Abstract
The transmission of Yersinia pseudotuberculosis in the pork production chain was followed from farm to slaughterhouse by studying the same 364 pigs from different production systems at farm and slaughterhouse levels. In all, 1,785 samples were collected, and the isolated Y. pseudotuberculosis strains were analyzed by pulsed-field gel electrophoresis. The results of microbial sampling were combined with data from an on-farm observation and questionnaire study to elucidate the associations between farm factors and the prevalence of Y. pseudotuberculosis. Following the same pigs in the production chain from farm to slaughterhouse, we were able to show similar Y. pseudotuberculosis genotypes in live animals, pluck sets (containing tongue, tonsils, esophagus, trachea, heart, lungs, diaphragm, liver, and kidneys), and carcasses and to conclude that Y. pseudotuberculosis contamination originates from the farms, is transported to slaughterhouses with pigs, and transfers to pluck sets and carcasses in the slaughter process. The study also showed that the high prevalence of Y. pseudotuberculosis in live pigs predisposes carcasses and pluck sets to contamination. When production types and capacities were compared, the prevalence of Y. pseudotuberculosis was higher in organic production than in conventional production and on conventional farms with high rather than low production capacity. We were also able to associate specific farm factors with the prevalence of Y. pseudotuberculosis by using a questionnaire and on-farm observations. On farms, contact with pest animals and the outside environment and a rise in the number of pigs on the farm appear to increase the prevalence of Y. pseudotuberculosis.
Yersinia pseudotuberculosis is a food-borne pathogen that can cause serious illness in humans (15, 29). Symptoms of the illness, which include fever and acute abdominal pain caused by mesenteric lymphadenitis, are often clinically indistinguishable from those of acute appendicitis (32, 33). Systemic complications, such as erythema nodosum and reactive arthritis, are also relatively common (13, 15). In the last few years, the incidence of Y. pseudotuberculosis per 100,000 inhabitants in Finland has been 0.6 to 5 (20-22). Recent Y. pseudotuberculosis outbreaks in Finland have been linked to vegetables, e.g., carrots and iceberg lettuce (15, 16, 29), but worldwide milk, water, and pork have also been suspected sources of Y. pseudotuberculosis infections (10, 28, 36).
Y. pseudotuberculosis is frequently found in tonsils and intestinal contents of clinically healthy pigs at slaughterhouses around the world. The prevalence of Y. pseudotuberculosis in the tonsils and intestinal contents of fattening pigs has ranged from 0.03% to 6% (3, 11, 25, 38) and from 0.6% to 3% (3, 12, 19, 34, 35, 37), respectively. Y. pseudotuberculosis has also been isolated from pork (6), indicating a possible route from pigs to humans.
Differences in pig husbandry practices can affect the prevalence of such pathogens as Yersinia. The prevalence of Yersinia enterocolitica has been shown to be higher in specialized slaughter pig production than in conventional farrow-to-finish production and in conventional production than in organic production (27, 31). Because Y. pseudotuberculosis has been isolated from soil and a multitude of wild animals (8, 9, 24, 26), the prevalence of Y. pseudotuberculosis may be increased in production systems, such as organic pig production, where pigs have contact with the outside environment.
Pigs are often asymptomatic carriers of Y. pseudotuberculosis, and infected pigs cannot immediately be identified in the slaughter process. Understanding factors affecting the prevalence of Y. pseudotuberculosis is therefore important in order to identify potential measures to control the occurrence of Y. pseudotuberculosis both on farms and at slaughterhouses. To this end, we followed 364 individual fattening pigs from the farms to the slaughterhouse and showed using pulsed-field gel electrophoresis analysis of isolated strains that carcasses and pluck sets are contaminated with Y. pseudotuberculosis strains that pigs acquired on the farms. We also analyzed the associations between different farm factors and the prevalence of Y. pseudotuberculosis in different pig production systems at farm and slaughterhouse levels.
MATERIALS AND METHODS
Sampling.
A total of 15 farms, five organic (median, 338 fattening pigs), five conventional with production capacity of under 1,000 fattening pigs (median, 350) per year, and five conventional farms with production capacity of 1,000 fattening pigs or more (median, 2,600) per year, were selected from southwestern Finland and sampled between June 2003 and January 2005. The organic farms are registered as organic and inspected according to European Union regulations (2). On each farm, 21 to 26 pigs were sampled for Y. pseudotuberculosis. Samples were collected at the farm and slaughterhouse, and all samples were logistically connected to the corresponding pig. At the farm, rectal swabs were obtained from pigs, and the pigs were ear tagged for further sampling at the slaughterhouse. The time between farm and slaughterhouse sampling was 1 to 2 weeks. At the slaughterhouse, intestinal content, tonsils, pluck set (containing tongue, tonsils, esophagus, trachea, heart, lungs, diaphragm, liver, and kidneys), and carcass swabs were collected after meat inspection. Rectal swabs were obtained using sterile cotton wool sticks, and the samples were transferred into tubes containing 10 ml of PMB (phosphate-buffered saline supplemented with 1% mannitol and 0.15% bile salts). Intestinal content was collected from an incision into a bowel with a sterile spoon, and pluck sets were sampled by swabbing lungs, heart, liver, and kidneys with a 7.5- by 7.5-cm gauze square moistened with 10 ml of peptone water. Peptone water-moistened gauze was also used for swabbing thoracic and pelvic cavities of both halves of the carcass. Samples were stored cold during transportation and delivered to the laboratory on the same day. Analyses were started immediately after delivery or on the following day.
Determination of Y. pseudotuberculosis.
A total of 1,785 microbiological samples were examined using two different enrichment methods. Samples were examined using selective enrichment in irgasan-ticarcillin-potassium chloride (ITC broth base [Merck, Darmstadt, Germany] supplemented with ticarcillin and irgasan [Abtek Biologicals Ltd., United Kingdom] and 1 mg/ml KClO3) broth and cold enrichment in PMB for 7 and 14 days. In brief, samples were diluted 1:9 in PMB and mixed thoroughly. A 1-ml volume of PMB was inoculated in 9 ml of ITC broth and incubated at 25°C for 2 to 3 days. PMB broth was cold enriched at 4°C for 7 and 14 days. Alkali treatment (0.5 ml of the sample mixed with 4.5 ml of 0.25% KOH solution for 20 s before cultivation) was used after 14 days of cold enrichment. A quantity of 100 μl of sample was streaked after each enrichment step onto a cefsulodin-irgasan-novobiocin (CIN) agar plate (Yersinia Selective Agar Base and Yersinia Selective Supplement; Oxoid, Basingstoke, United Kingdom) and incubated at 30°C for 18 to 20 h and further at 22°C for 24 h. From each CIN agar plate, one to five suspect colonies were streaked onto tryptic soy agar (Difco, Maryland) plates for pure culture. All isolates on tryptic soy agar plates were tested for urea hydrolysis using a urea agar slant, and isolates that hydrolyzed urea were identified and tested for pathogenicity using a PCR assay targeting the chromosomal virulence gene inv and the virF gene in a virulence plasmid (pYV) according to the method of Nakajima et al. (18) with modifications as described by Niskanen et al. (25). All inv- and virF-positive isolates were identified as pathogenic Y. pseudotuberculosis and further tested using the API 20 E test (BioMérieux, Marcy l'Etoile, France) according to the manufacturer's instructions, with the exception of incubation at 25°C for 18 to 20 h.
Serotyping.
Isolates were serotyped with slide agglutination using commercial O:1 to O:6 antisera (Denka Seiken, Tokyo, Japan).
PFGE.
A total of 286 isolates were characterized by pulsed-field gel electrophoresis (PFGE) as described by Niskanen et al. (25) using SpeI and NotI restriction enzymes (New England Biolabs, Ipswich, MA). One isolate from each isolation step, i.e., one to three isolates per sample, was characterized, except on farm A, where all isolates were analyzed. Restriction patterns were analyzed visually and with the assistance of BioNumerics (Applied Maths, Sint-Martens-Latem, Belgium) version 4.61. Applied Dice coefficient correlation was applied to identify similarities among PFGE types, and a dendrogram was constructed with the unweighted-pair group method using arithmetic averages. The position tolerance was set to 1.40%, with the optimization value at 1.50%.
Association between Y. pseudotuberculosis and farm factors.
Data on farm management practices were collected from the farms with a questionnaire and on-farm observations, as described earlier (30), to assess different features associated with the presence of Y. pseudotuberculosis in pigs. SPSS 12.0.1 (SPSS Inc., Chicago, IL) was used to calculate correlations between farm factors and the prevalence of Y. pseudotuberculosis-positive pigs on different farms. In addition, a two-level (farm and pig) multivariate logistic regression model was constructed with MLwiN 2.02 (Centre for Multilevel Modeling, University of Bristol, United Kingdom). Building on the finding that fattening pigs can carry Y. enterocolitica in tonsils without shedding it into feces at the farm (23) and assuming that the same applies to Y. pseudotuberculosis, the association between Y. pseudotuberculosis and farm factors was tested with combined on-farm rectal swab results and tonsil results from the slaughterhouse. A pig was considered positive if either or both the rectal and tonsil samples were positive. A pig was excluded from analyses if either the rectal or the tonsil sample was missing. A total of 14 pigs were excluded from the analyses.
RESULTS
Altogether 103 of 1,785 samples investigated (6%) were Y. pseudotuberculosis positive including farm and slaughterhouse samples (Tables 1 and 2). Y. pseudotuberculosis was isolated from 62 pigs (17%) in either rectal, intestinal, or tonsil samples. Y. pseudotuberculosis was isolated from six farms (40%), i.e., 3 of 10 conventional (30%) and three of five organic (60%) farms. The within-farm prevalence of Y. pseudotuberculosis on all farms in rectal swabs, intestinal contents, tonsils, pluck sets, and carcass samples varied from 0% to 56%, 0% to 52%, 0% to 75%, 0% to 21%, and 0% to 38%, respectively.
TABLE 1.
Yersinia pseudotuberculosis in conventionally and organically produced pigs at farm level
| Production type | Value for rectal swab samples
|
|||
|---|---|---|---|---|
| n | No. (%) positive | 95% CIa
|
||
| Random samplingb | Clustering at farmsc | |||
| Organic | 121 | 23 (19) | 12-27 | 5-48 |
| Conventional (total) | 243 | 6 (3) | 1-6 | 0-14 |
| High capacityd | 125 | 6 (5) | 2-11 | 0-27 |
| Low capacitye | 118 | 0 (0) | 0-3 | NCf |
| Total | 364 | 29 (8) | 6-12 | 2-21 |
CI, confidence interval.
Ninety-five percent confidence interval of prevalence when sampling was assumed to be randomized (exact binomial estimates).
Ninety-five percent confidence interval of prevalence when the fact that 21 to 26 pigs were sampled from each farm was considered (Fleiss quadratic 95% confidence interval).
Production capacity of 1,000 fattening pigs or more per year.
Production capacity of fewer than 1,000 fattening pigs per year.
NC, could not be calculated.
TABLE 2.
Yersinia pseudotuberculosis in conventionally and organically produced pigs at slaughterhouse level
| Production type | Intestinal content
|
Tonsil
|
Pluck set
|
Carcass
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | No. (%) pos.b | 95% CIa
|
n | No. (%) pos. | 95% CI
|
n | No. (%) pos. | 95% CI
|
n | No. (%) pos. | 95% CI
|
|||||
| Random samplingc | Clustering at farmsd | Random samplingc | Clustering at farmsd | Random samplingc | Clustering at farmsd | Random samplingc | Clustering at farmsd | |||||||||
| Organic | 119 | 11 (9) | 5-16 | 2-31 | 119 | 28 (24) | 17-33 | 4-62 | 120 | 5 (4) | 1-9 | 0-24 | 120 | 10 (8) | 4-15 | 1-37 |
| Conventional (total) | 239 | 13 (5) | 3-9 | 0-28 | 231 | 6 (3) | 1-6 | 1-9 | 234 | 1 (0.4) | 0-2 | 0-3 | 239 | 0 (0) | 0-2 | NCe |
| High capacityf | 121 | 13 (11) | 6-19 | 0-49 | 117 | 4 (3) | 1-9 | 0-18 | 119 | 0 (0) | 0-3 | NC | 121 | 0 (0) | 0-3 | NC |
| Low capacityg | 118 | 0 (0) | 0-3 | NC | 114 | 2 (2) | 0-6 | 0-6 | 115 | 1 (1) | 0-6 | 0-6 | 118 | 0 (0) | 0-3 | NC |
| Total | 358 | 24 (7) | 5-10 | 2-20 | 350 | 34 (10) | 7-14 | 3-27 | 354 | 6 (2) | 1-4 | 0-7 | 359 | 10 (3) | 2-5 | 0-14 |
CI, confidence interval.
Pos., positive.
Ninety-five percent confidence interval of prevalence when sampling was assumed to be randomized (exact binomial estimates).
Ninety-five percent confidence interval of prevalence when the fact that 21 to 26 pigs were sampled from each farm was considered (Fleiss quadratic 95% confidence interval).
NC, could not be calculated.
Production capacity of 1,000 fattening pigs or more per year.
Production capacity of fewer than 1,000 fattening pigs per year.
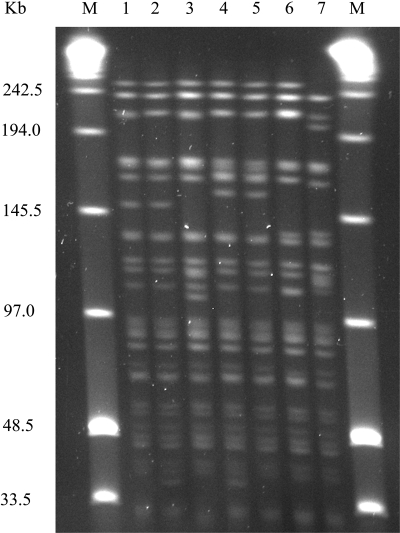
All Y. pseudotuberculosis isolates were melibiose negative and agglutinated with O:3 antiserum. Altogether seven and four different PFGE patterns were obtained from 286 Y. pseudotuberculosis isolates harboring inv and virF genes using SpeI and NotI enzymes, respectively (Fig. 1 and 2). Eight different genotypes (gI to gVIII) were gained by combining SpeI and NotI profiles. Genotypes gII and gIII were found from three and two farms, respectively (Table 3). The other genotypes were found from only one farm each. From three farms, only one genotype was recovered.
FIG. 1.
Seven different SpeI profiles (lanes 1 to 7) of Yersinia pseudotuberculosis strains obtained. M designates midrange PFGE markers.
FIG. 2.
Four different NotI profiles (lanes 1 to 4) of Yersinia pseudotuberculosis strains obtained. M designates midrange PFGE markers.
TABLE 3.
Yersinia pseudotuberculosis genotypes and numbers of positive samples from Yersinia pseudotuberculosis-positive farms
| Farm | Farm type | Genotype(s) (no. of positive samples) from sample type:
|
|||||
|---|---|---|---|---|---|---|---|
| Rectal swaba | Intestinal content | Tonsils | Pluck set | Carcass | Total | ||
| A | Organic | gII (12),A gIV (2),A,B gVII (1),B gVIII (1) | gII (8) | gII (7), gIV (1), gVIII (1) | gII (4), gIV (1) | gII (6), gIV (2), gVIII (1) | gII (36), gIV (6), gVII (1), gVIII (3) |
| D | Organic | gIII (6) | gIII (1) | gIII (17), gV (1) | gIII (1) | gIII (25), gV (1) | |
| F | Conventional | gIII (6) | gIII (13) | gII (3), gIII (1) | gII (3), gIII (20) | ||
| K | Conventional | gVI (1) | gVI (1) | ||||
| M | Organic | gI (3) | gI (2) | gI (1) | gI (6) | ||
| O | Conventional | gII (1) | gII (1) | gII (2) | |||
The same capital superscript letter indicates that the two different genotypes were isolated from the same sample.
All of the genotypes isolated from pluck sets and carcasses were also isolated from pigs of the same farm (Table 3). In addition, genotypes obtained from carcasses were also found in rectal swab, intestinal content, or tonsil samples from the same individual pigs (Table 4). Four of the six Y. pseudotuberculosis-positive pluck sets were from pigs in which the same Y. pseudotuberculosis genotype was also obtained from other samples.
TABLE 4.
Distribution of Yersinia pseudotuberculosis genotypes in carcass- or pluck set-positive pigs
| Farm and pig | Genotype(s) from sample type:
|
||||
|---|---|---|---|---|---|
| Rectal swab | Intestinal content | Tonsils | Pluck set | Carcass | |
| A | |||||
| 273 | gII | gII | gII | ||
| 274 | gIV, gVII | gIV | |||
| 275 | gII, gVIII | gII | gVIII | ||
| 276 | gII | gII | |||
| 280 | gII | ||||
| 286 | gII | gII | gII | ||
| 287 | gII, gIV | gII | gIV | gIV | |
| 289 | gII | gII | |||
| 291 | gII | gII | gII | ||
| 292 | gII | gII | gII | ||
| 297 | gII | gII | |||
| D | |||||
| 204 | gIII | gIII | |||
| O | |||||
| 304 | gII | ||||
The prevalence of Y. pseudotuberculosis was higher in all sample types in organic pork production than in conventional pork production (Tables 1 and 2). A significant difference was present in the total number of positive rectal swab (chi-square test, P < 0.001), tonsil (P < 0.001), pluck set (P < 0.010), and carcass (P < 0.001) samples between the organic and conventional production types when sampling was assumed to be randomized but not between farms from the two systems when the fact that 21 to 26 pigs were sampled from each farm was considered (Mann-Whitney U test). The prevalence of Y. pseudotuberculosis was higher in rectal swab, intestinal content, and tonsil samples on conventional farms with high production capacity (≥1,000 pigs) than on those with low production capacity (<1,000 pigs) (Tables 1 and 2). The difference between farms with low and high production capacities in conventional production was significant in the total numbers of positive rectal swab (chi-square test, P < 0.050) and intestinal content (P < 0.001) samples when sampling was assumed to be randomized but not when the fact that 21 to 26 pigs were sampled from each conventional farm was considered (Mann-Whitney U test).
In correlation and logistic regression analyses, contact with pets and pest animals and the outside environment was associated with a high prevalence of Y. pseudotuberculosis on farms (Tables 5 and 6). In correlation analyses, organic production and farm management practices, such as large group size on conventional farms and the use of troughs for drinking, were also associated with high prevalence (Table 5).
TABLE 5.
Farm factors associated with the prevalence of Yersinia pseudotuberculosis in herds with correlation analyses
| Variable | All farms
|
Conventional
|
Organic
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | ra | P | n | ra | P | n | ra | P | |
| Moderate or large numbers of rodents in the piggery | 15 | 0.931 | 0.000 | 10 | NCc | NC | 5 | 0.984 | 0.002 |
| Low stocking density (m2/pig) | 15 | 0.806 | 0.000 | 10 | −0.084 | 0.817 | 5 | 0.845 | 0.072 |
| Number of mo that pigs have access to outdoor area | 15 | 0.704 | 0.003 | 10 | NC | NC | 5 | 0.598 | 0.286 |
| Pest and pet animals hygiene scoreb | 15 | 0.673 | 0.006 | 10 | 0.278 | 0.436 | 5 | 0.628 | 0.257 |
| Slaughter pigs have access to outdoor areas | 15 | 0.647 | 0.009 | 10 | NC | NC | 5 | 0.482 | 0.411 |
| Birds have access to piggery | 15 | 0.636 | 0.011 | 10 | 0.650 | 0.050 | 5 | 0.590 | 0.295 |
| Dogs have access to piggery | 9 | 0.722 | 0.028 | 5 | −0.250 | 0.685 | 4 | 0.985 | 0.015 |
| Slaughter pigs drink from trough | 15 | 0.560 | 0.030 | 10 | 0.550 | 0.099 | 5 | 0.590 | 0.295 |
| Organic production | 15 | 0.529 | 0.043 | 10 | NC | NC | 5 | NC | NC |
| Moist area around drinker | 15 | 0 | 1.000 | 10 | 0.002 | 0.999 | 5 | 0.984 | 0.002 |
| Large group size | 15 | −0.202 | 0.471 | 10 | 0.939 | 0.047 | 5 | −0.546 | 0.341 |
Pearson correlation coefficient.
From the work of Siekkinen et al. (30).
NC, could not be calculated.
TABLE 6.
Results of a two-level multivariate logistic regression analysis for farm factors associated with the prevalence of Yersinia pseudotuberculosis in herdsa
| Variable | Estimate | Odds ratio | 95% CI of odds ratio | P |
|---|---|---|---|---|
| Constant | −4.353 | 0.01 | 0.003-0.051 | 0.000 |
| Birds have access to piggery | 2.575 | 13.13 | 1.966-87.72 | 0.008 |
| Number of mo that pigs have access to outdoor area | 0.269 | 1.31 | 1.041-1.646 | 0.021 |
Herd variance (σ2farm), 1.660 (standard error = 1.016), P value = 0.103 (Wald test); intraclass correlation coefficient = 0.492. CI, confidence interval.
Y. pseudotuberculosis was isolated from 76 samples after 7 days of cold enrichment in PMB (Table 7). An additional 27 positive samples were found after an enrichment of 14 days in PMB and alkali treatment. Y. pseudotuberculosis was isolated from 48 samples with both (7- and 14-day) cold enrichment steps. Plating onto CIN agar after ITC enrichment did not recover any new positive samples, and Y. pseudotuberculosis was isolated from only 12 samples. None of the isolation steps produced all genotypes: 7 days of enrichment produced seven of eight different genotypes (gI to gV, gVII, and gVIII) and 14 days of enrichment produced seven of eight different genotypes (gI to gVI and gVIII). ITC enrichment recovered only two genotypes (gII and gIII).
TABLE 7.
Number of Yersinia pseudotuberculosis-positive samples after cold enrichment
| Sample material | n | No. (%) of positive samples in different isolation steps
|
Total no. (%) of positive samples | |
|---|---|---|---|---|
| 7 days in PMB and CIN | 14 days in PMB, KOH, and CIN | |||
| Rectal swab | 364 | 28 (8) | 20 (5) | 29 (8) |
| Intestinal content | 358 | 20 (6) | 17 (5) | 24 (7) |
| Tonsils | 350 | 21 (6) | 22 (6) | 34 (10) |
| Pluck set | 354 | 3 (1) | 6 (2) | 6 (2) |
| Carcass | 359 | 4 (1) | 9 (3) | 10 (3) |
| Total | 1,785 | 76 (4) | 74 (4) | 103 (6) |
DISCUSSION
In this study, we were able to demonstrate the transmission of Y. pseudotuberculosis from pigs to carcasses and pluck sets by using PFGE analyses of isolated strains. On all farms where Y. pseudotuberculosis was isolated from both on-farm and slaughterhouse samples, the same genotypes were found at both farm and slaughterhouse levels, indicating that the contamination detected in the slaughterhouse originates from the farms in question. All carcass-positive pigs harbored the same Y. pseudotuberculosis genotype in either rectal, intestinal, or tonsil samples, and it is therefore likely that Y. pseudotuberculosis contamination in the carcass derives from Y. pseudotuberculosis carriage by the same pig during the fattening period. Cross-contamination at least with pluck sets at the slaughterhouse is also possible, since two of the six pluck set-positive pigs were not Y. pseudotuberculosis positive in rectal, intestinal, or tonsil samples. To our knowledge, this is the first time that farms have been demonstrated to be a source of Y. pseudotuberculosis contamination for carcasses and pluck sets. However, since not all Y. pseudotuberculosis strains from contaminated pluck sets could be linked to a certain pig, the potential role of the slaughterhouse environment in cross-contamination with Y. pseudotuberculosis should be further explored.
All of the positive carcasses originated from farms A and D, where the prevalence of Y. pseudotuberculosis in pigs (rectal swabs, intestinal content, and tonsils) was high. On the other hand, on farm F, where the prevalence was also very high, no Y. pseudotuberculosis was recovered from carcasses or pluck sets. Apparently, the high prevalence in live pigs predisposes carcasses and pluck sets to contamination, but strict hygiene practices in slaughterhouses can reduce contamination.
The prevalence of Y. pseudotuberculosis was higher in organically produced pigs than in conventionally produced pigs, but the wide range of within-farm prevalence suggests that there are some farm-specific factors that affect the prevalence of Y. pseudotuberculosis on farms, even within the same production system. These farm factors seem to include contact with animals and with the outside environment. Y. pseudotuberculosis has been isolated from many different animals and soil (8, 9, 24, 26), and some Y. pseudotuberculosis strains from wild animals, the environment, and pigs have been shown to have the same patterns in restriction endonuclease analysis of virulence plasmids (9). Pest animals and the environment are therefore a possible initial source of Y. pseudotuberculosis for pigs on farms. However, some authors have proposed that, e.g., Y. enterocolitica would more likely move from pigs to rodents than from rodents to pigs (1, 17). Irrespective of the initial source of Y. pseudotuberculosis, pest animals seem to have a substantial role in spreading and maintaining the Y. pseudotuberculosis contamination on the farm. The higher prevalence of Y. pseudotuberculosis on organic farms may be explained by the large number of pest and pet animal contacts of organically farmed pigs. Pigs in conventional farming are kept indoors year round, having fewer outdoor contacts than organically farmed pigs and therefore fewer possibilities of receiving Y. pseudotuberculosis from the environment.
Farm management practices, such as large group size on conventional farms or the use of troughs for drinking, can spread the infection from one pig to another at the piggery: pigs in a pen can contaminate the water in the trough with feces, spreading Y. pseudotuberculosis to other pigs in a manner similar to that for Salmonella strains (4, 39). Moreover, one carrier can infect numerous other pigs with Y. pseudotuberculosis in pig-to-pig contacts. Production capacity also seems to affect the prevalence of Y. pseudotuberculosis: in this study the prevalence was higher on conventional farms with high production capacity than on low-capacity farms. In a previous study (1), high within-farm prevalence of Yersinia was associated with poor hygiene conditions, but no such relationship was seen in our study or in an on-farm observation and questionnaire study by Siekkinen et al. (30), which evaluated the same organic and conventional pig farms as those in our study.
On-farm management of pet and pest animals seems to be an integral component in maintaining Y. pseudotuberculosis-negative farms. The possibility of spreading Y. pseudotuberculosis contamination via troughs and pig-to-pig contacts should also be minimized. Because of the significance of pest animals and outdoor contacts in the introduction or maintenance of Y. pseudotuberculosis contamination on farms, producing and maintaining Y. pseudotuberculosis-free pig farms, especially in an organic production system, can be difficult. Slaughter hygiene and slaughter methods are therefore important in preventing carcass contamination.
The two cold enrichment steps used were almost equally effective, and neither of the isolation steps can be omitted without a marked reduction in isolation rate. ITC enrichment performed poorly in the isolation of Y. pseudotuberculosis. ITC enrichment is part of the International Organization for Standardization standard for isolation of Y. enterocolitica (14) and has been used efficiently in isolation of this pathogen (5), but no data on the use of ITC in isolation of Y. pseudotuberculosis are available. In agreement with our results, Niskanen et al. (25) reported that all Y. pseudotuberculosis strains from tonsils were isolated after cold enrichment when direct plating, overnight enrichment in tryptic soy broth, and selective enrichment in modified Rappaport broth for 3 days were used. A possible reason for poor performance in ITC enrichment is weak growth of Y. pseudotuberculosis with strongly selective media. CIN, for example, has been shown to inhibit the growth of Y. pseudotuberculosis (7), and two selective media (ITC and CIN) may have overwhelmed the bacteria.
Following the same pigs from farm to slaughterhouse, we were able to conclude that Y. pseudotuberculosis contamination originates from the farms, is transported to slaughterhouses with pigs, and transfers to pluck sets and carcasses in the slaughter process. The prevalence of Y. pseudotuberculosis was higher in organic production than in conventional production and on conventional farms with high rather than low production capacity. We were also able to associate specific farm factors with the prevalence of Y. pseudotuberculosis by using questionnaires and on-farm observations. On farms, contact with pest animals and the outside environment and the rise in the number of pigs on the farm appear to increase the prevalence of Y. pseudotuberculosis.
Acknowledgments
This study was supported by research funding from the Ministry of Agriculture and Forestry, Finland.
We thank farmers, slaughterhouses, and the Finnish Association for Organic Farming for their cooperation in sampling. We also thank Jari Aho, Erja Merivirta, Anu Ranta-Reniers, Anu Seppänen, and Maija Summa for technical support.
Footnotes
Published ahead of print on 18 July 2008.
REFERENCES
- 1.Aldová, E., B. Skorkovský, J. Kapinus, M. Pejhovská, and G. Soukupová. 1980. On the ecology of Yersinia enterocolitica O 3. Yersinia in synanthropic animals. Zentralbl. Bakteriol. A 246:344-352. [PubMed] [Google Scholar]
- 2.Anonymous. 1991. Council regulation (EEC) no. 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs. Official Journal L 198 22/07/1991, p. 1-15.
- 3.Chiesa, C., L. Pacifico, F. Nanni, A. M. Renzi, and G. Ravagnan. 1993. Yersinia pseudotuberculosis in Italy. Attempted recovery from 37,666 samples. Microbiol. Immunol. 37:391-394. [DOI] [PubMed] [Google Scholar]
- 4.Feder, I., J. C. Nietfeld, J. Galland, T. Yeary, J. M. Sargeant, R. Oberst, M. L. Tamplin, and J. B. Luchansky. 2001. Comparison of cultivation and PCR-hybridization for detection of Salmonella in porcine fecal and water samples. J. Clin. Microbiol. 39:2477-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredriksson-Ahomaa, M., and H. Korkeala. 2003. Low occurrence of pathogenic Yersinia enterocolitica in clinical, food, and environmental samples: a methodological problem. Clin. Microbiol. Rev. 16:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukushima, H. 1985. Direct isolation of Yersinia enterocolitica and Yersinia pseudotuberculosis from meat. Appl. Environ. Microbiol. 50:710-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukushima, H., and M. Gomyoda. 1986. Growth of Yersinia pseudotuberculosis and Yersinia enterocolitica biotype 3B serotype O3 inhibited on cefsulodin-irgasan-novobiocin agar. J. Clin. Microbiol. 24:116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukushima, H., and M. Gomyoda. 1991. Intestinal carriage of Yersinia pseudotuberculosis by wild birds and mammals in Japan. Appl. Environ. Microbiol. 57:1152-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukushima, H., M. Gomyoda, S. Kaneko, M. Tsubokura, N. Takeda, T. Hongo, and F. N. Shubin. 1994. Restriction endonuclease analysis of virulence plasmids for molecular epidemiology of Yersinia pseudotuberculosis infections. J. Clin. Microbiol. 32:1410-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukushima, H., M. Gomyoda, K. Shiozawa, S. Kaneko, and M. Tsubokura. 1988. Yersinia pseudotuberculosis infection contracted through water contaminated by a wild animal. J. Clin. Microbiol. 26:584-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukushima, H., K. Hoshina, R. Nakamura, Y. Ito, and M. Gomyoda. 1987. Epidemiological study of Yersinia enterocolitica and Yersinia pseudotuberculosis in Shimane Prefecture, Japan. Contrib. Microbiol. Immunol. 9:103-110. [PubMed] [Google Scholar]
- 12.Fukushima, H., K. Maruyama, I. Omori, K. Ito, and M. Iorihara. 1989. Role of the contaminated skin of pigs in faecal Yersinia contamination of pig carcasses at slaughter. Fleischwirtschaft 69:369-372. [Google Scholar]
- 13.Hannu, T., L. Mattila, J. P. Nuorti, P. Ruutu, J. Mikkola, A. Siitonen, and M. Leirisalo-Repo. 2003. Reactive arthritis after an outbreak of Yersinia pseudotuberculosis serotype O:3 infection. Ann. Rheum. Dis. 62:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Organization for Standardization. 2003. Microbiology of food and animal feeding stuffs—horizontal method for the detection of presumptive pathogenic Yersinia enterocolitica. ISO 10273. International Organization for Standardization, Geneva, Switzerland.
- 15.Jalava, K., M. Hakkinen, M. Valkonen, U. M. Nakari, T. Palo, S. Hallanvuo, J. Ollgren, A. Siitonen, and J. P. Nuorti. 2006. An outbreak of gastrointestinal illness and erythema nodosum from grated carrots contaminated with Yersinia pseudotuberculosis. J. Infect. Dis. 194:1209-1216. [DOI] [PubMed] [Google Scholar]
- 16.Jalava, K., S. Hallanvuo, U. M. Nakari, P. Ruutu, E. Kela, T. Heinäsmäki, A. Siitonen, and J. P. Nuorti. 2004. Multiple outbreaks of Yersinia pseudotuberculosis infections in Finland. J. Clin. Microbiol. 42:2789-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko, K. I., S. Hamada, Y. Kasai, and E. Kato. 1978. Occurrence of Yersinia enterocolitica in house rats. Appl. Environ. Microbiol. 36:314-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima, H., M. Inoue, T. Mori, K. Itoh, E. Arakawa, and H. Watanabe. 1992. Detection and identification of Yersinia pseudotuberculosis and pathogenic Yersinia enterocolitica by an improved polymerase chain reaction method. J. Clin. Microbiol. 30:2484-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narucka, U., and J. F. Westendorp. 1977. Studies for the presence of Yersinia enterocolitica and Yersinia pseudotuberculosis in clinically normal pigs. Tijdschr. Diergeneeskd. 102:299-303. (In Dutch.) [PubMed] [Google Scholar]
- 20.National Public Health Institute. 2005. Infectious diseases in Finland 1995-2004. National Public Health Institute (KTL), Helsinki, Finland.
- 21.National Public Health Institute. 2006. Infectious diseases in Finland 2005. National Public Health Institute (KTL), Helsinki, Finland.
- 22.National Public Health Institute. 2007. Infectious diseases in Finland 2006. National Public Health Institute (KTL), Helsinki, Finland.
- 23.Nesbakken, T., T. Iversen, K. Eckner, and B. Lium. 2006. Testing of pathogenic Yersinia enterocolitica in pig herds based on the natural dynamic of infection. Int. J. Food Microbiol. 111:99-104. [DOI] [PubMed] [Google Scholar]
- 24.Nikolova, S., Y. Tzvetkov, H. Najdenski, and A. Vesselinova. 2001. Isolation of pathogenic yersiniae from wild animals in Bulgaria. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:203-209. [DOI] [PubMed] [Google Scholar]
- 25.Niskanen, T., M. Fredriksson-Ahomaa, and H. Korkeala. 2002. Yersinia pseudotuberculosis with limited genetic diversity is a common finding in tonsils of fattening pigs. J. Food Prot. 65:540-545. [DOI] [PubMed] [Google Scholar]
- 26.Niskanen, T., J. Waldenström, M. Fredriksson-Ahomaa, B. Olsen, and H. Korkeala. 2003. virF-positive Yersinia pseudotuberculosis and Yersinia enterocolitica found in migratory birds in Sweden. Appl. Environ. Microbiol. 69:4670-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak, B., T. V. Mueffling, K. Caspari, and J. Hartung. 2006. Validation of a method for the detection of virulent Yersinia enterocolitica and their distribution in slaughter pigs from conventional and alternative housing systems. Vet. Microbiol. 117:219-228. [DOI] [PubMed] [Google Scholar]
- 28.Nowgesic, E., M. Fyfe, J. Hockin, A. King, H. Ng, A. Paccagnella, A. Trinidad, L. Wilcott, R. Smith, A. Denney, L. Struck, G. Embree, K. Higo, J. I. Chan, P. Markey, S. Martin, and D. Bush. 1999. Outbreak of Yersinia pseudotuberculosis in British Columbia—November 1998. Can. Commun. Dis. Rep. 25:97-100. [PubMed] [Google Scholar]
- 29.Nuorti, J. P., T. Niskanen, S. Hallanvuo, J. Mikkola, E. Kela, M. Hatakka, M. Fredriksson-Ahomaa, O. Lyytikäinen, A. Siitonen, H. Korkeala, and P. Ruutu. 2004. A widespread outbreak of Yersinia pseudotuberculosis O:3 infection from iceberg lettuce. J. Infect. Dis. 189:766-774. [DOI] [PubMed] [Google Scholar]
- 30.Siekkinen, K.-M., L. Nuotio, J. Ranta, R. Laukkanen, S. Hellström, H. Korkeala, and R. Maijala. 2006. Assessing hygiene proficiency on organic and conventional pig farms regarding pork safety: a pilot study in Finland. Livestock Sci. 104:193-202. [Google Scholar]
- 31.Skjerve, E., B. Lium, B. Nielsen, and T. Nesbakken. 1998. Control of Yersinia enterocolitica in pigs at herd level. Int. J. Food Microbiol. 45:195-203. [DOI] [PubMed] [Google Scholar]
- 32.Tertti, R., K. Granfors, O. P. Lehtonen, J. Mertsola, A. L. Mäkelä, I. Välimäki, P. Hänninen, and A. Toivanen. 1984. An outbreak of Yersinia pseudotuberculosis infection. J. Infect. Dis. 149:245-250. [DOI] [PubMed] [Google Scholar]
- 33.Tertti, R., R. Vuento, P. Mikkola, K. Granfors, A. L. Mäkelä, and A. Toivanen. 1989. Clinical manifestations of Yersinia pseudotuberculosis infection in children. Eur. J. Clin. Microbiol. Infect. Dis. 8:587-591. [DOI] [PubMed] [Google Scholar]
- 34.Toma, S., and V. R. Deidrick. 1975. Isolation of Yersinia enterocolitica from swine. J. Clin. Microbiol. 2:478-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsubokura, M., K. Otsuki, T. Fukuda, M. Kubota, and M. Imamura. 1976. Studies on Yersinia pseudotuberculosis. IV. Isolation of Y. pseudotuberculosis from healthy swine. Nippon Juigaku Zasshi 38:549-552. [DOI] [PubMed] [Google Scholar]
- 36.Tsubokura, M., K. Otsuki, Y. Kawaoka, and T. Maruyama. 1984. Characterization and pathogenicity of Yersinia pseudotuberculosis isolated from swine and other animals. J. Clin. Microbiol. 19:754-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber, A., and W. Knapp. 1981. Demonstration of Yersinia enterocolitica and Yersinia pseudotuberculosis in fecal samples of healthy slaughter swine depending on the season. Zentralbl. Veterinarmed. B 28:407-413. [PubMed] [Google Scholar]
- 38.Weber, A., and W. Knapp. 1981. Seasonal isolation of Yersinia enterocolitica and Yersinia pseudotuberculosis from tonsils of healthy slaughter pigs. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 250:78-83. (In German.) [PubMed] [Google Scholar]
- 39.Zheng, D. M., M. Bonde, and J. T. Sørensen. 2007. Associations between the proportion of Salmonella seropositive slaughter pigs and the presence of herd level risk factors for introduction and transmission of Salmonella in 34 Danish organic, outdoor (nonorganic) and indoor finishing-pig farms. Livestock Sci. 106:189-199. [Google Scholar]