Abstract
Heavy metals have been shown to be strong inhibitors of nitrification in wastewater treatment plants. In this research, the effects of cadmium, copper, and mercury on Nitrosomonas europaea were studied in quasi-steady-state batch reactors. When cells were exposed to 1 μM CdCl2, 6 μM HgCl2, or 8 μM CuCl2, ammonia oxidation rates were decreased by about 90%. Whole-genome transcriptional and proteomic responses of N. europaea to cadmium were used to identify heavy metal stress response genes. When cells were exposed to 1 μM CdCl2 for 1 h, 66 genes (of the total of 2,460 genes) were upregulated, and 50 genes were downregulated more than twofold. Of these, the mercury resistance genes (merTPCADE) averaged 277-fold upregulation under 1 μM CdCl2, with merA (mercuric reductase) showing 297-fold upregulation. In N. europaea cells exposed to 6 μM HgCl2 or to 8 μM CuCl2, merA showed 250-fold and 1.7-fold upregulation, respectively. Cells showed the ability to recover quickly from Hg2+-related toxic effects, apparently associated with upregulation of the mercury resistance genes and amoA, but no such recovery was evident in Cd2+-exposed cells even though merTPCADE were highly upregulated. We suggest that the upregulation of merA in response to CdCl2 and HgCl2 exposure may provide a means to develop an early-warning indicator for inhibition of nitrification by these metals.
Nitrifying bacteria, such as Nitrosomonas europaea (ATCC 19718), are important in the removal of nitrogen in wastewater reclamation plants. N. europaea obtains essential reductants for energy and biosynthesis from the oxidation of ammonia (NH3+) to nitrite (NO2−) and uses CO2 as its carbon source (1). Oxidation of NH3+ by N. europaea is a two-step reaction catalyzed by ammonia monooxygenase ([AMO] a membrane-bound enzyme) and hydroxylamine oxidoreductase ([HAO] a periplasmic protein), generating NO2− as the final product (1). Nitrifying bacteria are sensitive to various environmental contaminants and generally have low growth rates, with doubling times of about 8 to 12 h (46), making them the critical step in biological nitrogen removal (BNR). Heavy metals, such as Cd2+ (12, 28), Hg2+, and Cu2+ (30), are extensively used in industry (e.g., in the fabrication of pigments, batteries, and electronics), and improper disposal of the metals or their by-products tends to contaminate the environment (40). These metals may inhibit nitrification in the reclamation of wastewater (4, 5, 27, 44).
Inhibition of nitrification has been best documented in the model bacterium N. europaea. N. europaea is sensitive to inorganic compounds such as Cd2+ (7, 21) and organic compounds such as chlorinated aliphatic hydrocarbons (23) and to pH shifts (14), among other factors. With the sequencing of the N. europaea genome, transcriptomics studies to characterize N. europaea stress responses to inhibitors are now possible. Exposure to Zn2+ caused inhibition of ammonia oxidation and, concomitantly, expression of specific genes encoding membrane transporter and putative metal resistance proteins (38). Exposure to chlorinated aliphatic hydrocarbons increased the expression of genes encoding heat shock proteins, sigma factors of the extracytoplasmic function (ECF) subfamily, and toxin-antitoxin loci (15). Such genes potentially may be useful as early-warning toxicity indicators to prevent excessive inhibition of nitrification and in BNR processes.
Failure or inhibition of BNR processes caused by heavy metals, such as Cd2+, Hg2+, and Cu2+, can lead to high ammonia discharges and contribute to eutrophication of water bodies (44). Because current methods to monitor nitrification are laborious and cannot identify causes of inhibition, quick and sensitive methods are required for early detection of nitrification inhibition and for identification of its causes. Such methods could rely on “sentinel genes” that are highly upregulated in the presence of a certain heavy metal or group of heavy metals. In a previous study, merTPCAD were identified as putative sentinel genes in N. europaea exposed to Zn2+ (38). The main objective of the current study was to look for similar sentinel genes by examining whole-genome transcriptional changes in N. europaea exposed to Cd2+. For comparison, transcriptional changes of selected genes were also determined in cells exposed to Hg2+ and Cu2+.
MATERIALS AND METHODS
Batch reactor experiments.
N. europaea cells were grown in batch cultures with 25 mM (NH4)2SO4 as described previously (38), harvested in mid- to late-exponential phase (optical density at 600 nm [OD600] of ≅0.07), and washed two times with 40 mM NaH2PO4 (pH 7.8). Washed cells were resuspended in 1 liter of 50 mM HEPES buffer (pH 7.8) containing (NH4)2SO4 (2.5 mM). The 1-liter cell suspension was evenly divided into two, gas-tight, 1.67-liter reactor vessels (Wheaton double-sidearm cell stir), and experiments were conducted without further additions of either buffer or ammonia. Cell suspensions prepared in this way sustained a quasi-steady-state condition during 4-h incubations comparable to N. europaea batch reactor experiments previously reported using phosphate-buffered medium (15). For the experiments, cells were stirred for 1 h to allow them to reach quasi-steady state, at which point either 1 μM CdCl2, 6 μM HgCl2, or 8 μM CuCl2 was injected into the treatment reactor vessels.
Oxygen uptake measurement and nitrite assay.
The ammonia-dependent specific oxygen uptake rate (A-SOUR), hydrazine-dependent specific oxygen uptake rate (H-SOUR), and NO2− production rates were tested every 30 min during the incubations, as described previously (23). An aliquot of 1 ml drawn from a batch reactor was centrifuged immediately and analyzed to determine the NO2− concentration (22). The A-SOUR was measured in a 1.8-ml glass water-jacketed reaction vessel at 30°C using a heated circulating water bath (10). The H-SOUR was then determined by blocking ammonia-dependent oxygen uptake with 100 μM allylthiourea and adding 750 μM hydrazine as an alternative substrate for HAO. The SOUR of the cells was calculated based on the saturated oxygen concentration in water (10).
Affymetrix microarray experiments.
A volume of 180 ml of N. europaea culture (OD600 of ≅0.07) was obtained from three independent experiments (three control reactors and three Cd2+-treatment reactors). Trizol (Ambion Inc., Austin, TX) was used to extract total RNA from the suspension, following the manufacturer's instructions. After purification with an RNeasy Mini kit (Qiagen Inc., Valencia, CA), the quality and quantity of the RNA samples were measured using an RNA 6000 Nano LabChip kit on an Agilent Bioanlyzer 2100 (Agilent Technologies, Inc., Palo Alto, CA). All of the 2,460 annotated N. europaea genes were represented on the custom Nimble Express made-to-order arrays (NimbleGen Systems, Inc.). cDNA synthesis, labeling, and hybridization were carried out by the Center for Genome Research and Biocomputing Core Laboratories at Oregon State University. GeneSpring software (version 7.2; Silicon Genetics) was used to identify genes that were upregulated or downregulated more than twofold under Cd2+, applying an unpaired two-sample t test with a cutoff P value of 0.05.
qRT-PCR.
Quantitative reverse transcription-PCR (qRT-PCR) was used to evaluate the transcriptional levels of selected up- or downregulated genes identified in the microarrays and to determine transcriptional levels of merA in N. europaea cells exposed to Cu2+ and Hg2+. cDNA was synthesized using an IScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA) with total RNA extracted from cells treated with or without Cd2+, Cu2+, or Hg2+, as described previously (38). qRT-PCR was performed on an ABI 7500 instrument (Applied Biosystems, Foster City, CA) with IQ Sybr Green Supermix (Bio-Rad). Primers were designed using Primer3 software and manufactured commercially (Invitrogen, Carlsbad, CA). The qRT-PCR efficiency was determined using standard curves created by serial dilution of RNA samples. The relative changes to show ideal amplification efficiency were calculated using the formula 2−ΔΔCT (where CT is cycle threshold) (39).
2D SDS-PAGE.
To find proteins that were differentially expressed under Cd2+ stress, total proteins, including membrane-bound proteins, were prepared from N. europaea cells after a 3-h incubation with and without 1 μM CdCl2 using a ReadyPrep Sequential Extraction Kit (Bio-Rad). The extracted total proteins were then compared by two-dimensional (2D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (38). ReadyStrip immobilized pH gradient strips (13 cm; Bio-Rad) with a nonlinear pH gradient from 3 to 10 were used for isoelectric focusing. SDS-PAGE was carried out on 12.5% precast SDS-PAGE gels (Bio-Rad) at 200 V for 45 min. The upregulated proteins in Cd2+-exposed cells were excised from SDS-PAGE gels stained with Sypro Ruby (Cambrex Bioscience, Rockland, ME) and identified by nano-liquid chromatography-tandem mass spectrometry (Midwest Bio Services, LLC, Overland, KS).
Microarray data accession number.
The microarray data are available at the Gene Expression Omnibus database under accession number GSE9221 (http://www.ncbi.nih.gov/geo).
RESULTS
Nitrite production and SOURs.
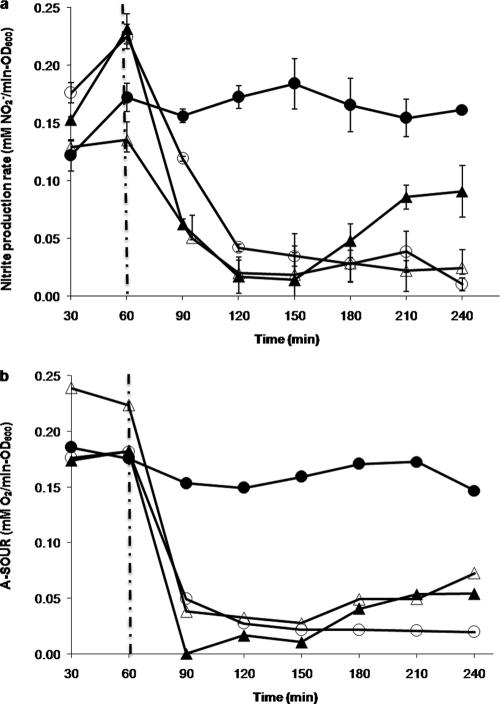
To test the toxicity of the heavy metals in this study, N. europaea cells were placed in two batch reactors (a treatment reactor and a control reactor). In the control reactors, the cells consistently reached quasi-steady state in about 1 h (constant NO2− production rate of about 0.2 mM/min·OD600) and were stable for up to 4 h (Fig. 1a). In the treatment reactors, 1 μM CdCl2, 6 μM HgCl2, or 8 μM CuCl2 was added at 1 h in quasi-steady state. After 1 h of further incubation with Cd2+, the cells showed about an 88% decrease in the NO2− production rate (Fig. 1a) and an 82% decrease in A-SOUR (Fig. 1b). N. europaea exposed to 6 μM HgCl2 or 8 μM CuCl2 for 1 h showed about 90% and 82% inhibition, respectively, of A-SOUR (Fig. 1b), while their corresponding nitrite production rates decreased by about 88% and 76%, respectively.
FIG. 1.
N. europaea nitrite production rate (a) and A-SOUR (b) under 1 μM CdCl2, 6 μM HgCl2, or 8 μM CuCl2 treatments for 4 h. Nitrite production rate and AMO activity were normalized by cell density. Closed circles, open circles, closed triangles, and open triangles represent control condition, 8 μM CuCl2, 6 μM HgCl2, and 1 μM CdCl2, respectively. The vertical dashed lines indicate the injection time of inhibitors. The error bars indicate 95% confidence intervals.
Determinations of H-SOUR showed that hydrazine-dependent oxygen uptake activity remained unaffected in N. europaea cells exposed to 1 μM CdCl2 and 6 μM HgCl2 but that it decreased by 71% in cells exposed to 8 μM CuCl2 (data not shown).
Interestingly, 6 μM HgCl2 almost completely stopped NO2− production (Fig. 1a) and ammonia-dependent oxygen uptake (Fig. 1b), but both nitrite production and A-SOUR began to rebound within 60 min of further incubation (Fig. 1a and b), suggesting that the cells were able to recover from the Hg2+ exposure.
Transcriptomic responses to Cd2+.
Using microarrays, we determined the global transcriptional changes of N. europaea exposed to Cd2+. The analysis revealed that 1 μM CdCl2 caused significant changes (greater than twofold) in the transcript levels of 116 genes. The genes included 66 genes with known functions (39 upregulated and 27 downregulated), 21 open reading frames with no known functions (12 upregulated and 9 downregulated), and 29 intergenic regions (15 upregulated and 14 downregulated). The up- or downregulated genes with known function were grouped by functional classification based on the N. europaea genome database (http://genome.ornl.gov) (Tables 1 and 2). Among these, the mercury resistance genes and two genes upstream of that operon were upregulated more than 100-fold. Various genes involved in coenzyme metabolism, translation, DNA replication, and nucleotide transport showed intensities slightly above twofold over the control. The arrays also showed 50 downregulated genes slightly above twofold and included genes that function in signal transduction mechanisms and cell processes. Interestingly, the membrane-bound metallopeptidase encoded by NE2218 showed about 80-fold downregulation in response to Cd2+ toxicity exposure, perhaps to prevent cytotoxicity by self-digestion when the cells slow down their metabolism.
TABLE 1.
Selected upregulated genes under cadmium stressa
| Gene function and N. europaea locus tag | Gene name | Description | Fold change | P value (<0.05) |
|---|---|---|---|---|
| Mercury resistance pathway | ||||
| NE0838 | merD* | Bacterial regulatory protein (MerR family) | 107.4 | 1.9 × 10−5 |
| NE0839 | merA* | Mercuric reductase | 296.7 | 9.1 × 10−6 |
| NE0840 | merC* | Putative mercury transport protein | 171.8 | 9.2 × 10−5 |
| NE0841 | merP* | Mercury scavenger protein | 438.6 | 9.6 × 10−7 |
| NE0842 | merT* | Mercuric transport protein | 370.3 | 4.8 × 10−5 |
| Inorganic ion transport mechanism, NE0852 | yvgQ | Nitrite and sulfite reductase | 2.0 | 2.1 × 10−3 |
| Efflux pump, NE1640 | czcC | Outer membrane efflux protein | 2.4 | 1.4 × 10−3 |
| Oxidative stress, NE1034 | trxA | Thioredoxin domain-containing protein | 2.1 | 2.6 × 10−2 |
| ABC transporter, NE1899* | ATPase component ABC-type transport system | 2.4 | 2.5 × 10−3 | |
| Coenzyme metabolism | ||||
| NE0856 | Flavin adenine dinucleotide biosynthesis | 2.0 | 4.9 × 10−3 | |
| NE0634 | cobO | Cobalamine biosynthesis | 2.7 | 2.8 × 10−4 |
| NE0636 | Outer membrane cobalamin receptor protein | 2.0 | 2.6 × 10−4 | |
| Posttranslational modification, protein turnover, chaperones | ||||
| NE0221 | Organic radical activating enzymes | 2.1 | 3.3 × 10−4 | |
| NE2206 | ppiD | Peptidyl-prolyl isomerase | 2.0 | 8.0 × 10−3 |
| Cell envelope biosynthesis | ||||
| NE0378 | Sugar transferases involved in lipopolysaccharide synthesis | 2.1 | 4.7 × 10−4 | |
| NE2279 | yccZ | Periplasmic protein involved in polysaccharide export | 2.0 | 2.6 × 10−4 |
| Translation, ribosomal structure, biogenesis | ||||
| NE2072 | gatA | Amidase:glutamyl-tRNA (Gln), amidotransferase A subunit | 2.9 | 3.0 × 10−4 |
| NE2073 | gatB | GatB:glutamyl-tRNA(Gln) amidotransferase, B subunit | 2.1 | 4.7 × 10−4 |
| NE0389 | rnpA* | RNase P protein component | 2.4 | 2.6 × 10−4 |
| NE1457 | Ribonucleases G and E | 2.4 | 9.2 × 10−5 | |
| NE2363 | glnS | Glutamyl- and glutaminyl-tRNA synthetases | 2.1 | 3.3 × 10−4 |
| DNA replication, recombination, repair | ||||
| NE0835 | tnpA | Transposase and inactivated derivatives TnpA family | 25.5 | 9.1 × 10−6 |
| NE0836 | tnpR | Site-specific recombinase DNA | 36.7 | 3.0 × 10−6 |
| NE0837* | Domain of unknown function 2 | 157.7 | 1.4 × 10−5 | |
| NE2207 | hupB | Bacterial histone-like DNA binding protein | 2.1 | 2.5 × 10−5 |
| Transcription | ||||
| NE2324 | rnc | Double-stranded-RNA-specific RNase | 2.4 | 1.4 × 10−4 |
| NE1035 | Transcription termination factor | 2.6 | 2.5 × 10−3 | |
| NE0854 | cysB | Transcriptional regulator | 2.2 | 5.0 × 10−3 |
| NE0951 | Predicted transcriptional regulators (MerR family) | 2.0 | 1.2 × 10−3 | |
| Amino acid transport and metabolism | ||||
| NE1005 | argB | Acetylglutamate kinase | 2.7 | 3.4 × 10−4 |
| NE0872 | hisD | Histidinol dehydrogenase | 2.1 | 5.0 × 10−3 |
| Nucleotide transport and metabolism, NE0277 | XTP pyrophosphatase | 2.2 | 1.4 × 10−3 | |
| Carbohydrate transport and metabolism, NE1691 | Phosphogluconolactonase/glucosamine-6-phosphate isomerase/deaminase | 3.8 | 6.2 × 10−4 | |
| Fatty acid biosynthesis, NE1646 | Fatty acid synthesis | 2.1 | 3.1 × 10−4 | |
| Signal transduction mechanism, NE0848 | Phosphoglycerate mutase family | 2.1 | 3.1 × 10−4 | |
| Others | ||||
| NE1176* | Peptidoglycan binding | 2.6 | 3.0 × 10−4 | |
| NE0090 | Predicted ATPase | 2.0 | 2.6 × 10−4 | |
| NE2325 | Transmembrane protein | 2.4 | 5.1 × 10−5 | |
| NE2326 | lepB | Signal peptidase I | 2.0 | 3.0 × 10−4 |
| NE0218 | tolB | Periplasmic component of the Tol biopolymer transport system | 2.0 | 5.3 × 10−4 |
Commonly upregulated genes under Cd2+ and Zn2+ treatment are indicated by an asterisk.
TABLE 2.
Selected downregulated genes under cadmium stressa
| Gene function and N. europaea locus tag | Gene name | Description | Fold change | P value (<0.05) |
|---|---|---|---|---|
| Inorganic ion transport mechanism | ||||
| NE0730 | Ferric uptake regulator family | 2.6 | 4.7 × 10−2 | |
| NE0731 | TonB-dependent receptor protein | 2.4 | 4.4 × 10−2 | |
| NE0999 | Phosphate transport system permease protein | 3.5 | 2.4 × 10−2 | |
| NE1000 | ABC-type phosphate transport system permease component | 2.9 | 4.6 × 10−2 | |
| NE1001 | pstB | Phosphate transport system ATP-binding protein | 2.7 | 2.4 × 10−2 |
| NE1531* | TonB-dependent receptor protein | 2.6 | 2.4 × 10−2 | |
| NE0345 | Acriflavin resistance protein; heavy metal efflux pump CzcA | 5.9 | 2.4 × 10−2 | |
| RubisCO | ||||
| NE1918 | cbbO* | von Willebrand factor type A domain | 7.3 | 2.4 × 10−2 |
| NE1919* | Nitric oxide reductase NorQ protein | 5.7 | 2.4 × 10−2 | |
| Cell processes | ||||
| NE2290 | Bacterial type II secretion system protein E; GAF domain | 2.0 | 4.0 × 10−2 | |
| NE1298* | Tetratricopeptide repeat | 2.2 | 4.0 × 10−2 | |
| NE2315 | pilN | Putative type 4 fimbrial biogenesis protein | 2.2 | 2.7 × 10−2 |
| NE2488 | flhA | Bacterial export FHIPEP family | 2.1 | 2.4 × 10−2 |
| NE0346 | Possible cation transporter transmembrane protein | 5.2 | 2.4 × 10−2 | |
| NE2218* | Membrane-bound metallopeptidase | 79.78 | 2.7 × 10−2 | |
| NE1538 | Chromosome segregation ATPases | 2.2 | 2.4 × 10−2 | |
| Transcription | 2.4 × 10−2 | |||
| NE2435 | fecI | Specialized sigma subunits of RNA polymerase | 2.2 | 2.4 × 10−2 |
| NE1217 | Sigma 70 factor, ECF subfamily | 2.4 | 2.4 × 10−2 | |
| NE0533 | Sigma 70 factor, ECF subfamily | 2.3 | 2.4 × 10−2 | |
| NE1452 | Transcriptional regulator | 2.1 | 4.7 × 10−2 | |
| NE0787* | Response regulator containing a CheY-like receiver domain and a helix-turn-helix DNA-binding domain | 2.1 | 2.7 × 10−2 | |
| Signal transduction mechanism | ||||
| NE1923 | cheY* | Response regulator receiver domain | 3.0 | 2.7 × 10−2 |
| NE0534 | Transmembrane sensor | 2.2 | 2.7 × 10−2 | |
| Posttranslational modification, protein turnover, chaperone, NE1529 | Signal peptide protein | 2.0 | 2.7 × 10−2 | |
| Others | ||||
| NE0315 | mnxG* | Possible multicopper oxidase | 2.1 | 4.0 × 10−2 |
| NE2038* | Myeloperoxidase, thyroid peroxidase, cyclooxygenase catalytic domain | 2.2 | 4.4 × 10−2 | |
| NE0353 | exbB1 | MotA TolQ ExbB proton channel family | 2.3 | 2.7 × 10−2 |
| NE1545 | Pirin-related protein | 3.0 | 2.4 × 10−2 |
Commonly downregulated genes under Cd2+ and Zn2+ toxicity are indicated with an asterisk.
To confirm expression changes observed in the microarrays, selected genes were analyzed by qRT-PCR. The relative changes measured by qRT-PCR were consistent and in agreement with the relative changes in the microarrays (Fig. 2).
FIG. 2.
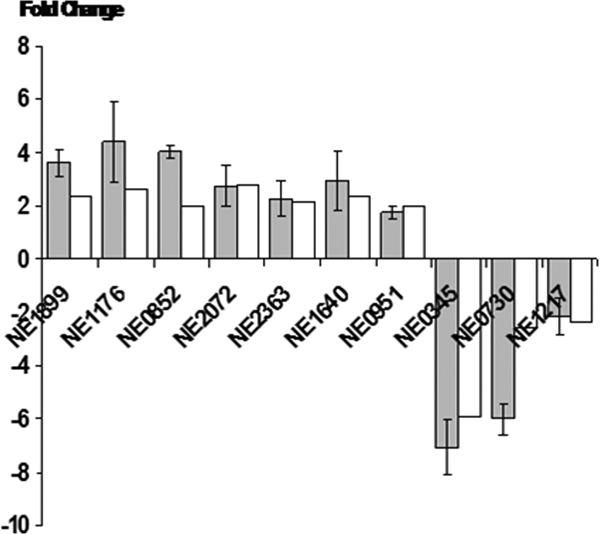
Comparison of mRNA expression levels of selected up- or downregulated genes determined by qRT-PCR (black bars) and microarray (white bars). The positive value represents upregulation, and the negative value represents downregulation. Error bars represent standard errors of the means.
Expression of merA and amoA under Cu2+ and Hg2+ toxicity.
The merTPCADE cluster was upregulated 297-fold in cells exposed to Cd2+ and 46-fold in cells exposed to Zn2+ (38). To examine whether merTPCADE respond similarly to other heavy metals, we used qRT-PCR to examine time-dependent transcriptional responses of merA to 6 μM HgCl2 and 8 μM CuCl2. The transcript level of merA increased 150-fold in the first 30 min in response to Hg2+ and continued to increase up to 250-fold upregulation (Table 3). Consistent with detoxification, during the recovery of nitrification activity, the transcript level of merA decreased to 21-fold upregulation in the span of 3 h (Table 3). In contrast, the transcript level of merA did not show a significant change in response to Cu2+ (Table 3) at levels that inhibited nitrification (Fig. 1).
TABLE 3.
Expression change of merA and amoA determined by qRT-PCR under Hg2+ and Cu2+ treatment
| Treatment and gene | Fold change in expression after incubation fora:
|
|||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| Hg2+ treatment | ||||
| merA | 157.1 ± 0.22 | 254.5 ± 0.72 | 115.7 ± 0.01 | 21.6 ± 0.26 |
| amoA | 1.0 ± 0.02 | 2.0 ± 0.02 | 1.9 ± 0.05 | 3.7 ± 0.12 |
| Cu2+ treatment | ||||
| merA | 1.8 ± 0.50 | 0.9 ± 0.01 | 1.4 ± 0.35 | 1.7 ± 0.12 |
| amoA | 2.0 ± 0.07 | 2.0 ± 0.33 | 0.5 ± 0.03 | 0.4 ± 0.02 |
The error (± value) indicates the 95% confidence interval.
We also examined the time-dependent transcriptional responses of amoA under Cu2+ and Hg2+ stress (Table 3). The transcript level of amoA increased twofold in Cu2+-exposed cells, which may have caused uptake of a small amount of the Cu2+ in the first 60 min, but decreased twofold by 180 min. N. europaea exposed 30 min to 6 μM HgCl2 lost almost all AMO activity and, consequently, NO2− production (Fig. 1b). However, as the transcript level of merA increased within 1 h, AMO transcript also increased, and nitrite production rates began to rebound.
2D SDS-PAGE.
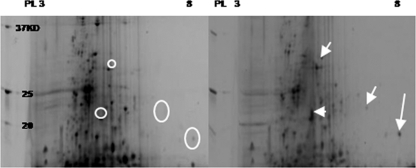
To determine changes in protein expression, we used 2D SDS-PAGE, using protein extracts from control and Cd2+-exposed cells (1 μM CdCl2) taken after a 3-h incubation. Several proteins showed higher intensity in the Cd2+-exposed cells than in control cells. The differentially expressed proteins were excised from the gels for identification by nano-liquid chromatography-tandem mass spectrometry and to deduce the genes that encode them. Identified proteins included nitrite reductase, encoded by NE0924, Rieske iron-sulfur protein, encoded by NE1503, and two hypothetical proteins encoded by NE2057 and NE1752 (Fig. 3).
FIG. 3.
Comparison in 2D SDS-PAGE of the soluble protein fraction of N. europaea treated without (left) and with 1 μM CdCl2 (right) for 3 h. Circles represent absent or low translation under control conditions. Arrows represent protein spots upregulated under cadmium stress.
DISCUSSION
Physiological responses to Cd2+, Hg2+, or Cu2+.
Our results (Fig. 1) suggest that the inhibitory effects of Cd2+ and Hg2+ exposure were confined mainly to AMO while apparently causing little or no damage to other elements of the electron transport chain. However, the decrease in H-SOUR with 8 μM CuCl2 suggests that Cu2+-related inhibition was not confined only to AMO. Cu2+ has been reported to induce cytotoxicity by producing hydroperoxide and by causing losses of intracellular K+, an indication of loss of membrane integrity, in nitrifying autotrophic bacteria (20; also T. S. Radniecki and R. L. Ely, unpublished data). Decreases in both A-SOUR and H-SOUR, as observed in this study, would be consistent with compromised membrane integrity associated with Cu2+ exposure.
We showed previously in batch reactors that Zn2+ caused 50% nitrification inhibition at a concentration of 3.4 μM (38). Compared to Zn2+, Cd2+ and Hg2+ were more toxic to ammonia oxidizers, but Cu2+ was less toxic, with 1 μM Hg2+ or 6 μM Cu2+ causing 50% inhibition (data not shown) and 1 μM Cd2+ causing 90% inhibition. Other studies have shown the inhibition of nitrification by heavy metals in wastewater sludge (27, 33), consistent with our results showing the sensitivity of ammonia oxidizers (e.g., N. europaea) to Cd2+, Hg2+, or Cu2+.
Proteomic responses to Cd2+.
In microarray experiments (1-h incubation), transcript levels of the genes NE0924, NE1503, NE2057, and NE1752, corresponding to the overexpressed peptides (3-h incubation) (Fig. 3), did not show detectable increases. Similar discrepancies between transcript and protein levels have been seen in studies with human cells (6), Plasmodium falciparum (29), iron-regulated genes of Vibrio anguillarum (8), and with Escherichia coli (25). Posttranscriptional splicing and posttranslation modifications have been suggested as possible reasons for different responses in transcription and translation (6, 29). In our experiments, microarray data showed upregulation of the genes that encode posttranslational modification proteins, NE0221 and NE2206, consistent with the possibility that posttranslational modifications may have been important in cellular responses to Cd2+ toxicity. Overexpression of NirK (nitrite reductase) in N. europaea exposed to Cd2+ might support the metal tolerance hypothesis in soil microorganisms (16, 43). It has been reported that NirK is involved in heavy metal tolerance in denitrifying bacteria (16, 43). Therefore, in addition to conferring nitrite tolerance in N. europaea (3), perhaps NirK could play a role in Cd2+ tolerance as well.
Expression of merTPCADE under Cd2+ toxicity.
Of the 66 upregulated genes detected in the microarrays of cells exposed to 1 μM CdCl2, the highest upregulated genes (more than 100-fold) were those encoding mercury resistance proteins (merTPCADE). This observation is consistent with higher transcript levels of the merTPCADE operon seen previously in Zn2+-exposed cells (38), suggesting that the mercury resistance operon in N. europaea may play an important role in protecting the cells from toxic heavy metals. In addition, the transposase encoded by tnpA (NE0835) and the resolvase encoded by tnpR (NE0836) were upregulated under Cd2+ (31-fold) and Hg2+ (54-fold) stress but not under Zn2+ and Cu2+ stress (data not shown). The transposase and resolvase are thought to form a transposase-related protein, known as the mercury resistance transposon (34, 35), that may increase antibiotic resistance in gram-negative facultative bacteria (31). In N. europaea, tnpA and tnpR are located downstream of the operon merTPCADE (Fig. 4). The role of the mercury resistance transposon with respect to Cd2+ toxicity in N. europaea remains unclear, as we did not observe increased Cd2+ tolerance or adaptation in our experiments. However, we can suggest that the upregulation of merTPCADE and tnpAR may occur in concert when exposed to highly toxic heavy metals (e.g., Cd2+ and Hg2+) but not when exposed to less toxic metals (e.g., Zn2+ and Cu2+). We also observed 2.4-fold upregulation of NE1640, which putatively encodes CzcC, the outer membrane protein of the CzcCBA efflux pump thought to be involved in Cd2+ detoxification in Ralstonia eutropha and other gram-negative bacteria (37). However, because N. europaea cells did not recover from Cd2+ treatment as they did from Hg2+ treatment, a role for this gene in the removal of Cd2+ seems unlikely. The accumulation of Cd2+ in the cytoplasm would eventually become deleterious to the cell and inhibit nitrification completely.
FIG. 4.
Organization of merR, merTPCADE, and transposase-related genes (tnpMRA) in N. europaea. Arrows indicate the genes and their orientation. The numbers in parentheses indicate the relative changes of upregulated merTPCADE and tnpMRA under 1 μM CdCl2 treatments.
Transcriptional responses of merA and amoA in cells exposed to Hg2+ and Cu2+.
In cells exposed to Zn2+ (38), Cd2+, or Cu2+, nitrite production and ammonia-dependent oxygen uptake rates did not rebound from heavy metal toxicity within the 3-h time period used in this study even though merTPCAD were upregulated 277-fold in Cd2+-exposed cells, perhaps due to severe oxidative stress (7, 12). In contrast, cells exposed to Hg2+ did recover, with nitrite production rates rebounding from 0.02 mM NO2−/min·OD600 to 0.09 mM NO2−/min·OD600 in 90 min (Fig. 1), accompanied by increased expression of merA (Table 3). Recovery of N. europaea from Hg2+ toxicity is thought to be related to the mercury resistance genes (merTPCAD), in particular, to merA because the merA gene product can reduce Hg2+ to a volatile form (Hg0) (2, 17, 37), as follows: Hg2+ + NADPH → Hg0 + NADP+ + H+ (36). Hg0 then can be volatilized by the cells (36).
Transcript levels of amoA increased in the early stages of Cu2+-induced toxicity, but they did not translate into increases in A-SOUR. This indicates that Cu2+, although essential for AMO activity (11), can be detrimental even at relatively low concentrations.
Increases in the A-SOUR in cells exposed to Hg2+ were reflected in the transcript levels of amoA (Table 3). While it did not change appreciably during the first 30 min of Hg2+ exposure, amoA transcription increased fourfold during the time that cells showed recovery. These observations suggest also that monitoring amoA expression can be valuable for tracking cellular responses to toxicity.
Shared up- or downregulated genes in Cd2+ and Zn2+ treatment.
Several genes that may be related to detoxification of heavy metals showed transcript level changes with Cd2+ or Zn2+ exposure. NE1176, encoding peptidoglycan binding protein, known to interact with integral outer membrane proteins (13), was upregulated 2.4- to 2.6-fold. A similar observation has been reported with Caulobacter crescentus exposed to Cd2+ and Cr2+ (19). Other upregulated genes included NE1899, encoding an ATPase component ABC-type metal transporter or an arsenite resistance protein (37), and NE0389, encoding an RNase P protein that processes a precursor of tRNA (18). Genes that were downregulated included NE2218, encoding a membrane-bound metallopeptidase (80-fold under Cd2+ and 8-fold under Zn2+ treatments), which requires a small amount of a transition metal such as Zn2+ or Co2+ in its active site (26). The genes cbbQO (NE1919/1918), encoding ribulose bisphosphate carboxylase/oxygenase (RubisCO), were downregulated in both Zn2+ and Cd2+ treatments. Carbon sequestration by RubisCO is an energy-intensive process (47). Under starvation conditions in N. europaea (48) and under Cu2+ stress in Nitrosococcus mobilis (41), genes encoding RubisCO were downregulated, possibly to conserve energy for NH3+ metabolism.
Heavy metals cause oxidative stress in gram-negative bacteria (37), and in this work, the microarrays detected changes in transcript levels of genes encoding proteins associated with oxidative stress. Myeloperoxidase, encoded by NE2038, is thought to be a reactive oxygen species-generating enzyme (24) and was downregulated, perhaps to reduce oxidative stress under heavy metal toxicity. NE0315 (mnxG), encoding a multicopper oxidase known to catalyze Mn2+ oxidation, was downregulated under the Zn2+ and Cd2+ treatments, again perhaps to reduce oxidative stress (9). Genes that were also downregulated under both Zn2+ and Cd2+ stress include NE1298, encoding tetratricopeptide repeat, involved in protein-protein interactions (32); NE1531, encoding a TonB-dependent protein; and NE0787, encoding CheY protein. NE1923, encoding CheY (a flagellar protein) in N. europaea, was previously seen to be downregulated in response to NO, as NO promoted the formation of biofilm, and mobility was no longer necessary (42). It could be that the expression of CheY decreases during metal stress to promote the formation of cell agglomerates to protect some of the cells from further exposure.
Candidate genes to detect Cd2+ stress.
Of the N. europaea genes that were up- or downregulated under Cd2+ treatment, some potentially may serve as specific indicators of Cd2+ exposure. Because Cd2+ causes oxidative stress by producing reactive oxygen species that deplete glutathione and protein-bound sulfhydryl groups (37), the upregulation of NE1034, encoding thioredoxin (disulfide reductase) (19), could help the cell to resist oxidative stresses. NE1005 (argB) and NE0872 (hisD), encoding an amino acid transport, were upregulated under Cd2+. Biosynthesis of amino acids would use more energy than their uptake through an amino acid transport; therefore, these genes might be upregulated to conserve energy in a toxic environment (45). NE0221, encoding an organic radical-activating enzyme, and NE2206 (ppiD), encoding a peptidyl-prolyl isomerase that belongs to a posttranslational modification protein, showed upregulation under Cd2+ treatment (ppiD was also upregulated under chloroform treatment) (15). NE2324 (rnc), NE1035, NE0854 (cysB), and NE0951, which relates to transcription, showed upregulation, while NE2435 (fecI), NE1217, NE0533, NE1452, and NE0787 showed downregulation (Table 2). Transcript of NE1217, belonging to the ECF family, was also increased in response to chloroform toxicity (15). NE0835, NE0836, NE0837, and NE2207 (hupB), involved in DNA replication, recombination, and repair, showed upregulation. NE0378 and NE2279 (yccZ), involved in cell envelope biosynthesis, were upregulated, while six genes in the groups were downregulated to statistically significant degrees (Table 2). Although the role(s) of these genes in heavy metal stress was not clear, they could be important for finding heavy metal toxicity mechanisms or general stress genes in the future.
Acknowledgments
This research was supported by NSF Biocomplexity grant 0412711.
We thank Barbara O. Gvakharia and Tyler S. Radniecki for many helpful discussions regarding experimental design. We especially acknowledge Daniel J. Arp, Peter J. Bottomley, and Luis Sayavedra-Soto for giving us the benefit of their vast expertise regarding N. europaea and for helping with manuscript preparation. We also thank the Center for Genome Research and Biocomputing at Oregon State University for services provided for RNA quality testing and microarrays.
Footnotes
Published ahead of print on 7 July 2008.
REFERENCES
- 1.Arp, D. J., L. A. Sayavedra-Soto, and N. G. Hommes. 2002. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch. Microbiol. 178:250-255. [DOI] [PubMed] [Google Scholar]
- 2.Barkay, T., S. M. Miller, and A. O. Summers. 2003. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 27:355-384. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont, H. J., N. G. Hommes, L. A. Sayavedra-Soto, D. J. Arp, D. M. Arciero, A. B. Hooper, H. V. Westerhoff, and R. J. van Spanning. 2002. Nitrite reductase of Nitrosomonas europaea is not essential for production of gaseous nitrogen oxides and confers tolerance to nitrite. J. Bacteriol. 184:2557-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benmossa, H., G. Martin, Y. Richard, and A. Leprince. 1986. Inhibition of nitrification by heavy metal cations. Water Res. 20:1333-1339. [Google Scholar]
- 5.Braam, F., and A. Klapwijk. 1981. Effect of copper on nitrification in activated sludge. Water Res. 15:1093-1098. [Google Scholar]
- 6.Celis, J. E., M. Kruhoffer, I. Gromova, C. Frederiksen, M. Ostergaard, T. Thykjaer, P. Gromov, J. Yu, H. Palsdottir, N. Magnusson, and T. F. Orntoft. 2000. Gene expression profiling: monitoring transcription and translation products using DNA microarrays and proteomics. FEBS Lett. 480:2-16. [DOI] [PubMed] [Google Scholar]
- 7.Chandran, K., and N. G. Love. 2008. Physiological state, growth mode, and oxidative stress play a role in Cd(II)-mediated inhibition of Nitrosomonas europaea 19718. Appl. Environ. Microbiol. 74:2447-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosa, J. H. 1997. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol. Mol. Biol. Rev. 61:319-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick, G. J., J. W. Torpey, T. J. Beveridge, and B. M. Tebo. 2008. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl. Environ. Microbiol. 74:1527-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ely, R. L., M. R. Hyman, D. J. Arp, R. B. Guenther, and K. J. Williamson. 1995. A cometabolic kinetics model incorporating enzyme inhibition, inactivation, and recovery. 2: Trichloroethylene degradation experiments. Biotechnol. Bioeng. 46:232-245. [DOI] [PubMed] [Google Scholar]
- 11.Ensign, S. A., M. R. Hyman, and D. J. Arp. 1993. In vitro activation of ammonia monooxygenase from Nitrosomonas europaea by copper. J. Bacteriol. 175:1971-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferianc, P., A. Farewell, and T. Nystrom. 1998. The cadmium-stress stimulon of Escherichia coli K-12. Microbiology 144:1045-1050. [DOI] [PubMed] [Google Scholar]
- 13.Grizot, S., and S. K. Buchanan. 2004. Structure of the OmpA-like domain of RmpM from Neisseria meningitidis. Mol. Microbiol. 51:1027-1037. [DOI] [PubMed] [Google Scholar]
- 14.Groeneweg, J., B. Sellner, and W. Tappe. 1994. Ammonia oxidation in Nitrosomonas at NH3 concentrations near Km: effects of pH and temperature. Water Res. 28:2561-2566. [Google Scholar]
- 15.Gvakharia, B. O., E. A. Permina, M. S. Gelfand, P. J. Bottomley, L. A. Sayavedra-Soto, and D. J. Arp. 2007. Global transcriptional response of Nitrosomonas europaea to chloroform and chloromethane. Appl. Environ. Microbiol. 73:3440-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtan-Hartwig, L., M. Bechmann, T. R. Høyås, R. Linjordeta, and L. R. Bakkenb. 2002. Heavy metals tolerance of soil denitrifying communities: N2O dynamics. Soil Biol. Biochem. 34:1181-1190. [Google Scholar]
- 17.Horn, J. M., M. Brunke, W. D. Deckwer, and K. N. Timmis. 1994. Pseudomonas putida strains which constitutively overexpress mercury resistance for biodetoxification of organomercurial pollutants. Appl. Environ. Microbiol. 60:357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh, J., A. J. Andrews, and C. A. Fierke. 2004. Roles of protein subunits in RNA-protein complexes: lessons from ribonuclease P. Biopolymers 73:79-89. [DOI] [PubMed] [Google Scholar]
- 19.Hu, P., E. L. Brodie, Y. Suzuki, H. H. McAdams, and G. L. Andersen. 2005. Whole-genome transcriptional analysis of heavy metal stresses in Caulobacter crescentus. J. Bacteriol. 187:8437-8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, Z., K. Chandran, D. Grasso, and B. F. Smets. 2004. Comparison of nitrification inhibition by metals in batch and continuous flow reactors. Water Res. 38:3949-3959. [DOI] [PubMed] [Google Scholar]
- 21.Hu, Z., K. Chandran, D. Grasso, and B. F. Smets. 2002. Effect of nickel and cadmium speciation on nitrification inhibition. Environ. Sci. Technol. 36:3074-3078. [DOI] [PubMed] [Google Scholar]
- 22.Hyman, M. R., and D. J. Arp. 1995. Effects of ammonia on the de novo synthesis of polypeptides in cells of Nitrosomonas europaea denied ammonia as an energy source. J. Bacteriol. 177:4974-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman, M. R., S. A. Russell, R. L. Ely, K. J. Williamson, and D. J. Arp. 1995. Inhibition, inactivation, and recovery of ammonia-oxidizing activity in cometabolism of trichloroethylene by Nitrosomonas europaea. Appl. Environ. Microbiol. 61:1480-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imlay, J. A., and S. Linn. 1986. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J. Bacteriol. 166:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaacs, F. J., D. J. Dwyer, C. Ding, D. D. Pervouchine, C. R. Cantor, and J. J. Collins. 2004. Engineered riboregulators enable post-transcriptional control of gene expression. Nat. Biotechnol. 22:841-847. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, G. D., and J. S. Bond. 1999. Cell-associated metalloproteinases. Birkhäuser Verlag, Basel, Switzerland.
- 27.Juliastuti, S. R., J. Baeyens, C. Creemers, D. Bixio, and E. Lodewyckx. 2003. The inhibitory effects of heavy metals and organic compounds on the net maximum specific growth rate of the autotrophic biomass in activated sludge. J. Hazard Mater. 100:271-283. [DOI] [PubMed] [Google Scholar]
- 28.Koller, L. D. 1998. Immunotoxicology of environmental and occupational metals. CRC Press, Boca Raton, FL.
- 29.Le Roch, K. G., J. R. Johnson, L. Florens, Y. Zhou, A. Santrosyan, M. Grainger, S. F. Yan, K. C. Williamson, A. A. Holder, D. J. Carucci, J. R. Yates III, and E. A. Winzeler. 2004. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 14:2308-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung, W. C., M.-F. Wong, H. Chua, W. Lo, P. H. F. Yu, and C. K. Leung. 2000. Removal and recovery of heavy metals by bacteria isolated from activated sludge treating industrial effluents and municipal wastewater. Water Sci. Technol. 41:233-240. [Google Scholar]
- 31.Liebert, C. A., R. M. Hall, and A. O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim, H., K. Kim, D. Han, J. Oh, and Y. Kim. 2007. Crystal structure of TTC0263, a thermophilic TPR protein from Thermus thermophilus HB27. Mol. Cells 24:27-36. [PubMed] [Google Scholar]
- 33.Madoni, P., D. Davoli, and L. Guglielmi. 1999. Response of SOUR and AUR to heavy metal contamination in activated sludge. Water Res. 33:2459-2464. [Google Scholar]
- 34.Mindlin, S., G. Kholodii, Z. Gorlenko, S. Minakhina, L. Minakhin, E. Kalyaeva, A. Kopteva, M. Petrova, O. Yurieva, and V. Nikiforov. 2001. Mercury resistance transposons of gram-negative environmental bacteria and their classification. Res. Microbiol. 152:811-822. [DOI] [PubMed] [Google Scholar]
- 35.Mindlin, S., L. Minakhin, M. Petrova, G. Kholodii, S. Minakhina, Z. Gorlenko, and V. Nikiforov. 2005. Present-day mercury resistance transposons are common in bacteria preserved in permafrost grounds since the Upper Pleistocene. Res. Microbiol. 156:994-1004. [DOI] [PubMed] [Google Scholar]
- 36.Nazaret, S., W. H. Jeffrey, E. Saouter, R. Von Haven, and T. Barkay. 1994. merA gene expression in aquatic environments measured by mRNA production and Hg(II) volatilization. Appl. Environ. Microbiol. 60:4059-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nies, D. H. 1999. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51:730-750. [DOI] [PubMed] [Google Scholar]
- 38.Park, S., and R. L. Ely. 2008. Genome-wide transcriptional responses of Nitrosomonas europaea to zinc. Arch. Microbiol. 189:541-548. [DOI] [PubMed] [Google Scholar]
- 39.Peirson, S. N., J. N. Butler, and R. G. Foster. 2003. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 31:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Principi, P., F. Villa, M. Bernasconi, and E. Zanardini. 2006. Metal toxicity in municipal wastewater activated sludge investigated by multivariate analysis and in situ hybridization. Water Res. 40:99-106. [DOI] [PubMed] [Google Scholar]
- 41.Radniecki, T. S., and R. L. Ely. 2008. Zinc chloride inhibition of Nitrosococcus mobilis. Biotechnol. Bioeng. 99:1085-1095. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, I., P. J. Steenbakkers, H. J. op den Camp, K. Schmidt, and M. S. Jetten. 2004. Physiologic and proteomic evidence for a role of nitric oxide in biofilm formation by Nitrosomonas europaea and other ammonia oxidizers. J. Bacteriol. 186:2781-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Throback, I. N., M. Johansson, M. Rosenquist, M. Pell, M. Hansson, and S. Hallin. 2007. Silver (Ag+) reduces denitrification and induces enrichment of novel nirK genotypes in soil. FEMS Microbiol. Lett. 270:189-194. [DOI] [PubMed] [Google Scholar]
- 44.U.S. Environmental Protection Agency. 1993. Process design manual: nitrogen control. Report 625/R-93/010. U.S. Environmental Protection Agency, Washington, DC.
- 45.Wang, A., and D. E. Crowley. 2005. Global gene expression responses to cadmium toxicity in Escherichia coli. J. Bacteriol. 187:3259-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson, S. W., F. W. Valois, and J. B. Waterbury. 1981. The family Nitrobacteraceae, p. 1005-1022. In M. P. Starr, J. Stolp, H. G. Truper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, New York, NY.
- 47.Wei, X., L. A. Sayavedra-Soto, and D. J. Arp. 2004. The transcription of the cbb operon in Nitrosomonas europaea. Microbiology 150:1869-1879. [DOI] [PubMed] [Google Scholar]
- 48.Wei, X., T. Yan, N. G. Hommes, X. Liu, L. Wu, C. McAlvin, M. G. Klotz, L. A. Sayavedra-Soto, J. Zhou, and D. J. Arp. 2006. Transcript profiles of Nitrosomonas europaea during growth and upon deprivation of ammonia and carbonate. FEMS Microbiol. Lett. 257:76-83. [DOI] [PubMed] [Google Scholar]