s. The slope of the retraction force curves in the region where the probe and sample are in contact was used to convert the voltage into cantilever deflection. The conversion of deflection data to force data was carried out as has been previously described by others (8). The maximum adhesion peak upon retraction was recorded as a function of contact time between the bacterium-coated AFM cantilever and the surface. Subsequently, for each bacterial probe, the maximum adhesion forces were plotted as a function of the surface delay time t and fitted to the following equation:
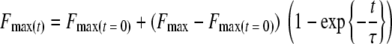 |
with Fmax(t = 0) as the maximum adhesion force at 0 s contact time, Fmax as the maximum adhesion force after bond strengthening, and τ as the characteristic time needed for the adhesion force to strengthen.
Each strain was cultured in triplicate, and for each culture, 5 force-distance curves were measured for each surface delay time, resulting in a total of 15 force-distance curves for each surface delay. In order to check whether the bacterium-coated cantilever was not contaminated or had not suffered bacterial detachment, adhesion forces with a surface delay time of 0 s were measured in between each two measurements of a force-distance curve with a given surface delay time. As long as the measured adhesion forces with a 0-s surface delay were within range, this was taken as an indication that the cantilever could be used again. When aberrant values were found, a new bacteria-coated cantilever was prepared. In addition, scanning electron micrographs were occasionally taken to establish whether tips were completely covered by bacteria, while also tips were checked after dead/live staining (BacLight; Molecular Probes Europe BV) in a confocal scanning laser microscope which avoids dehydration artifacts.
Statistics.
The difference in adhesion to a salivary conditioning film and BSA for the various strains were compared using Student's t test. A 95% confidence interval (P < 0.05) was adapted for statistical significance.
RESULTS
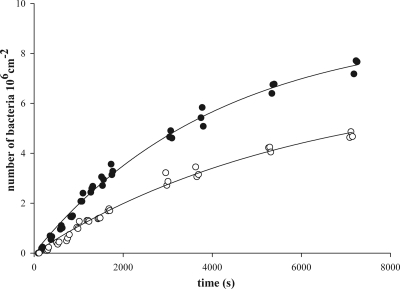
Adhesion kinetics of the strains involved were initially linear during 1,000 to 1,500 s and then slowly leveled off due to blocking (Fig. 1). Initial bacterial deposition rates, derived from the initial, linear trajectory were significantly (P < 0.05) higher for salivary conditioning films than for BSA coatings (Table 1), except for S. oralis J22, for which similar results were observed for both surfaces. The preference of the strains for adhesion to salivary conditioning films was maintained also after 2 h of adhesion at a statistically significant level (P < 0.05), except again for S. oralis J22.
FIG. 1.
Example of the numbers of A. naeslundii T14V-J1 cells adhering to a salivary conditioning film (closed symbols) and to a BSA coating (open symbols) as a function of time in a parallel plate flow chamber.
TABLE 1.
Initial deposition rates, j0, and number of bacteria adhering after 2 h, n2 h, to salivary conditioning films or BSA coatings for six oral bacterial strainsa
| Bacterial strain | Protein coating | j0 (cm−2 s−1) | n2 h (106 cm−2) |
|---|---|---|---|
| A. naeslundii T14V-J1 | Saliva | 1,745 | 7.5 |
| BSA | 380 | 4.2 | |
| S. mitis ATCC 9811 | Saliva | 867 | 5.6 |
| BSA | 18 | 0.1 | |
| S. mutans NS | Saliva | 881 | 4.1 |
| BSA | 378 | 1.5 | |
| S. oralis J22 | Saliva | 1,045 | 7.0 |
| BSA | 1,001 | 7.1 | |
| S. sanguinis ATCC 10556 | Saliva | 992 | 5.7 |
| BSA | 110 | 2.6 | |
| S. sobrinus HG1025 | Saliva | 175 | 0.7 |
| BSA | 41 | 0.2 |
All experiments were done in triplicate in a parallel plate flow chamber with independent bacterial cultures, yielding a mean SD of 30% for both j0, and n2 h.
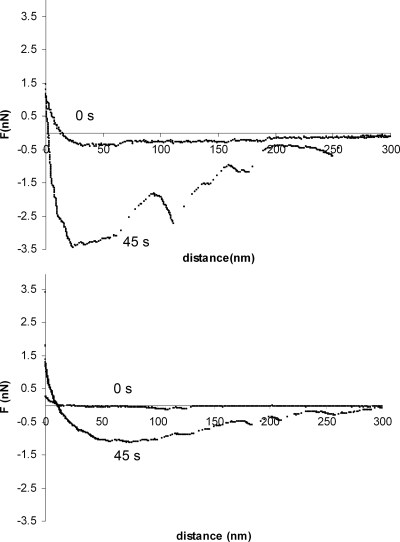
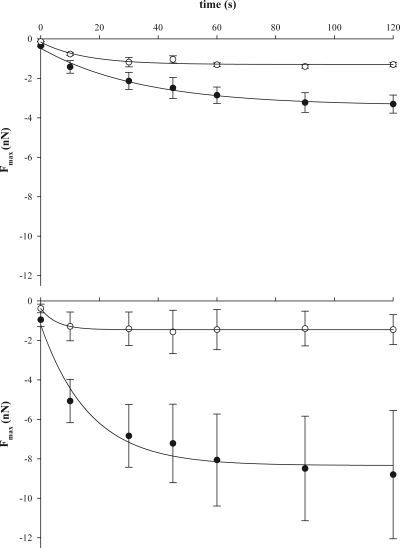
The retraction force-distance curves (Fig. 2) showed major differences when bacteria-coated AFM cantilevers were removed from the surfaces, while furthermore, a delay time of the bacteria-coated AFM cantilever at the surface clearly stimulated bond strengthening (Fig. 3). Bond strengthening as a function of the delay time was analyzed for three parameters using an exponential rise to maximum function as follows: an initial adhesion force, Fmax(t = 0), a final adhesion force, Fmax, and a characteristic time constant for the strengthening process, τ. Initial adhesion forces Fmax(t = 0) were significantly (P < 0.05) stronger on a salivary conditioning film than on a BSA coating for four out of six strains, with the exception of S. mutans NS and S. sanguinis ATCC 10556, for which equal initial adhesion forces were observed on both substrata (Table 2). Adhesion forces became stronger (P < 0.05) for all strains and both surfaces upon increasing the contact time between the bacteria-coated cantilevers and the surfaces, but in all cases, adhesion forces remained weaker on BSA coatings than on salivary conditioning films. Bond strengthening occurred over a time period of several tens of seconds and was faster on BSA coatings than on salivary conditioning films for all strains. Bond strengthening proceeded over the longest time scale for A. naeslundii T14V-J1 (significant at P < 0.05).
FIG. 2.
Example of the force-distance curves in the retracting mode between S. sanguinis ATCC 10556 attached to the AFM cantilever and a salivary conditioning film (top) and a BSA coating (bottom) after a surface delay time of 0 s and 45 s.
FIG. 3.
The maximum adhesion force upon retraction of an AFM cantilever with attached S. sanguinis ATCC 10556 (top) and S. mitis ATCC 9811 (bottom) from a salivary conditioning film (closed symbols) and a BSA coating (open symbols) as a function of the surface delay time. Error bars denote the standard deviation (SD) over 15 force-distance curves, taken from three independent bacterial cultures.
TABLE 2.
Initial adhesion forces, Fmax(t = 0), and after bond strengthening, Fmax, as measured during retraction of bacterium-coated AFM cantilever away from salivary conditioning films or BSA coatings together with the characteristic time constants, τ, of the strengthening processa
| Bacterial strain | Protein coating | Fmax(t = 0) (nN) | Fmax (nN) | τ |
|---|---|---|---|---|
| A. naeslundii T14V-J1 | Saliva | −1.3 | −6.1 | 83 |
| BSA | −0.2 | −1.3 | 66 | |
| S. mitis ATCC 9811 | Saliva | −1.2 | −8.4 | 17 |
| BSA | −0.3 | −1.3 | 5 | |
| S. mutans NS | Saliva | −0.5 | −4.4 | 51 |
| BSA | −0.4 | −2.5 | 38 | |
| S. oralis J22 | Saliva | −0.5 | −2.9 | 26 |
| BSA | −0.2 | −1.3 | 16 | |
| S. sanguinis ATCC 10556 | Saliva | −0.5 | −3.3 | 30 |
| BSA | −0.3 | −1.9 | 18 | |
| S. sobrinus HG1025 | Saliva | −0.4 | −3.5 | 28 |
| BSA | −0.1 | −1.3 | 11 |
All data were based on 15 force curves taken from three independent bacterial cultures of each strain, yielding a mean SD of 30%, 20%, and 50% for Fmax(t = 0) and after bond strengthening Fmax and τ, respectively.
DISCUSSION
In this study, we compared the strengthening of bonds between oral bacterial strains and salivary conditioning films with that of bonds between these bacteria and BSA coatings. Bacterial adhesion is generally considered to be an interplay between overall, physicochemical properties of the interacting surfaces, as mediated by Lifshitz-van der Waals, electrostatic and acid-base interactions (nonspecific contribution), and interactions between highly localized, microscopic domains (specific contribution). Nevertheless, both specific and nonspecific contributions originate from the same basic physicochemical forces (21). Our data show that the specific contributions operative between salivary conditioning films and bacterial cell surfaces are stronger than the nonspecific contributions operative between BSA coatings and the bacterial cell surfaces, while furthermore, they strengthen over more-extended periods of time.
On BSA coatings, only nonspecific contributions toward bacterial adhesion are operative, whereas on salivary conditioning films, nonspecific and specific contributions work together (5). Although it is outside the scope of this paper to summarize and discuss all specific ligand-receptor combinations described in the literature for oral bacterial adhesion to salivary conditioning films, it is important to notice that for all strains in this study, namely, A. naeslundii, S. mitis, S. oralis, (11, 16), S. mutans, S. sanguinis (12, 16), and S. sobrinus (16), specific receptors for salivary components have been described. Specific receptors are of utmost importance for the early colonizers as they belong to the category of the so-called initial salivary pellicle colonizers (6) that must be able to adhere strongly to salivary conditioning films in order to withstand oral shear-off forces.
The study was carried out with bacterial AFM probes, which required careful controls to ascertain that bacteria did not detach from the cantilever or that proteinaceous remnants did not contaminate the bacterial cell surfaces after a measurement. In our opinion, the force controls carried out are more telling than any type of microscopy that could reveal bacterial detachment from a cantilever but not the potential presence of conditioning film proteins on a cell surface after retraction. Also, in our experience, the preparation of bacterial probes for AFM with a tipless cantilever is preferable to using a regular AFM with a tip, as a regular AFM tip concentrates the pressure, with the associated danger that it is the tip that contacts a substratum rather than the bacteria themselves.
The forces mediating the nonspecific contribution to bacterial interactions with substratum surfaces originate from all atoms in the interacting entities and work instantaneously and always in concert with specific contributions. They can best be compared with the gravitational forces that are ubiquitously present on the earth but weaken with distance above the earth. Exactly the same thing happens with the nonspecific interactions, and they clearly strengthen over time, which must be associated with the entropically favorable removal of interfacial water from in between the interacting surfaces, allowing their closer approach. A closer approach facilitates stereochemical interactions, of which hydrogen bonding is the most common example. However, also complex key-lock interactions, as in specific bacterial adhesion, require a close approach. Possibly, bacterial cell surface structures may reorient themselves to face the substratum surface with their most favorable sites. This would explain why the bond strengthening of spherical streptococci occurs faster than that of the more irregularly shaped actinomyces, which may develop multiple contacts.
Effective specific contributions work only at close approach and may require extensive unfolding of surface structures and conformational changes of cell surface proteins in order for effective interactions to occur. Evidently, this slows down the strengthening of bonds between bacteria and a salivary conditioning film compared to that of bonds between bacteria and BSA coatings, where specific interactions are absent. It is interesting to note that after bond strengthening on salivary conditioning films, as opposed to that on BSA coatings, multiple adhesion peaks develop (Fig. 2), similar to that observed by Abu-Lail et al. in the interaction between Escherichia coli and silicon nitride AFM tips (1). After Poisson analysis of the adhesion peaks and comparison with thermodynamic analyses, these authors (1) concluded that such multiple peaks were due to acid-base interactions (“hydrogen bonding”) with an individual force value of −0.125 nN. By combination, the current results show that these acid-base interactions develop over time and in general do not operate instantaneously after contact, which is logical due to the required stereochemistry between the interacting groups and the need for close approach. Moreover, it shows that specific interactions are at least partly acid-base in nature. The formation of an acid-base bond as such is unlikely to require tens of seconds, but likely the processes of water removal and structural rearrangements are responsible for the relatively long time constants observed.
Adhesion forces are especially important under flow for the adhering bacteria to maintain their positions at a substratum surface, and indeed, by comparing Tables 1 and 2, it can be seen that for each individual bacterial strain, the stronger adhesion forces on salivary conditioning films compared with BSA coatings coincide with higher numbers of adhering bacteria in the parallel plate flow chamber. There appears to be no general relationship between adhesion forces and numbers valid over the entire collection of strains involved in this study, likely because the approach of a bacterium to a substratum surface is generally hampered by a repulsive electrosteric force, as demonstrated by AFM studies. Analysis of these electrosteric repulsive forces upon approach can be done in AFM in combination with the measurement of adhesion forces upon retraction (20), but this analysis has not been attempted in the present study due to its focus on the effects of surface delay times on adhesion forces upon retraction. Electrosteric forces upon approach and the distance over which they operate, however, are highly strain dependent (22), which explains why there is no general relationship between the AFM data in the retraction mode and adhesion in the parallel plate flow chamber.
In summary, this paper shows for the first time that nonspecific interactions mediating bacterial adhesion strengthen on a faster time scale than specific interactions, although at all points in time, nonspecific interactions remain weaker than specific interactions. Stronger adhesion forces were associated with higher numbers of adhering bacteria under flow.
Acknowledgments
This study was supported by the University Medical Center Groningen-University of Groningen, Groningen, The Netherlands.
We thank ZonMw for grant 91105005, enabling the purchase of the Nanoscope IV (Digital Instruments).
Footnotes
Published ahead of print on 18 July 2008.
REFERENCES
- 1.Abu-Lail, N. I., and T. A. Camesano. 2006. Specific and nonspecific interaction forces between Escherichia coli and silicon nitride, determined by Poisson statistical analysis. Langmuir 22:7296-7301. [DOI] [PubMed] [Google Scholar]
- 2.Adamczyk, Z., and T. G. M. van de Ven. 1984. Kinetics of particle accumulation at collector surfaces. I. Approximate analytical solutions. J. Colloid Interface Sci. 97:68-90. [Google Scholar]
- 3.Adamczyk, Z., T. Dabros, J. Czarnecki, and T. G. M. van de Ven. 1984. Kinetics of particle accumulation at collector surfaces. II. Exact numerical solutions. J. Colloid Interface Sci. 97:91-104. [Google Scholar]
- 4.Busscher, H. J., and H. C. van der Mei. 2006. Microbial adhesion in flow displacement systems. Clin. Microbiol. Rev. 19:127-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busscher, H. J., M. M. Cowan, and H. C. van der Mei. 1992. On the relative importance of specific and non-specific approaches to (oral) microbial adhesion. FEMS Microbiol. Rev. 88:199-210. [DOI] [PubMed] [Google Scholar]
- 6.Diaz, P. I., N. I. Chalmers, A. H. Rickard, C. Kong, C. L. Milburn, R. J. Palmer, Jr., and P. E. Kolenbrander. 2006. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 72:2837-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dige, I., H. Nilsson, M. Kilian, and B. Nyvad. 2007. In situ identification of streptococci and other bacteria in initial dental biofilm by confocal laser scanning microscopy and fluorescence in situ hybridization. Eur. J. Oral Sci. 115:459-467. [DOI] [PubMed] [Google Scholar]
- 8.Dufrêne, Y. F. 2003. Recent progress in the application of atomic force microscopy imaging and force spectroscopy to microbiology. Curr. Opin. Microbiol. 6:317-323. [DOI] [PubMed] [Google Scholar]
- 9.Flemming, H.-C., T. R. Neu, and D. J. Wozniak. 2007. The EPS matrix: the “house of biofilm cells.” J. Bacteriol. 189:7945-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutter, J. L., and J. Bechhoefer. 1993. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 64:1868-1873. [Google Scholar]
- 11.Li, J., E. J. Helmerhorst, C. W. Leone, R. F. Troxler, T. Yaskell, A. D. Haffajee, S. S. Socransky, and F. G. Oppenheim. 2004. Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 97:1311-1318. [DOI] [PubMed] [Google Scholar]
- 12.Loimaranta, V., N. S. Jakubovics, J. Hytönen, J. Finne, H. F. Jenkinson, and N. Strömberg. 2005. Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infect. Immun. 73:2245-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamee, C. E., N. Pyo, S. Tanaka, I. U. Vakarelski, Y. Kanda, and K. Higashitani. 2006. Parameters affecting the adhesion strength between a living cell and a colloid probe when measured by the atomic force microscope. Colloids Surf. B 48:176-182. [DOI] [PubMed] [Google Scholar]
- 14.Meinders, J. M., and H. J. Busscher. 1993. Influence of ionic strength and shear rate on the desorption of polystyrene particles from a glass collector as studied in a parallel plate flow chamber. Colloids Surf. A 80:279-285. [Google Scholar]
- 15.Meinders, J. M., H. C. van der Mei, and H. J. Busscher. 1994. Physicochemical aspects of deposition of Streptococcus thermophilus B to hydrophobic and hydrophilic substrata in a parallel plate flow chamber. J. Colloid Interface Sci. 164:355-363. [Google Scholar]
- 16.Murray, P. A., A. Prakobphol, T. Lee, C. I. Hoover, and S. J. Fisher. 1992. Adherence of oral streptococci to salivary proteins. Infect. Immun. 60:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyvad, B., and M. Kilian. 1987. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 95:369-380. [DOI] [PubMed] [Google Scholar]
- 18.Palmer, R. J., Jr., P. I. Diaz, and P. E. Kolenbrander. 2006. Rapid succession within the Veillonella population of a developing human oral biofilm in situ. J. Bacteriol. 188:4117-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, R. J., Jr., S. M. Gordon, J. O. Cisar, and P. E. Kolenbrander. 2003. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J. Bacteriol. 185:3400-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vadillo-Rodríguez, V., H. J. Busscher, W. Norde, J. de Vries, and H. C. van der Mei. 2004. Atomic force microscopic corroboration of bond aging for adhesion of Streptococcus thermophilus to solid substrata. J. Colloid Interface Sci. 278:251-254. [DOI] [PubMed] [Google Scholar]
- 21.Van Oss, C. J. 1995. Hydrophobicity of biosurfaces—origin, quantitative-determination and interaction energies. Colloids Surf. B 5:91-110. [Google Scholar]
- 22.Yongsunthon, R., V. G. J. Fowler, B. H. Lower, F. P. Vellano, E. Alexander, L. B. Reller, G. R. Corey, and S. K. Lower. 2007. Correlation between fundamental binding forces and clinical prognosis of Staphylococcus aureus infections of medical implants. Langmuir 23:2289-2292. [DOI] [PubMed] [Google Scholar]