Abstract
Two freshwater strains of the gammaproteobacterium Beggiatoa alba, B18LD and OH75-2a, are able to use methanol as a sole carbon and energy source under microoxic conditions. Genes encoding a methanol dehydrogenase large-subunit homolog and four enzymes of the tetrahydromethanopterin-dependent C1 oxidation pathway were identified in B18LD. No evidence of methanotrophy was detected.
Methylotrophs are an ecologically significant group of microorganisms that can use reduced one-carbon (C1) compounds, such as methane, methanol, methylated amines, and methylated sulfur compounds, as their sole source of carbon and as an oxidizable energy source. These microorganisms are globally important as a major sink for greenhouse gases and as key members in microbial communities around cold seeps, mud volcanoes, and other environments rich in C1 compounds (3, 8, 24, 29).
Members of the filamentous, colorless, sulfide-oxidizing genus Beggiatoa are often found in methane-rich environments, where they may form conspicuous mats (1, 25), but their abundance has been assumed to be linked to high rates of biogenic sulfide production due to anaerobic methane oxidation. Metabolism of individual Beggiatoa strains ranges from obligate chemoautotrophy to organoheterotrophy (20, 21). Certain marine Beggiatoa assemblages incorporate CO2 derived from methane oxidation (13, 18), but to date there has been no demonstration that they are capable of directly oxidizing and incorporating methane, methanol, or other reduced C1 compounds. Here we describe for the first time the ability of a Beggiatoa strain (B18LD) to grow as a methylotroph, which, coupled with prior documentation of its ability to grow on multicarbon compounds, renders it a facultative methylotroph.
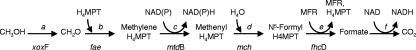
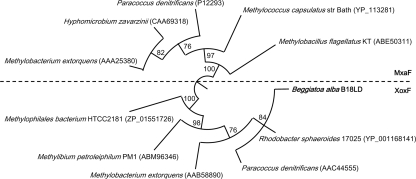
Our first evidence of methylotrophy in Beggiatoa alba strain B18LD (ATCC 33555) came from the accidental amplification and cloning of a gene encoding formaldehyde-activating enzyme (fae) from acetate-grown cells. The nucleic acid sequence amplified by PCR using degenerate nifH3 primers (32)—here serving as both forward and reverse primers—had 75% identity to the fae gene of Methylobacillus flagellatus KT. This gene encodes a tetrahydromethanopterin (H4MPT)-dependent enzyme that may function in either methylotrophy or formaldehyde detoxification. The genetic potential for methylotrophy in B18LD was explored by seeking genes within the linear C1 oxidation pathway (Fig. 1), using previously published primers and PCR conditions (10, 11, 15, 23, 30). The partial sequences of NAD-linked methylene-H4MPT dehydrogenase (encoded by mtdB; 47% nucleic acid identity to top NCBI match), methenyl-H4MPT cyclohydrolase (encoded by mch; 60%), and the D subunit of the formyltransferase/hydrolase complex (encoded by fhcD; 71%) were identified. Primers for mxaF, encoding the large alpha subunit of pyrroloquinoline quinone-linked methanol dehydrogenase (MDH), amplified a 513-bp sequence from B18LD, which when translated had significant homology to both MxaF (71%) and to the MxaF homolog XoxF (83%). To resolve this ambiguity, the phylogenetic relationship was examined in an unrooted tree using the distance method with the unweighted-pair group method with arithmetic mean (UPGMA) and a 1,000 bootstrap analysis (Fig. 2). The B18LD sequence clustered with the XoxF sequences.
FIG. 1.
Proposed pathway for oxidation of methanol to CO2 in Beggiatoa alba. a, pyrroloquinoline quinone-linked methanol dehydrogenase homolog; b, H4MPT-linked formaldehyde activating enzyme; c, NAD(P)-linked methenyl-H4MPT dehydrogenase; d, methenyl-H4MPT cyclohydrolase; e, formyltransferase/hydrolase complex; f, formate dehydrogenase. Adapted from the work of Lidstrom (14).
FIG. 2.
Phylogenetic distance tree of MxaF and XoxF. Nodes supported by bootstrap values greater than 70% have been retained.
The genomes of three other species capable of growth on methanol, Rhodobacter sphaeroides, Methylibium petroleiphilum PM1, and Methylophilales bacterium HTCC2181, contain only xoxF, whereas the genomes of Paracoccus denitrificans and Methylobacterium extorquens contain both mxaF and xoxF (2, 4, 12, 28, 31). Until recently, XoxF was not believed to be involved in methylotrophy, and the function was unknown (12, 28). Wilson et al. (31) demonstrated MDH activity in R. sphaeroides XoxF, a protein that shares 83% identity with the translated xoxF sequence from B18LD. In contrast, the B18LD sequence shares only 50% identity with M. extorquens XoxF, a protein that does not posses MDH activity (2).
We do not know if the B18LD genome also contains mxaF. To date, the only Beggiatoa partial genome sequence available, obtained by repeated sequencing of single filaments, belongs to a marine morphotype (17). An NCBI BLAST search of those contigs did not reveal any homology to xoxF or to the H4MPT-linked fae, mtdB, mch, or fhcD nucleic acid or translated amino acid sequences identified in this study.
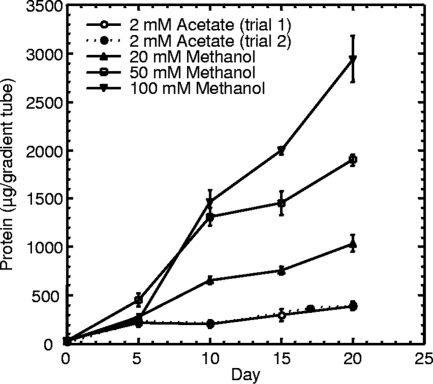
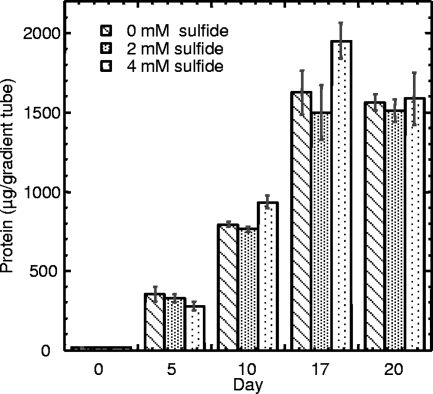
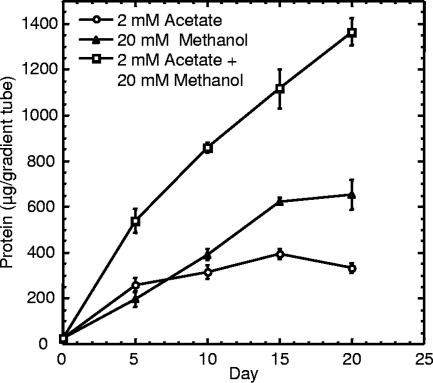
A previous attempt by Mezzino et al. (16) to culture strain B18LD aerobically on methanol in liquid medium was unsuccessful, which matches the inconsistent results we obtained for liquid cultivation (data not shown). Therefore, the ability of strain B18LD to grow as a methylotroph was examined using sulfide-gradient medium (19) prepared with SuperPure agar (U.S. BioTech Sources LLC, EI Sobrante, CA) and supplemented with methanol or acetate as the sole carbon source. Growth, measured as total protein in the horizontal Beggiatoa “plate” (22), was determined as previously described (6). The purity of the B18LD cultures was confirmed by phase-contrast microscopy (400×) throughout the experiment. The amount of biomass strain B18LD produced (Fig. 3) was positively correlated with the methanol concentration in the medium. The possibility of mixotrophy, with sulfide serving as an electron donor and methanol serving solely as the carbon source, was also examined (Fig. 4). With the pH of the bottom agar in the medium lacking sulfide carefully adjusted to match that of the medium containing sulfide, no significant difference in yields (day 17 or day 20) could be attributed to the presence (2 or 4 mM) or absence of sulfide. The yield on day 10 for 4 mM sulfide was significantly greater (P value of >0.02 and <0.05) than for the other two treatments; however, we observed that the dense, horizontal plates of Beggiatoa growth were more compact and easier to visualize, due to sulfur deposition, in the presence of 4 mM sulfide, and we speculate that this facilitated more-complete harvesting. Taken together, these data (Fig. 3 and 4) indicate that methanol can serve as both the carbon and energy source for B18LD and that biomass increases with the concentration, up to 100 mM. Methylotrophy was also demonstrated for the closely related Beggiatoa strain OH75-2a, which shares 99% 16S rRNA sequence identify with B18LD (Fig. 5). Using the data shown in this figure and from an identical experiment performed using B18LD (data not shown), we estimate the molar growth yield, Ymethanol, to be 6.6 and 9.0 g dry weight per mol methanol for OH75-2a and B18LD, respectively. Since metabolized substrates will form gradients in agar-gelled media (22), determining the diffusional limitation on consumption of added methanol is problematic. For both strains, the molar growth yield on acetate is known (5, 20, 27). Thus, by assuming equal diffusion coefficients for methanol and acetate, yields in acetate-supplemented gradient media can be translated into the quantity of methanol consumed (methanol or methanol plus acetate) (Fig. 5), thereby permitting calculation of corresponding methanol molar growth yields. With robust growth on methanol and acetate and on a variety of 2-, 3- and 4-carbon organic substrates (16), B18LD is a facultative methylotroph, a property uncommon among gammaproteobacterial methylotrophs (14).
FIG. 3.
Yield versus time for B18LD in gradient media with 4 mM sulfide in the bottom agar and methanol or acetate as the sole added carbon source in the top agar layer. Data shown are the means of protein determinations on triplicate cultures ± standard errors.
FIG. 4.
Comparison of yield versus time for strain B18LD in gradient media containing 20 mM methanol in the top agar and 0, 2, or 4 mM neutralized sodium sulfide in the bottom agar. Data shown are the means for triplicate cultures ± standard errors.
FIG. 5.
Yield for B. alba strain OH75-2a in gradient media with acetate and/or methanol as the carbon source in the top agar layer and 4 mM sulfide in the bottom layer. Data shown are the means for triplicate culture tubes ± standard errors.
Other potential substrates for methylotrophy—dimethylsulfoxide, dimethylsulfide, and methylamine—are initially metabolized by other microbes using separate pathways that merge with the methanol oxidation pathway at the level of formaldehyde. For strain B18LD, there was no evidence of growth in gradient medium containing dimethylsulfoxide, dimethylsulfide, or methylamine (20 mM each). This strain was also unable to grow in gradient medium supplemented with 2 mM formaldehyde. In addition, no evidence of methanotrophy, as determined by an increase in biomass when grown under a 50% air -50% methane atmosphere, was found for B18LD in the absence or presence of Cu2+, NH4+ or a limiting concentration of methanol. We were also unable to amplify PCR products with significant homology to methane monooxygenase genes using primers that target conserved regions (7, 26). Therefore, it appears that B18LD is incapable of methanotrophy.
It remains to be determined if methylotrophy is a widespread trait among freshwater and marine Beggiatoa species. Beggiatoa and relatives are already known, based on massive natural abundance in a variety of environments, to play prominent roles in marine sulfur and nitrogen cycles (9). Here we offer the added possibility that they have a measurable impact on the cycling of C1 compounds in addition to CO2.
Nucleotide sequence accession numbers.
The nucleic acid sequences determined in this study have been deposited in the GenBank database under accession numbers EU367933 to EU367937.
Acknowledgments
This research was supported in part by funds from the Biological Oceanography Program of the U.S. National Science Foundation (grant NSF 0526653).
Footnotes
Published ahead of print on 11 July 2008.
REFERENCES
- 1.Boetius, A., K. Ravenschlag, C. J. Schubert, D. Rickert, F. Widdel, A. Gieseke, R. Amann, B. B. Jørgensen, U. Witte, and O. Pfannkuche. 2000. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623-626. [DOI] [PubMed] [Google Scholar]
- 2.Chistoserdova, L., and M. E. Lidstrom. 1997. Molecular and mutational analysis of a DNA region separating two methylotrophy gene clusters in Methylobacterium extorquens AM1. Microbiology 143:1729-1736. [DOI] [PubMed] [Google Scholar]
- 3.Ding, H., and D. L. Valentine. 2008. Methanotrophic bacteria occupy benthic microbial mats in shallow marine hydrocarbon seeps, Coal Oil Point, California. J. Geophys. Res. 113:G01015. doi: 10.1029/2007JG000537. [DOI] [Google Scholar]
- 4.Giovannoni, S. J., D. H. Hayakawa, H. J. Tripp, U. Stingl, S. A. Givan, J. C. Cho, H. M. Oh, J. B. Kitner, K. L. Vergin, and M. S. Rappé. 2008. The small genome of an abundant coastal ocean methylotroph. Environ. Microbiol. 10:1771-1782. [DOI] [PubMed] [Google Scholar]
- 5.Güde, H., W. R. Strohl, and J. M. Larkin. 1981. Mixotrophic and heterotrophic growth of Beggiatoa alba in continuous culture. Arch. Microbiol. 129:357-360. [DOI] [PubMed] [Google Scholar]
- 6.Hagen, K. D., and D. C. Nelson. 1996. Organic carbon utilization by obligately and facultatively autotrophic Beggiatoa strains in homogeneous and gradient cultures. Appl. Environ. Microbiol. 62:947-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchens, E., S. Radajewski, M. G. Dumont, I. R. McDonald, and J. C. Murrell. 2004. Analysis of methanotrophic bacteria in Movile Cave by stable-isotope probing. Environ. Microbiol. 6:111-120. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki, F., U. Tsunogai, M. Suzuki, A. Kosaka, H. Machiyama, K. Takai, T. Nunoura, K. H. Nealson, and K. Horikoshi. 2004. Characterization of C1-metabolizing prokaryotic communities in methane seep habitats at the Kuroshima Knoll, Southern Ryukyu Arc, by analyzing pmoA, mmoX, mxaF, mcrA, and 16S rRNA genes. Appl. Environ. Microbiol. 70:7445-7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jørgensen, B. B., and D. C. Nelson. 2004. Sulfide oxidation in marine sediments: geochemistry meets microbiology, p. 63-81. Special paper 379. In J. Amend, K. J. Edwards, and T. W. Lyonns (ed.), Sulfur biogeochemistry—past and present. Geological Society of America, Boulder, CO.
- 10.Kalyuzhnaya, M. G., M. E. Lidstrom, and L. Chistoserdova. 2004. Utility of environmental primers targeting ancient enzymes: methylotroph detection in Lake Washington. Microb. Ecol. 48:463-472. [DOI] [PubMed] [Google Scholar]
- 11.Kalyuzhnaya, M. G., O. Nercessian, M. E. Lidstrom, and L. Chistoserdova. 2005. Development and application of polymerase chain reaction primers based on fhcD for environmental detection of methanopterin-linked C1-metabolism in bacteria. Environ. Microbiol. 7:1269-1274. [DOI] [PubMed] [Google Scholar]
- 12.Kane, S. R., A. Y. Chakicherla, P. S. G. Chain, R. Schmidt, M. W. Shin, T. C. Legler, K. M. Scow, F. W. Larimer, S. M. Lucas, P. M. Richardson, and K. R. Hristova. 2007. Whole-genome analysis of the methyl tert-butyl ether-degrading beta-proteobacterium Methylibium petroleiphilum PM1. J. Bacteriol. 189:1931-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin, J., M. C. Henk, and P. Aharon. 1994. Beggiatoa in microbial mats at hydrocarbon vents in the Gulf of Mexico and Warm Mineral Springs, Florida. Geo-Mar. Lett. 14:97-103. [Google Scholar]
- 14.Lidstrom, M. E. 2006. Aerobic methylotrophic prokaryotes, p. 618-634. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, NY.
- 15.McDonald, I. R., and J. C. Murrell. 1997. The methanol dehydrogenase structural gene mxaF and its use as a functional gene probe for methanotrophs and methylotrophs. Appl. Environ. Microbiol. 63:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezzino, M. J., W. R. Strohl, and J. M. Larkin. 1984. Characterization of Beggiatoa alba. Arch. Microbiol. 137:139-144. [Google Scholar]
- 17.Mussmann, M., F. Z. Hu, M. Richter, D. de Beer, A. Preisler, B. B. Jørgensen, M. Huntemann, F. O. Gloeckner, R. Amann, W. J. H. Koopman, R. S. Lasken, B. Janto, J. Hogg, P. Stoodley, R. Boissy, and G. D. Ehrlich. 2007. Insights into the genome of large sulfur bacteria revealed by analysis of single filaments. PLoS Biol. 5:e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauhaus, K., M. Albrecht, M. Elvert, A. Boetius, and F. Widdel. 2007. In vitro cell growth of marine archaeal-bacterial consortia during anaerobic oxidation of methane with sulfate. Environ. Microbiol. 9:187-196. [DOI] [PubMed] [Google Scholar]
- 19.Nelson, D. C. 1992. The genus Beggiatoa, p. 3171-3180. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, New York, NY.
- 20.Nelson, D. C., and R. W. Castenholz. 1981. Organic nutrition of Beggiatoa sp. J. Bacteriol. 147:236-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson, D. C., and H. W. Jannasch. 1983. Chemoautotrophic growth of a marine Beggiatoa in sulfide-gradient cultures. Arch. Microbiol. 136:262-269. [Google Scholar]
- 22.Nelson, D. C., B. B. Jørgensen, and N. P. Revsbech. 1986. Growth pattern and yield of a chemoautotrophic Beggiatoa sp. in oxygen-sulfide microgradients. Appl. Environ. Microbiol. 52:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neufeld, J. D., H. Schäfer, M. J. Cox, R. Boden, I. R. McDonald, and J. C. Murrell. 2007. Stable isotope probing implicates Methylophaga spp. and novel Gammaproteobacteria in marine methanol and methylamine metabolism. ISME J. 1:480-491. [DOI] [PubMed] [Google Scholar]
- 24.Niemann, H., T. Lösekann, D. de Beer, M. Elvert, T. Nadalig, K. Knittel, R. Amann, E. J. Sauter, M. Schlüter, M. Klages, J. P. Foucher, and A. Boetius. 2006. Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature 443:854-858. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaus, R., J. W. Ammerman, and I. R. MacDonald. 2003. Distinct pigmentation and trophic modes in Beggiatoa from hydrocarbon seeps in the Gulf of Mexico. Aquat. Microb. Ecol. 32:85-93. [Google Scholar]
- 26.Stoecker, K., B. Bendinger, B. Schöning, P. H. Nielsen, J. L. Nielsen, C. Baranyi, E. R. Toenshoff, H. Daims, and M. Wagner. 2006. Cohn's Crenothrix is a filamentous methane oxidizer with an unusual methane monooxygenase. Proc. Natl. Acad. Sci. USA 103:2363-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strohl, W. R., G. C. Cannon, J. M. Shively, H. Güde, L. A. Hook, C. M. Lane, and J. M. Larkin. 1981. Heterotrophic carbon metabolism by Beggiatoa alba. J. Bacteriol. 148:572-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Spanning, R. J. M., S. de Vries, and N. Harms. 2000. Coping with formaldehyde during C1 metabolism of Paracoccus denitrificans. J. Mol. Catal. B Enzym. 8:37-50. [Google Scholar]
- 29.Vila-Costa, M., D. A. del Valle, J. M. González, D. Slezak, R. P. Kiene, O. Sánchez, and R. Simó. 2006. Phylogenetic identification and metabolism of marine dimethylsulfide-consuming bacteria. Environ. Microbiol. 8:2189-2200. [DOI] [PubMed] [Google Scholar]
- 30.Vorholt, J. A., L. Chistoserdova, S. M. Stolyar, R. K. Thauer, and M. E. Lidstrom. 1999. Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclohydrolases. J. Bacteriol. 181:5750-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson, S. M., M. P. Gleisten, and T. J. Donohue. 2008. Identification of proteins involved in formaldehyde metabolism by Rhodobacter sphaeroides. Microbiology 154:296-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zehr, J. P., and P. J. Turner. 2001. Nitrogen fixation: nitrogenase genes and gene expression. In J. H. Paul (ed.), Methods in marine microbiology. Academic Press, New York, NY.