To attain the global goal of an environmentally sustainable society in which organic material is successfully recycled back to arable land, it is crucial to develop effective procedures for the treatment of sewage sludge. The term “sewage sludge” or “biosolids” represents the insoluble residue produced during wastewater treatment and subsequent sludge stabilization procedures, such as aerobic or anaerobic digestion (2). The composition of sludge generated from different geographical locations and individual treatment facilities varies appreciably. However, on average, a typical ton of dry sludge comprises about 80 pounds of nitrogen, 200 pounds of phosphate (P2O5), and 10 pounds of potash (K2O) (28). These substantial quantities of N and P, in combination with high levels of organic constituents supplying beneficial conditioning properties to soil, highlight the significant value of sewage sludge as a crop fertilizer. Indeed, the application of sludge to arable land enhances crop yield appreciably, commonly exceeding the effects of fertilized controls (25). In Sweden, about 1 million tons of sludge is produced annually in the municipal wastewater treatment plants (according to the Swedish Environmental Protection Agency [www.naturvardsverket.se]). While the sludge generated provides a great nutrient resource, its handling and processing remain a major challenge.
Stabilized sewage sludge intended for arable land use needs to be rigorously assessed for quality due to the high content of metals (cadmium, arsenic, copper, lead, mercury, and zinc), persistent organic pollutants (the organochlorines aldrin, dieldrin, heptachlor, dichlorodiphenyltrichloroethane, and lindane), and pathogenic microorganisms (bacteria, viruses, protozoa, and helminths) to ensure no transmission of harmful elements to humans through entry into the food chain via crops or grazing animals. According to European Union regulations (EEC 1774/2002), stabilized organic residues must be adequately treated and proven hygienically safe, prior to the application of sewage sludge to arable land. Numerous investigators have focused on the heavy metals and organic contaminants present in sludge derived from different sources. However, limited information is available on risk analyses and strategies to detect and eliminate human pathogenic bacteria. The human pathogenic bacterial constituents of sewage sludge naturally vary depending on several factors, including geographical location of the wastewater uptake area and population size. Additionally, the differences in bacterial communities are due to both spatial and temporal factors such as seasonal variations (78). Consequently, sludge from a single specific wastewater treatment cannot be approved for use on arable land based on the microbial quality assessment on isolated occasions but requires continuous observation and evaluation.
While the entire microbial community of sewage sludge is of considerable interest, the bacterial group has been most frequently assessed, mainly due to the relative ease of its analysis and quantification (16). Additionally, bacteria have epidemiological significance, making them the most interesting and well-characterized microbial group. The pathogenic bacterial level of sludge is apparently affected by the different stabilization procedures at various sewage treatment plants (77), emphasizing the importance of further development, optimization, and standardization of the existing techniques or, preferably, introduction of an additional sanitization step, established by global standards, in the processing of sewage sludge. As confirmation of the necessity for this type of treatment, published data that have been collated on the stabilization/sanitization of sewage sludge emphasize the lack of procedures to generate sludge of a sufficiently high microbial quality acceptable for arable land application. This article focuses on previous findings, identifies flaws among the standard procedures in eliminating human pathogenic bacteria, and offers suggestions for improvement. The review concludes with concrete proposals for future research opportunities, providing the next step forward in addressing the global need for the recycling of organic waste, such as sewage sludge.
PATHOGENIC BACTERIA FREQUENTLY PRESENT IN SEWAGE SLUDGE
Sewage sludge commonly contains high amounts of human pathogenic bacteria excreted in feces and urine. The enteric pathogenic bacterial constituents include Salmonella spp., Listeria spp., Escherichia coli (enterotoxigenic and enteropathogenic variants), Campylobacter spp., Clostridium spp., and Yersinia spp. (26, 48, 49, 54-56, 81, 86) (Table 1). A large proportion of these bacteria are both human pathogenic and zoonotic, meaning that they are able to cause infections in both humans and animals. Additionally, these organisms have a strong ability to persistently adapt to changes in the surrounding environment (52) for survival and can be relatively resistant to commonly employed sludge stabilization techniques (76). The health risks related to these pathogens in terms of spreading sewage sludge on arable land depend on the prior sludge treatments applied, as well as their ability to maintain virulence properties during both storage and distribution on a field used for grazing or food harvesting purposes (48, 51). These features are usually affected by surrounding environmental factors, including temperature, pH, moisture, and nutrient supply (36). Strauch (85) and the U.S. Environmental Protection Agency (89) stipulated that sewage sludge intended for use as a fertilizer on crops to be eaten raw or in contact with the public needs to be adequately treated to ensure that the pathogenic microorganisms are reduced to below detection limits (referred to as class A biosolids whereby Salmonella should be present at a most probable number of less than 3 per 4 g of dry weight solid) (16, 35). In cases where the sludge is distributed on arable land containing crops that are not consumed raw, the level of pathogenic microorganisms still requires significant reduction but is allowed to remain above detection limits (class B biosolids), necessitating restricted public access to the site, controlled animal grazing, and regulation of the time period between sludge application and crop harvesting (31, 35). No risk assessments of pathogenic microorganisms associated with the land application of sewage sludge are considered entirely complete, partly due to the culture-dependent nature of most analytical techniques used to date. For instance, certain pathogens enter the physiological stage as viable but nonculturable organisms (59); they still retain virulence factors and infectivity but are not detectable by traditional methods (75, 82). Therefore, the pathogens listed here should be considered only a subgroup of the entire bacterial community present in sewage sludge. Moreover, the actual concentrations of pathogens in sludge are probably often underestimated (82), further emphasizing the need for reliable qualitative and quantitative analytical tools for the evaluation of pathogenic microbial community compositions in sewage sludge.
TABLE 1.
Principal human pathogenic bacteria identified in municipal wastewater and sewage sludge
| Pathogen | Disease(s) and/or symptoms |
|---|---|
| Salmonella spp. | Salmonellosis, typhoid |
| Shigella spp. | Bacillary dysentery |
| Escherichia coli (enteropathogenic strains) | Gastroenteritis |
| Pseudomonas aeruginosa | Otitis externa, skin infections (opportunistic pathogen) |
| Yersinia enterocolitica | Acute gastroenteritis |
| Clostridium perfringens | Gastroenteritis (food poisoning) |
| Clostridium botulinum | Botulism |
| Bacillus anthracis | Anthrax |
| Listeria monocytogenes | Listeriosis |
| Vibrio cholera | Cholera |
| Mycobacterium spp. | Leprosy, tuberculosis |
| Leptospira spp. | Leptospirosis |
| Campylobacter spp. | Gastroenteritis |
| Staphylococcus | Impetigo, wound infections, food poisoning |
| Streptococcus | Sore throat, necrotizing fasciitis, scarlet fever |
Members of the genus Salmonella are the most extensively studied bacteria with regard to survival in sewage sludge (30, 60, 87). Interestingly, these bacteria survive for more than 1 year in this environment (64). Salmonella species are annually responsible for 1 to 2 million cases of human disease in the United States (5, 82), and all serovars of the bacterium potentially infect both humans and domestic animals (7). Danielsson (22) identified Salmonella spp. in the majority of sewage sludge samples from Swedish treatment plants, including raw and digested sludge. Specifically, Salmonella spp. were detected in 53% of the digested sludge samples (22). These bacteria have been consistently identified in several additional studies analyzing sludge (27, 33, 46, 62, 74, 78); the bacteria reportedly survived up to 16 months on grass treated with sludge in Switzerland (43), clearly highlighting the urgent need for the international development of a proper sanitization step before sewage sludge can be used as a crop fertilizer. Spore-forming bacteria, such as Clostridium spp. and Bacillus spp., grow as vegetative cells when the ambient environmental conditions are ideal (high moisture and low temperatures) but enter structures as extremely persistent endospores under poor growth conditions (7). Bacteria in the spore form present in sludge are extremely difficult to destroy using conventional sanitization procedures due to their resistance to heat and desiccation and persist in the environment for several years (76). Thus, the elimination of spore-forming bacteria in sewage sludge remains a major problem. For example, Clostridium tyrobutyricum causes big economic losses to the dairy industry (23, 76) by transferal to cows and eventually to milk via silage fertilized by digested residues (76).
As the difficulty in detection and quantification of enteric pathogens is mainly attributed to low concentrations in sludge, indicator organisms are commonly used in their place for monitoring temporal fluctuations. The presence of these indicator organisms signifies the potential existence of specific pathogenic bacteria (82). Accordingly, indicator organisms need to meet a number of criteria, such as consistent presence in large quantities in unprocessed raw sludge and comparable resistance as the corresponding pathogens to the lethal parameters of sludge treatment. Additional advantages would include ease of culture and distinguishable features from other bacteria present in the sludge. No single bacterium meets all of these criteria for predicting the existence of all pathogenic bacteria of interest (82), and, consequently, several indicator organisms are commonly employed. For instance, E. coli and Enterococcus spp. may be used as indicator bacteria (37, 45) since E. coli is closely related to Salmonella, whereas members of the Enterococcus genus are more resistant to sludge treatment processes (7, 57). Overall, the use of indicator organisms is a useful technique, albeit not optimal in view of the potential differences among indicators and pathogens. However, the development and application of novel sensitive detection and quantification molecular tools should allow direct focus on the pathogens of interest, leading to more reliable and cost- and time-effective working strategies.
PROCEDURES FOR STABILIZATION OF SEWAGE SLUDGE
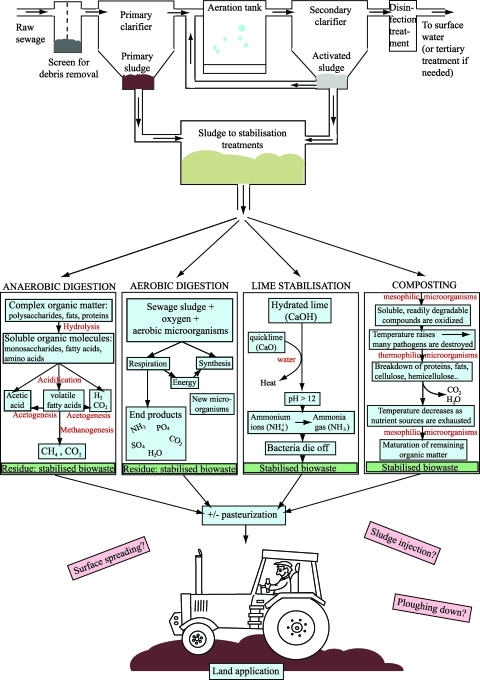
Prior to land application, sewage sludge needs to be stabilized (Fig. 1). The stabilization procedure commonly reduces organic matter and water content, emission of unpleasant odors, and concentrations of pathogenic microorganisms (82). Stabilization should result in either an end product containing pathogens below detection limits (class A) or, alternatively, sludge whereby the pathogen numbers are reduced but still detectable (class B). Common stabilization approaches include anaerobic (mesophilic or thermophilic) and aerobic digestion, lime stabilization, composting, and heat drying (39). These procedures differ dramatically in their ability to reduce the pathogenic microbial content in sewage sludge (33). Traditionally, storage of sewage sludge was applied as the sole treatment, with the aim of sanitization in terms of destroying pathogenic microorganisms, a method proven not effective (16, 37) and therefore discontinued. The most frequently used stabilization methods for sewage sludge are biological anaerobic and aerobic digestion (39). However, neither of these two procedures generates sludge that is better quality than class B grade, promoting a future shift to the use of alternative methods, such as alkaline stabilization and heat drying, to further reduce pathogen levels (39), resulting in class A sludge with fewer user restrictions. An additional promising option of producing hygienically safe material for arable recycling is to combine stabilization procedures, such as digestion, with pasteurization or liquid composting.
FIG. 1.
Processing flowchart of sewage sludge showing potential steps in the treatment procedure, with the view to yielding an end product with high microbial quality for subsequent use as a crop fertilizer.
ANAEROBIC AND AEROBIC DIGESTION
Anaerobic digestion or decomposition produces methane, carbon dioxide, and a number of other gases in small amounts, minor quantities of heat, and an end product of stabilized sludge with higher nitrogen content than that produced by aerobic digestion (61). Aerobic digestion generates carbon dioxide, ammonia, and some additional gases in small quantities, heat in large quantities, and a final sludge product (61). However, while the sewage sludge subjected to anaerobic digestion contains higher concentrations of nitrogen, aerobically digested sludge displays higher rates of N mineralization (19 to 50% and 16 to 41%, respectively) after 16 weeks of incubation at 30°C (24, 34). This finding may be explained by carbon deficiencies in anaerobically treated sludge, leading to insufficient C for the decomposing microbial biomass to mineralize the N present in the soil. The differences in N mineralization may also depend on specific groups or compounds present in the sludge, such as polyphenols (which delay mineralization by binding to N in proteins), soluble carbohydrates, or soil water content (15).
The digestion procedures are either mesophilic (30 to 38°C) or thermophilic (50 to 60°C) (82), a critical parameter in the inactivation of pathogens. Indeed, most bacteria in vegetative growth stages are inactivated during heat exposure, provided the temperature is well above the optimum growth temperature and the period of exposure is sufficient (84, 85). Thermophilic waste treatment is clearly more efficient in reducing the content of vegetative pathogenic bacteria and intestinal parasites than the mesophilic option (27, 32, 33, 57, 68, 71, 77). For instance, Olsen and Larsen (69) demonstrated that Salmonella enterica serovar Typhimurium and Mycobacterium paratuberculosis are inactivated within 24 h in a thermophilic anaerobic digester, but the process takes weeks and months in its mesophilic counterpart (69).
A major advantage of anaerobic over aerobic digestion is that methane and carbon dioxide (biogas) are generated as end products, thus supplying the energy needs of the treatment facility (11). Biogas normally contains about 60 to 70% methane, 30 to 40% carbon dioxide, and small amounts of other gases, including ammonia, hydrogen sulfide, and mercaptans (42), making it an extremely valuable gas that is rich in easily extractable energy. Additionally, anaerobic digestion does not require input of air or oxygen into the system, which is extremely cost-effective in relation to sludge treatment systems requiring oxygen (70).
Kearney et al. (50) utilized mesophilic anaerobic digestion to analyze the survival of pathogenic bacteria in animal waste. The group found that viable numbers of E. coli, S. enterica serovar Typhimurium, Yersinia enterocolitica, Listeria monocytogenes, and Campylobacter jejuni were reduced during processing and that indigenous bacterial strains survived better than laboratory strains, consistent with previous results (65, 69). Y. enterocolitica was the least resistant species to anaerobic digestion (time necessary to inactivate 90% of the population, 18.2 days), whereas C. jejuni was the most resistant (time necessary to inactivate 90% of the population, 438.6 days) (50), suggesting variations in the susceptibility to disinfection among the different bacterial species. Sahlström and colleagues (77) confirmed the unsuitability of sewage sludge produced in Swedish treatment plants for arable land due to its relatively high pathogenic bacterial content, irrespective of prior stabilization procedures, including thermophilic/mesophilic anaerobic digestion, composting, and sedimentation. These results are consistent with those reported by Jepsen et al. (46), who concluded that aerobic stabilization does not reduce the pathogens and indicator organisms to levels that are acceptable for the unrestricted use of sludge in agriculture. The data (46, 77) emphasize the emerging need for an additional sanitization step during sludge stabilization to achieve a product with sufficiently high microbial quality to enable recycling of organic residues.
CHEMICAL TREATMENT
Lime stabilization is an interesting alternative to anaerobic and aerobic digestion (21), mainly due to its cost-effective and functional nature. Hydrated lime (calcium hydroxide) is added to liquid sewage sludge at a concentration sufficient to raise the pH to 12.0 for at least 2 h. At pH 12.0, NH4+ ions present in the sludge are deprotonated, generating ammonia gas (82) that acts bactericidally by diffusing through the cell membranes of microorganisms (63). The combination of high pH and ammonia reduces the presence of coliform indicator bacteria by 2 to 7 orders of magnitude (18) and fecal streptococci indicator bacteria to a minor extent (18, 82). Several studies validate the necessity of stable pH at 12.0 for 20 to 60 days for effective Salmonella elimination from sewage sludge (1, 72), potentially classifying lime stabilization as a relatively time-consuming treatment option. In contrast, Strauch (83) reported elimination of Salmonella within 24 h at a stable pH of 10. The author concluded that removal of these pathogens depends on the pH obtained, period of liming activity, and dryness of the sludge (83). In keeping with previous data, Bina et al. (10) showed that the microbial quality of sewage sludge met the requirements for class B within 2 h at pH 12, whereas class A sludge was obtained for Salmonella and fecal coliforms after 2 and 24 h, respectively, at the same pH (10). An alternative to hydrated lime is quicklime (calcium oxide), which produces an exothermic reaction with water (67). The release of heat commonly elevates the temperature of the sludge to 70°C, comparable to that obtained during pasteurization.
An additional advantage of lime stabilization is that the high pH potentially precipitates most metals present in the sludge, thereby reducing their solubility and mobility. Free calcium ions provided by hydrated lime form complexes with odorous sulfur species, such as hydrogen sulfide and organic mercaptans, resulting in sludge with less odor. Additionally, lime induces an increase in the solid content, making the sludge easier to handle and store (66).
Vinnerås and coworkers (92) evaluated the effects of urea or peracetic acid (PAA) on pathogens in fecal material and showed that urea provides an efficient disinfection climate against E. coli, Enterococcus spp., and Salmonella spp. within 3 weeks. On the other hand, PAA reduced the viable fraction of all the bacteria within 12 h after application, including the vegetative fraction of Clostridia spp. To avoid bacterial regrowth in PAA-treated sludge, the treatment needs to be performed immediately prior to distribution on soil. In the case of urea, no ammonia is consumed during treatment, thereby avoiding the risk of bacterial regrowth. Despite the inconsistent results reported for these stabilization techniques, the chemicals involved are cheap and easily accessible, do not harm the environment, and even improve the fertilization effects of sludge (14, 91, 92). Thus, chemical stabilization methods require further investigation, potentially in combination with other promising procedures, for evaluation of their potential as effective sewage sludge sanitization treatments.
COMPOSTING
In composting, liquid sludge is treated with a bulking agent, such as wood chips, dry compost, or municipal refuse (82). Indigenous microorganisms in the compost pile oxidize the utilizable substrates present in sludge, leading to an extreme temperature increase, particularly in the center of the pile (up to 60°C or higher) (6, 70, 82). The temperature of the compost pile decreases to ambient as soon as the nutrient sources are exhausted and the organic content of sludge has been mineralized to CO2 and H2O or converted to humic-like substances (82). There are several different composting procedures, and the results yielded are not necessarily comparable, making it difficult to assess the effectiveness of this technique for eliminating human pathogenic bacteria in sewage sludge. However, the main factors controlling pathogen inactivation are temperature and time (82), highlighting the importance of homogenized compost reaching high temperatures, both in the central part of the pile and the edge. Additionally, the microbial inactivation procedure is affected by other agents, such as ammonia, organic constituents, dissolved solids, and hydroxide anions (91). Reduction of the number of pathogenic bacteria in compost may also potentially depend on biological control (e.g., antagonism) or competition between bacterial species present in the pile. Hussong et al. (44) observed an increase in the level of Salmonella in sludge treated with irradiated compost compared to that treated with nonirradiated compost. The authors suggested that the differences in Salmonella levels could be explained by competing activities among microorganisms within the compost. Additionally, a number of lactic acid bacteria inhibit several human pathogenic bacteria present in sewage sludge (58), an observation that further indicates the significant potential of composting with standard microbiological agents, such as antibacterial metabolites. In another study, Christensen et al. (17) analyzed the survival of E. coli, Enterococcus faecalis (indicators), and Salmonella during composting performed in four countries (Denmark, Sweden, Norway, and Finland). The mean temperatures of the composting procedures in Denmark, Sweden, Norway, and Finland were 50 to 66°C, 45 to 74°C, 62 to 66°C, and 43 to 57°C, respectively. E. coli concentrations were reduced in all facilities, in general, by 4.9 to 6.6 log units. The concentration of Enterococcus was reduced in all processes (within the range of 4.1 to 5.7 log units). Salmonella was detected in the input material in all four countries but remained in the end product only in the Swedish facility (two out of five treated compost samples were positive), signifying the importance of a constant high temperature in the compost batch (17, 47). It appears that composting is a fairly effective sanitization procedure, provided the temperature reaches the desired level, is maintained for a sufficient period of time, and remains consistently high throughout the entire compost pile.
PASTEURIZATION
Pasteurization of biowaste at 70°C for at least 1 h is an effective approach to eliminate most pathogens (9, 12). For example, Salmonella is killed within 30 min in sludge heated up to 70°C (7, 8, 64). Ideally, pasteurization should be included either before or after the regular stabilization procedure (digestion, composting, or liming) to obtain a product suitable for use as a crop fertilizer. However, bacterial endospores present in sewage sludge (Clostridium spp. and Bacillus spp.) are not destroyed using standard pasteurization procedures (7, 64), and the potential presence of these bacteria requires evaluation prior to the field application of sludge. At least two separate rounds of pasteurization are needed to eliminate endospores, an expensive procedure. Primary heating should activate the spores into vegetative forms, which eventually start to germinate and grow. The secondary pasteurization step should kill these heat-labile bacteria, and the incubation period between the two pasteurization steps needs to be sufficiently short to prevent the formation of new endospores. Alternatively, irradiation technology (19, 20, 79, 90) for eliminating pathogenic bacteria and bacterial endospores in sewage sludge should be further assessed to determine whether it presents an effective alternative to pasteurization. Most endospore-forming bacterial species are already indigenously present in soil, and thus the issue of whether application of treated sewage sludge actually imposes an increased risk requires further investigation.
An additional aspect of pathogen contamination of sewage sludge is the potential regrowth of organisms from only partially disinfected sludge or recontamination of highly disinfected sludge (93). In the latter case, biowaste transport to and from the waste treatment facilities is a huge problem, since the same trucks are used for transportation of both fresh manure and sanitized sludge, presenting a considerable risk for microbial recontamination of treated sludge (7). Regrowth, on the other hand, occurs mainly within composting treatments where additives, such as wood chips and rice hulls, have been added. These materials are rich in soluble sugars and may therefore act as nutrient sources (33, 38, 40, 80). Additionally, studies monitoring enteropathogenic microorganisms in sludge applied to soil associate regrowth with rainy periods (33, 41) or hot weather (29, 33).
Overall, pasteurization is an efficient sanitization option although the method fails to eliminate bacterial endospores. Regardless of the effectiveness of sanitization, it is important to be aware of the basic microbial principles when organizing and handling biological products like sewage sludge in order to minimize bacterial recontamination and regrowth. Additionally, a drawback of pasteurization is its cost. Specifically, the heating step usually occurs with steam or a heat exchanger (7) and requires large amounts of energy, further highlighting the benefits of applying anaerobic digestion in combination with pasteurization, which would result in the generation of energy-rich biogas in the same facility.
NOVEL MOLECULAR TOOLS FOR THE EVALUATION OF PATHOGENIC BACTERIAL FATE
The microbial risks associated with sewage sludge spread on crop fields are frequently determined by detection of classical indicator bacteria. However, most recently identified pathogenic bacteria are scarcely related to the standard indicators. For example, Krovacek et al. (53) identified Aeromonas spp. as a representative new indicator bacterium and highlighted the ineffectiveness of fecal coliforms frequently used as indicator bacteria at present. Additionally, Rose (73) claimed that the negligible correlation between the amount of coliform bacteria present in water and the presence of enteric protozoa presents a problem. Nevertheless, the use of indicator bacteria would not be necessary if the methods used for detection and evaluation of pathogens had higher sensitivity. In this case, the actual pathogenic bacteria could be targeted in direct assays. To increase the possibility that specific bacteria in a sample reach the detection limit, the technique can be combined with prior selective enrichment of the bacteria of interest although this method is more expensive.
Evaluation of the microbial quality of sludge should greatly benefit from the application of new molecular approaches, which would provide valuable information on bacterial community structures, metabolic activities, and correlation of these parameters to the effectiveness of individual stabilization methods. Additionally, such molecular tools should aid in clarifying the fate of pathogenic bacteria in soil to which sludge is applied in terms of survival, leakage, and spread both to groundwater and surrounding crops. This information may eventually facilitate the development of reliable quantitative microbiological risk assessments/models that are invaluable in the prediction of the potential risks of land application of sewage sludge. Molecular methods also have a big advantage over traditional assays in terms of independence from cultivation of the bacteria of interest, resulting in an improved representative picture of a community, including the “viable but nonculturable” fraction. PCR, a technique that has been extensively used for environmental samples during the last 2 decades, is both sensitive and specific. However, a major drawback of PCR is its inability to differentiate between DNA derived from viable and nonviable organisms. For instance, DNA of organisms destroyed during sanitization treatments is still detectable in subsequent PCRs. One way to circumvent the detection of nonviable bacteria is bromodeoxyuridine (BrdU) incorporation (3, 4, 13, 88) prior to PCR with genus- or species-specific primers. The BrdU immunocapture approach permits the identification of microbial populations that grow under specific conditions and may be a good alternative for establishing the fate of specific bacteria that react differently to various sanitization treatments and for evaluating the efficiency of stabilization approaches. However, it is important to bear in mind that nonreplicating bacteria can still be activated when sludge is spread on soil, which provides an additional supply of nutrients and altered environmental conditions, confirming a need to evaluate the total bacterial community before sludge is distributed on crop fields.
Kearney et al. (52) determined the metabolic activity of a number of enteropathogenic bacterial strains by assessing their adenylate energy charge ratios and ability to incorporate [3H]thymidine. E. coli, S. enterica serovar Typhimurium, Y. enterocolitica, L. monocytogenes, and C. jejuni were analyzed within culture bags in laboratory anaerobic digesters. All bacteria were maintained at constant population levels after a period of anaerobic digestion and responded quickly in terms of growth on a fresh supply of nutrients. The authors suggest that the bacteria enter transient states between different stages of growth due to the fluctuating levels of nutrients during anaerobic digestion (52).
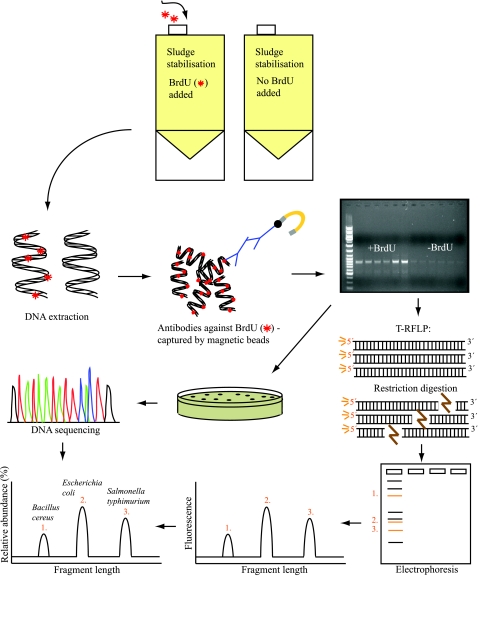
To establish sewage sludge bacterial community structures and potentially identify new bacteria in sludge, PCR should be combined with a fingerprinting approach that is able to visualize the community structure, such as terminal restriction fragment length polymorphism (T-RFLP). T-RFLP provides fingerprints of the dominant members of complex microbial communities and facilitates the identification of individual terminal restriction fragments in an electropherogram by comparison to clone libraries or predictions from existing sequence databases (3) (Fig. 2). Additionally, actively growing bacteria within a sludge bacterial community could be phylogenetically identified using a combination of BrdU immunocapture and T-RFLP, and newly discovered sludge pathogens could be evaluated further in terms of epidemiological status and risk of spread. To estimate the actual concentrations of individual pathogens, a quantitative assay based on molecular detection, such as real-time PCR targeting gene fragments of the bacteria of interest, should preferably be employed. The constant advances in detection techniques should facilitate the identification of novel pathogens as well as the reevaluation of the pathogenicity and significance of known pathogens. Consequently, fast and reliable detection/identification tools are essential to update the risk models for land distribution of sewage sludge.
FIG. 2.
Schematic drawing of a molecular approach involving BrdU immunocapture, T-RFLP, and clone libraries suitable for the evaluation of specific stabilization/sanitization procedures involving stimulation/inhibition of individual bacterial species. By comparing the BrdU-labeled (actively growing) bacterial fraction with the total fraction of the same community, it is possible to separate actively responding bacteria from those that have either been killed during specific treatments or are at least resting (viable but nonculturable). The proposed set of methods should provide invaluable information on the development and optimization of new stabilization/sanitization procedures and allow the rapid and sensitive determination of microbial sludge quality.
CONCLUSIONS AND FUTURE PROSPECTS
The majority of the standard sludge stabilization procedures aiming to reduce biochemical oxygen demand, solid content, and odor provide unsatisfactory results in terms of the reduction of the concentrations of human pathogenic bacteria for the safe use of sewage sludge as an appropriate crop fertilizer product. However, the use of pasteurization as a complement to the regular procedure is a relatively efficient alternative. The major drawbacks of pasteurization are high cost and the inability to kill bacterial endospores. Thus, the significance of a bacterial presence in soil distributed on crop fields requires urgent evaluation. Irradiation is a promising alternative for further development. Basically, more economical sanitization methods with greater efficiency of pathogen elimination and treatment duration need to be optimized. Another promising technique is the use of ammonia-based compounds that fulfill the above criteria and have high agronomic value.
Reliable risk assessment models estimating the spread of pathogenic microorganisms upon land application of sewage sludge are essential. To obtain these guidelines, new molecular tools with high sensitivity are required. Such methods may aid in evaluating the efficiencies of stabilization procedures and in examining the sludge environment for microbial activity and the subsequent fate of specific microorganisms following soil distribution. By using molecular techniques, such as a combination of BrdU immunocapture and T-RFLP, the growth responses (stimulation/inhibition) of individual pathogenic sludge bacteria mixed in soil may be ascertained. Additionally, the growth responses of individual bacteria could be correlated with functional parameters estimated in sludge/soil, and hence the effects of structural shifts in bacterial communities could be compared among stabilization procedures. Metabolically active bacteria within the sludge community should also be visualized and compared with the total bacterial consortium of the same community to provide valuable information about microbial interactions between species and possible evidence of potential antagonism and/or stimulation of specific bacterial species during certain treatments. This information may allow the improvement of stabilization/sanitization treatments to achieve more efficient destruction of the total pathogenic microbial consortium with lower cost and greater rapidity. The new procedures should significantly benefit the recycling of sewage sludge back to arable land, with the ultimate aim of implementation in municipal wastewater treatment facilities worldwide.
Footnotes
Published ahead of print on 7 July 2008.
REFERENCES
- 1.Amer, A. A. 1997. Destruction of sludge pathogenic bacteria using quick lime and cement dust. Egypt J. Soil Sci. 37:343-354. [Google Scholar]
- 2.Arcak, S., A. Karaca, E. Erdogan, and C. Türkmen. 2000. A study on potential agricultural use of sewage sludge of Ankara wastewater treatment plant, p. 345-349. In Proceedings of the International Symposium on Desertification, 13 to 17 June 2000, Konya, Turkey. The Soil Science Society of Turkey, Ankara, Turkey.
- 3.Artursson, V., R. D. Finlay, and J. K. Jansson. 2005. Combined bromodeoxyuridine immunocapture and terminal-restriction fragment length polymorphism analysis highlights differences in the active soil bacterial metagenome due to Glomus mosseae inoculation or plant species. Environ. Microbiol. 7:1952-1966. [DOI] [PubMed] [Google Scholar]
- 4.Artursson, V., and J. K. Jansson. 2003. Use of bromodeoxyuridine immunocapture to identify active bacteria associated with arbuscular mycorrhizal hyphae. Appl. Environ. Microbiol. 69:6208-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aserkoff, B., S. Schroeder, and P. Brachman. 1970. Salmonellosis in the United States—a five year review. Am. J. Epidemiol. 92:13-24. [DOI] [PubMed] [Google Scholar]
- 6.Atlas, R. M., and R. Bartha. 1987. Microbial ecology, 2nd ed. The Benjamin/Cummings Publishing Co., Menlo Park, CA.
- 7.Bagge, E., L. Sahlstrom, and A. Albihn. 2005. The effect of hygienic treatment on the microbial flora of biowaste at biogas plants. Water Res. 39:4879-4886. [DOI] [PubMed] [Google Scholar]
- 8.Bendixen, H. J. 1996. Hygienic recommendations and reduction of pathogenic microorganisms in Danish biogas plants. Dansk Vet. Tidskr. 11:475-482. [Google Scholar]
- 9.Bendixen, H. J. 1999. Hygienic safety—results of scientific investigations in Denmark, p. 27-47. In Proceedings of the IEA Bioenergy Workshop, Hohenheim, Germany. Deutsche Veterinarmedizinische Gesellschaft, Giessen, Germany.
- 10.Bina, B., H. Movahedian, and I. Kord. 2004. The effect of lime stabilization on the microbiological quality of sewage sludge. Iranian J. Env. Health Sci. Eng. 1:34-38. [Google Scholar]
- 11.Bitton, G. 1980. Fate of viruses in sewage treatment plants, p. 122-151. In G. Bitton (ed.), Introduction to environmental virology. Wiley, New York, NY.
- 12.Böhm, R., W. Martens, and W. Philipp. 1999. Regulation in Germany and results of investigations concerning hygienic safety of processing biowastes in biogas plants, p. 48-61. In Proceedings of the IEA Bioenergy workshop, Hohenheim, Germany. Deutsche Veterinarmedizinische Gesellschaft, Giessen, Germany.
- 13.Borneman, J. 1999. Culture-independent identification of microorganisms that respond to specified stimuli. Appl. Environ. Microbiol. 65:3398-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton, C. H., and C. Turner. 2003. Manure management. Treatment strategies for sustainable agriculture, 2nd ed. Lister and Durling, Flitwick, Bedford, United Kingdom.
- 15.Cabrera, M. L., D. E. Kissel, and M. F. Vigil. 2005. Nitrogen mineralization from organic residues: research opportunities. J. Environ. Qual. 34:75-79. [DOI] [PubMed] [Google Scholar]
- 16.Carrington, E. G. 2001. Evaluation of sludge treatments for pathogen reduction—final report. European Commission report no. CO 5026/1. Office for Official Publications of the European Community, Luxembourg.
- 17.Christensen, K. K., M. Carlsbaek, and E. Kron. 2002. Strategies for evaluating the sanitary quality of composting. J. Appl. Microbiol. 92:1143-1158. [DOI] [PubMed] [Google Scholar]
- 18.Counts, C. A., and A. J. Shuckrow. 1974. Lime stabilized sludge: its stability and effect on agricultural land. EPA 670/2-75-012. National Environmental Research Center, U.S. Environmental Protection Agency, Washington, DC.
- 19.Cuba, V., M. Pospisil, and V. Mucka. 2003. Electron beam/biological processing of anaerobic and aerobic sludge. Czech. J. Phys. 53:369-374. [Google Scholar]
- 20.Cuba, V., M. Pospisil, V. Mucka, P. Jenicek, R. Silber, M. Dohányos, and J. Zábranska. 2003. Impact of accelerated electrons on activating process and foaming potential of sludge. Rad. Phys. Chem. 67:545-548. [Google Scholar]
- 21.Czechowski, F., and T. Marcinkowski. 2006. Sewage sludge stabilisation with calcium hydroxide: effect on physicochemical properties and molecular composition. Water Res. 40:1895-1905. [DOI] [PubMed] [Google Scholar]
- 22.Danielsson, M. L. 1977. Salmonella in sewage and sludge. Acta Vet. Scand. Suppl. 65:1-126. [PubMed] [Google Scholar]
- 23.Dasgupta, A. P., and R. R. Hull. 1989. Late blowing of Swiss cheese: incidence of Clostridium tyrobutyricum in manufacturing milk. Aust. J. Dairy Technol. 44:82-87. [Google Scholar]
- 24.Douglas, B. F., and F. R. Magdoff. 1991. An evaluation of nitrogen mineralization indices for organic residues. J. Environ. Qual. 20:368-372. [Google Scholar]
- 25.Dowdy, R. H., W. E. Larson, J. M. Titrud, and J. J. Latterell. 1978. Growth and metal uptake of snap beans grown on sewage sludge-amended soil: a four-year field study. J. Environ. Qual. 7:252-257. [Google Scholar]
- 26.Dudley, D. J., M. N. Guentzel, M. J. Ibarra, B. E. Moore, and B. P. Sagik. 1980. Enumeration of potentially pathogenic bacteria from sewage sludges. Appl. Environ. Microbiol. 39:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumontet, S., A. Scopa, S. Kerje, and K. Krovacek. 2001. The importance of pathogenic organisms in sewage and sewage sludge. J. Air Waste Manage. Assoc. 51:848-860. [DOI] [PubMed] [Google Scholar]
- 28.Eberle, W. M., D. A. Whitney, and G. M. Powell. 1994. Sewage sludge use on agricultural land. Cooperative Extension Service, Kansas State University, Manhattan.
- 29.Edmonds, R. L. 1976. Survival of coliform bacteria in sewage sludge applied to a forest clearcut and potential movement into groundwater. Appl. Environ. Microbiol. 32:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feachem, R. G., D. J. Bradley, and H. Garelick. 1983. Sanitation and disease: health aspects of excreta and waste management. Wiley, New York, NY.
- 31.Federal Register. 1993. Standards for the use or disposal of sewage sludge, final rules. Fed. Regist. 58:9398-9400. [Google Scholar]
- 32.Gadre, R. V., D. R. Ranade, and S. H. Godbole. 1986. A note on survival of salmonellas during anaerobic digestion of cattle dung. J. Appl. Bacteriol. 60:93-96. [DOI] [PubMed] [Google Scholar]
- 33.Gantzer, C., P. Gaspard, L. Galvez, A. Huyard, N. Dumouthier, and J. Schwartzbrod. 2001. Monitoring of bacterial and parasitological contamination during various treatment of sludge. Water Res. 35:3763-3770. [DOI] [PubMed] [Google Scholar]
- 34.Garau, M. A., M. T. Felipó, and M. C. Ruiz de Villa. 1986. Nitrogen mineralization of sewage sludges in soils. J. Environ. Qual. 15:225-228. [Google Scholar]
- 35.Gerba, C. P., and J. E. Smith, Jr. 2005. Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 34:42-48. [PubMed] [Google Scholar]
- 36.Gerba, C. P., C. Wallis, and J. L. Melnick. 1975. Fate of wastewater bacteria and viruses in soil. J. Irrigation Drainage Div. 101:157-174. [Google Scholar]
- 37.Gibbs, R. A., C. J. Hu, G. E. Ho, P. A. Philips, and I. Unkovich. 1995. Pathogen die-off in stored wastewater sludge. Water Sci. Technol. 31:91-95. [Google Scholar]
- 38.Gibbs, R. A., C. J. Hu, G. E. Ho, and I. Unkovich. 1997. Regrowth of faecal coliforms and salmonellae in stored biosolids and soil amended with biosolids. Water Sci. Technol. 35:269-275. [Google Scholar]
- 39.Goldfarb, W., U. Krogmann, and C. Hopkins. 1999. Unsafe sewage sludge or beneficial biosolids? Liability, planning, and management issues regarding the land application of sewage treatment residuals. Boston Coll. Environ. Affairs Law Rev. 26:687-768. [Google Scholar]
- 40.Goldstein, N., W. A. Yanko, J. M. Walker, and W. Jakubowski. 1988. Determining pathogen levels in sludge products. Biocycle 29:44-47. [Google Scholar]
- 41.Hagedorn, C. 1980. Potential health hazard associated with the disposal of sewage sludge on agricultural soils in western Oregon. Water Research Institute, Oregon State University, Corvallis.
- 42.Hansen, R. W. 2007. Methane generation from livestock wastes. Colorado State University, Fort Collins, CO. http://www.ext.colostate.edu/PUBS/FARMMGT/05002.html.
- 43.Hess, E., and C. Breer. 1975. Epidemiology of salmonellae and fertilizing grassland with sewage sludge. Zentralbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. 16:154-160. [PubMed] [Google Scholar]
- 44.Hussong, D., W. D. Burge, and N. K. Enkiri. 1985. Occurrence, growth, and suppression of salmonellae in composted sewage sludge. Appl. Environ. Microbiol. 50:887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilsoe, B. 1993. Risk of infections by re-circulation of biowaste. Dansk Vet. Tidskr. 76:77-85. [Google Scholar]
- 46.Jepsen, S.-E., M. Krause, and H. Gruttner. 1997. Reduction of fecal streptococcus and Salmonella by selected treatment methods for sludge and organic waste. Water Sci. Technol. 36:203-210. [Google Scholar]
- 47.Jones, P., and M. Martin. 2003. A review of the literature on the occurrence and survival of pathogens of animals and humans in green compost. WRAP standards report. The Waste and Resources Action Programme, Oxon, United Kingdom.
- 48.Jones, P. W. 1980. Health hazards associated with the handling of animal wastes. Vet. Rec. 106:4-7. [DOI] [PubMed] [Google Scholar]
- 49.Jones, P. W., and P. R. Matthews. 1975. Examination of slurry from cattle for pathogenic bacteria. J. Hyg. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kearney, T. E., M. J. Larkin, J. P. Frost, and P. N. Levett. 1993. Survival of pathogenic bacteria during mesophilic anaerobic digestion of animal waste. J. Appl. Bacteriol. 75:215-219. [DOI] [PubMed] [Google Scholar]
- 51.Kearney, T. E., M. J. Larkin, and P. N. Levett. 1993. The effect of slurry storage and anaerobic digestion on survival of pathogenic bacteria. J. Appl. Bacteriol. 74:86-93. [DOI] [PubMed] [Google Scholar]
- 52.Kearney, T. E., M. J. Larkin, and P. N. Levett. 1994. Metabolic activity of pathogenic bacteria during semicontinuous anaerobic digestion. Appl. Environ. Microbiol. 60:3647-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krovacek, K., A. Farris, and I. Mansson. 1989. Enterotoxigenic and drug sensitivity of Aeromonas hydrophila isolated from well water in Sweden: a case study. Int. J. Food Microbiol. 8:149-154. [DOI] [PubMed] [Google Scholar]
- 54.Larsen, H. E. 1995. Bakteriologiske risici ved anvendelse af husdyrgödning of affald. Rev. Dansk Vet. Tidskrift. 78:763-766. [Google Scholar]
- 55.Larsen, H. E., and B. Munch. 1982. Occurrence and survival of pathogenic bacteria in cattle and pig slurry, p. 161-174. In J. R. Watton and E. G. White (ed.), Communicable diseases resulting from storage handling, transport and landspreading of manures. Commission of the European Communities, Brussels, Belgium.
- 56.Larsen, H. E., and B. Munch. 1986. Pathogenic bacteria in extraanimal environments, p. 57-66. In H. E. Larsen et al. (ed.), Ugeskrift for jordbrug, selective research reviews. Klampenbourg, Copenhagen, Denmark.
- 57.Larsen, H. E., B. Munch, and J. Schlundt. 1994. Use of indicators for monitoring the reduction of pathogens in animal waste treated in biogas plants. Zentralbl. Hyg. Umveltmed. 195:544-555. [PubMed] [Google Scholar]
- 58.Ligocka, A., and Z. Paluszak. 2005. Capability of lactic acid bacteria to inhibit pathogens in sewage sludge subjected to biotechnological processes. Bull. Vet. Inst. Pulawy 49:23-27. [Google Scholar]
- 59.Lowder, M., A. Unge, N. Maraha, J. K. Jansson, J. Swiggett, and J. D. Oliver. 2000. Effect of starvation and the viable-but-nonculturable state on green fluorescent protein (GFP) fluorescence in GFP-tagged Pseudomonas fluorescens A506. Appl. Environ. Microbiol. 66:3160-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucero-Ramirez, B., and J. F. Malina. 1998. The effect of standard municipal sludge treatment processes on the reduction of indicator and pathogenic organisms, p. 95-103. In Proceedings of the 71st Water Environment Federation Technical Exhibit Conference, Orlando, Florida. Water Environment Federation, Alexandria, VA.
- 61.Madur Electronics. 2003. Biogas. Madur Electronics, Vienna, Austria. http://www.habmigern2003.info/PDF/methane-digester.pdf.
- 62.Mavridou, A., P. Kouloubis, E. Vassalou, F. Rigas, and N. Vakalis. 2001. Microbiological quality of sewage sludge in Greece disposed for agricultural use. Int. J. Environ. Health Res. 11:275-279. [DOI] [PubMed] [Google Scholar]
- 63.Mendez, J. M., B. Jimenez, and C. Maya. 2004. Disinfection kinetics of pathogens in physicochemical sludge treated with ammonia. Water Sci. Technol. 50:67-74. [PubMed] [Google Scholar]
- 64.Mitscherlich, E., and E. H. Marth. 1984. Microbial survival in the environment. Springer, Berlin, Germany.
- 65.Munch, B., and J. Schlundt. 1983. On the reduction of pathogenic and indicator bacteria in animal slurry and sewage sludge subjected to anaerobic digestion or chemical disinfection, p. 130-149. In D. Strauch (ed.), Hygienic problems of animal manures. Proceedings of a Joint Workshop of Expert Groups of the Commission of the European Communities, German Veterinary Medical Society (DVG) and Food and Agricultural Organization, Stuttgart. University of Hohenheim, Stuttgart, Germany.
- 66.National Lime Association. 2008. Environmental uses of lime. National Lime Association, Arlington, VA. http://www.lime.org/ENV02/ENV802.htm.
- 67.Oates, J. A. H. 1998. Lime and limestone: chemistry and technology, production and uses. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 68.Olsen, J. E., J. B. Jörgensen, and P. Nansen. 1985. On the reduction of Mycobacterium paratuberculosis in bovine slurry subjected to batch mesophilic or thermophilic anaerobic digestion. Agric. Wastes 13:273-280. [Google Scholar]
- 69.Olsen, J. E., and H. E. Larsen. 1987. Bacterial decimation times in anaerobic digestions of animal slurries. Biol. Wastes 21:153-168. [Google Scholar]
- 70.Pederson, D. C. 1983. Effectiveness of sludge treatment processes in reducing levels of bacteria, viruses and parasites, p. 9-31. In P. M. Wallis and D. L. Lehman (ed.), Biological health risks of sludge disposal to land in cold climates. University of Calgary Press, Calgary, Alberta, Canada.
- 71.Plym-Forshell, L. 1995. Survival of Salmonella spp. and Ascaris suum eggs in a thermophilic biogas plant. Acta Vet. Scand. 36:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reimers, R. S., E. R. Desocio, W. S. Bankston, and J. A. Oleszkiewicz. 1998. Current/future advances in biosolids disinfection processing, p. 445-459. In Proceedings of the 71st Water Environment Federation Technical Exhibit Conference, Orlando, Florida. Water Environment Federation, Alexandria, VA.
- 73.Rose, B. J. 1997. Environmental ecology of Cryptosporidium and public health implications. Ann. Rev. Public Health 18:135-161. [DOI] [PubMed] [Google Scholar]
- 74.Rosef, O. 1999. Salmonella in sludge. Nor Vet. Tidskr. 111:795-799. [Google Scholar]
- 75.Roszak, D. B., and R. R. Colwell. 1987. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 51:365-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sahlström, L. 2003. A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour. Technol. 87:161-166. [DOI] [PubMed] [Google Scholar]
- 77.Sahlström, L., A. Aspan, E. Bagge, M.-L. Danielsson-Tham, and A. Albihn. 2004. Bacterial pathogen incidences in sludge from Swedish sewage treatment plants. Water Res. 38:1989-1994. [DOI] [PubMed] [Google Scholar]
- 78.Saleem, M., M. H. Al-Malack, and A. A. Bukhari. 2001. Seasonal variations in the microbial population density present in biological sludge. Environ. Technol. 22:255-259. [DOI] [PubMed] [Google Scholar]
- 79.Shin, K. S., and H. Kang. 2003. Electron beam pretreatment of sewage sludge before anaerobic digestion. Appl. Biochem. Biotechnol. 109:227-239. [DOI] [PubMed] [Google Scholar]
- 80.Skanavis, C., and W. A. Yanko. 1994. Evaluation of composted sewage sludge based soil amendments for potential risk of salmonellosis. Environ. Health 56:7. [Google Scholar]
- 81.Skirrow, M. B. 1990. Foodborne illness: Campylobacter. Lancet 336:921-923. [DOI] [PubMed] [Google Scholar]
- 82.Straub, T. M., I. L. Pepper, and C. P. Gerba. 1993. Hazards from pathogenic microorganisms in land-disposed sewage sludge. Rev. Environ. Contam. Toxicol. 132:55-91. [DOI] [PubMed] [Google Scholar]
- 83.Strauch, D. 1999. Improvements of the quality of sludge: microbial aspects, p. 160-169. In A. H. Dirkzawger and P. L. Hermitc (ed.), Sewage sludge treatment and use. Elsevier, London, United Kingdom.
- 84.Strauch, D. 1991. Microbiological treatment of municipal sewage waste and refuse as a means of disinfection prior to recycling in agriculture. Stud. Environ. Sci. 42:121-136. [Google Scholar]
- 85.Strauch, D. 1998. Pathogenic microorganisms in sludge. Anaerobic digestion and disinfection methods to make sludge usable as a fertilizer. Eur. Water Manage. 1:12-26. [Google Scholar]
- 86.Strauch, D. 1991. Survival of pathogenic micro-organisms and parasites in excreta, manure and sewage sludge. Rev. Sci. Technol. 10:813-846. [DOI] [PubMed] [Google Scholar]
- 87.Trad Rais, M., and R. Ben Aissa. 1998. Contamination bactérienne des boues d'épuration utilisées à des fins agricoles en Tunisie. J. Eur. Hydrol. 28:339-354. [Google Scholar]
- 88.Urbach, E., K. L. Vergin, and S. J. Giovannoni. 1999. Immunochemical detection and isolation of DNA from metabolically active bacteria. Appl. Environ. Microbiol. 65:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.U.S. Environmental Protection Agency. 1999. Environmental regulations and technology. Control of pathogens and vector attraction in sewage sludge. Report EPA/625/R-92/013. U.S. Environmental Protection Agency, Washington, DC.
- 90.Ward, R. L., J. G. Yeager, and C. S. Ashley. 1981. Response of bacteria in wastewater sludge to moisture loss by evaporation and effect of moisture content on bacterial inactivation by ionizing radiation. Appl. Environ. Microbiol. 41:1123-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venglovsky, J., J. Martinez, and I. Placha. 2006. Hygienic and ecological risks connected with utilization of animal manures and biosolids in agriculture. Livestock Sci. 102:197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vinneras, B., A. Holmqvist, E. Bagge, A. Albihn, and H. Jonsson. 2003. The potential for disinfection of separated faecal matter by urea and by peracetic acid for hygienic nutrient recycling. Bioresour. Technol. 89:155-161. [DOI] [PubMed] [Google Scholar]
- 93.Yeager, J. G., and R. L. Ward. 1981. Effects of moisture content on long-term survival and regrowth of bacteria in wastewater sludge. Appl. Environ. Microbiol. 41:1117-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]